

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laforin (Homo sapiens (Human)) | BDBM231167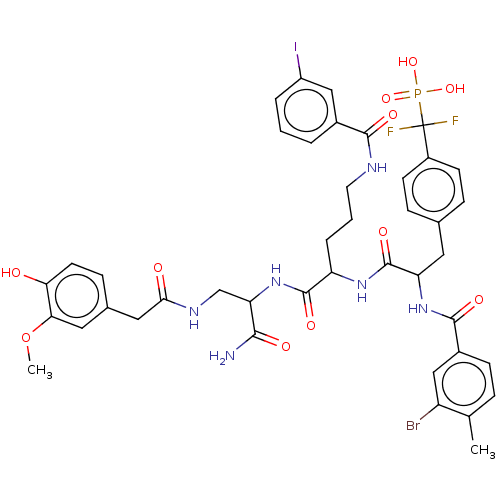 (US9340574, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50087856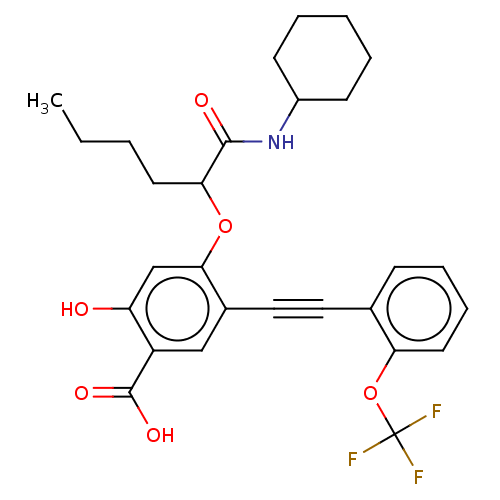 (CHEMBL3426913) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) using pNPP as substrate at pH 7 at 25 degC by spectrophotometric analysis | Bioorg Med Chem 23: 2798-809 (2015) Article DOI: 10.1016/j.bmc.2015.03.066 BindingDB Entry DOI: 10.7270/Q2VD7168 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50054344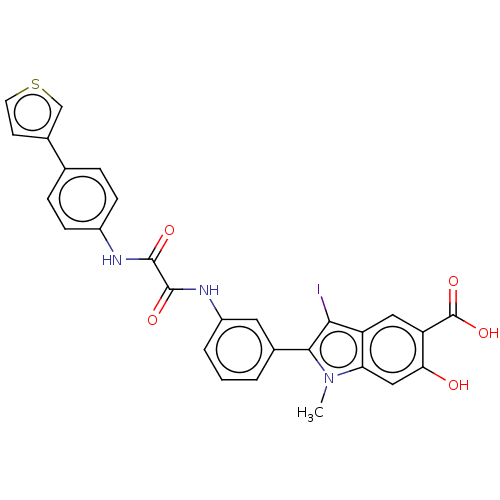 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description For selectivity studies, the PTPs, including LYP, mPTPA, SHP1-D1C, PTP1B, LMPTP, VHR, Laforin and PTPα-D1D2 were expressed and purified from E. ... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of laforin (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50544440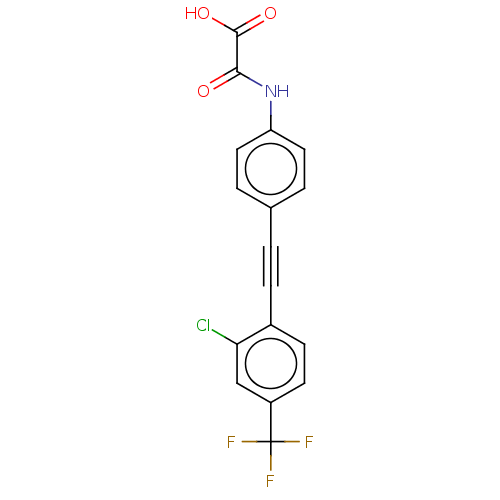 (CHEMBL4647367 | US11192850, Entry 4t) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50425808 (CHEMBL2316906) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50544431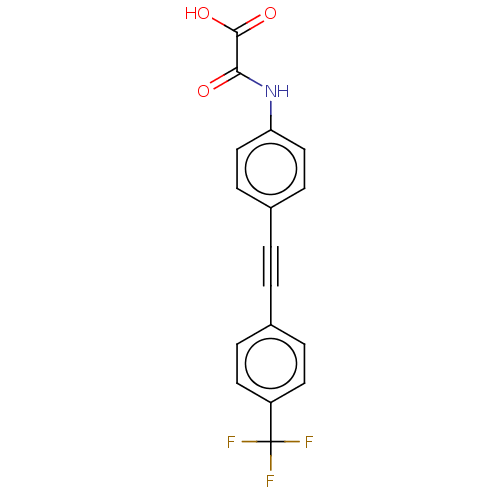 (CHEMBL4637459 | US11192850, Entry 4k) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50425806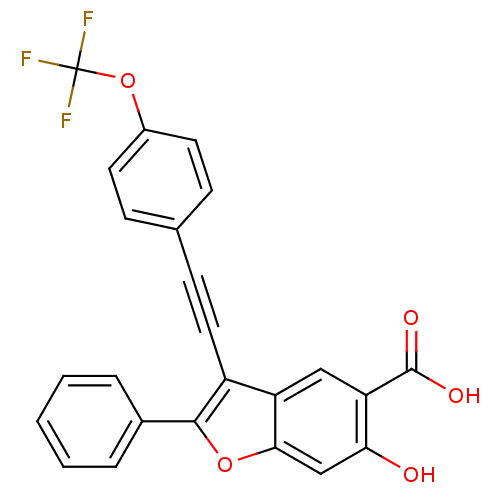 (CHEMBL2316907) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50425807 (CHEMBL2316902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50544427 (CHEMBL4632818 | US11192850, Entry 4g) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Laforin (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Laforin (Homo sapiens (Human)) | BDBM50498446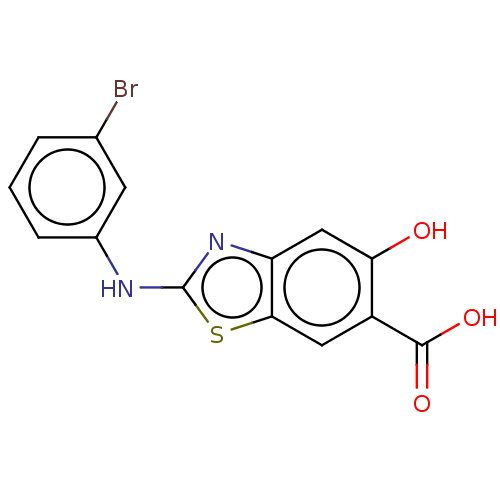 (CHEMBL3596431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human laforin using pNPP substrate by spectrophotometry | Medchemcomm 5: 1496-1499 (2014) Article DOI: 10.1039/c4md00099d BindingDB Entry DOI: 10.7270/Q25D8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||