

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427622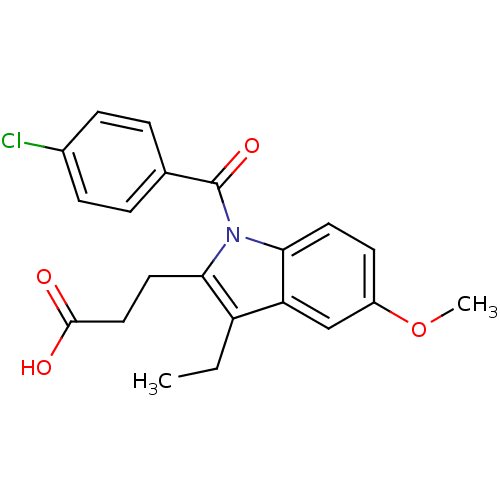 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427624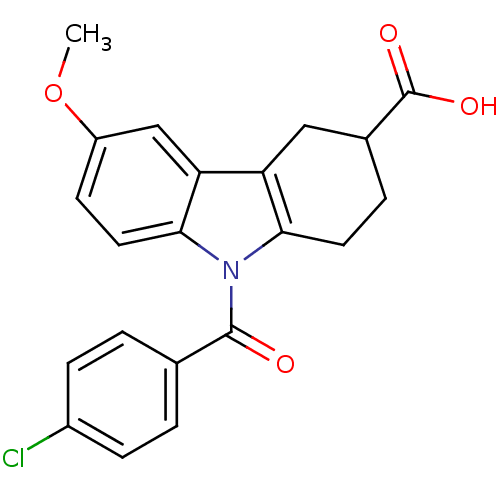 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427620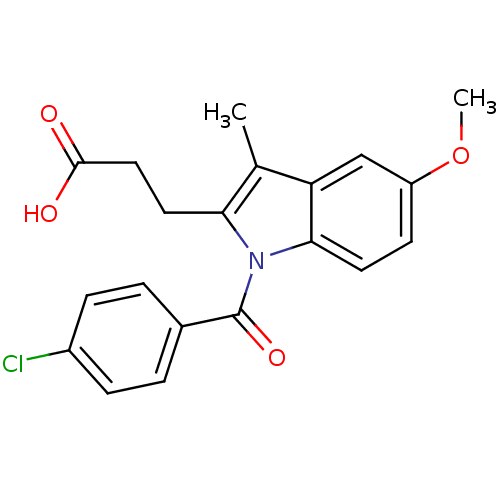 (CHEMBL2323507 | US9346803, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427619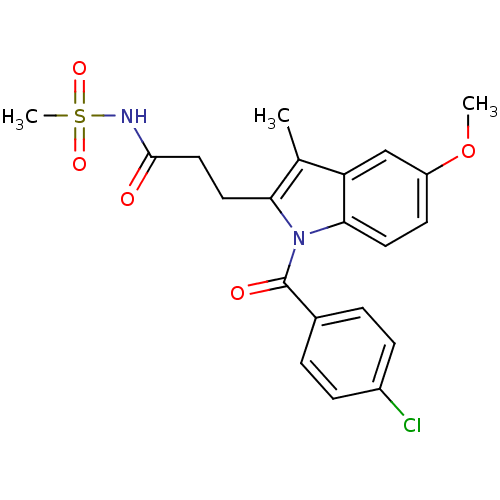 (CHEMBL2323511 | US9346803, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50293598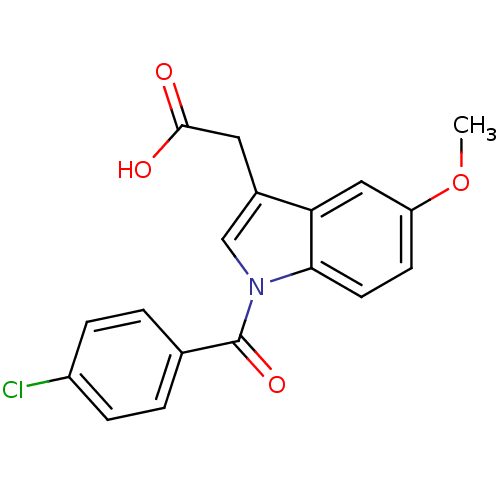 (2'-des-methyl indomethacin | CHEMBL503179 | US9346...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 48.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427628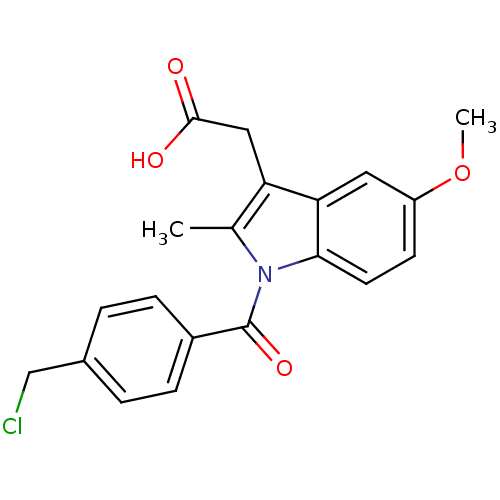 (CHEMBL2323472 | US9346803, Table 2, Compound 8: 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427621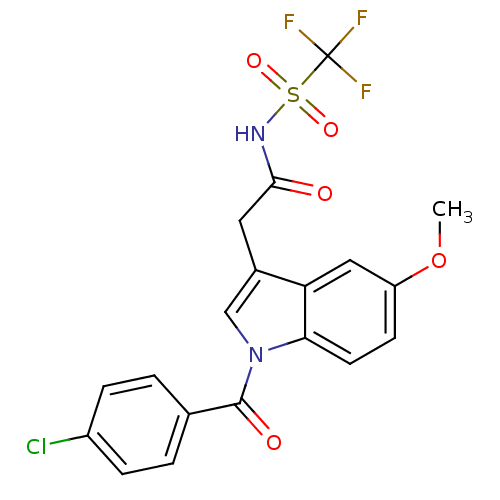 (CHEMBL2323490 | US9346803, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427627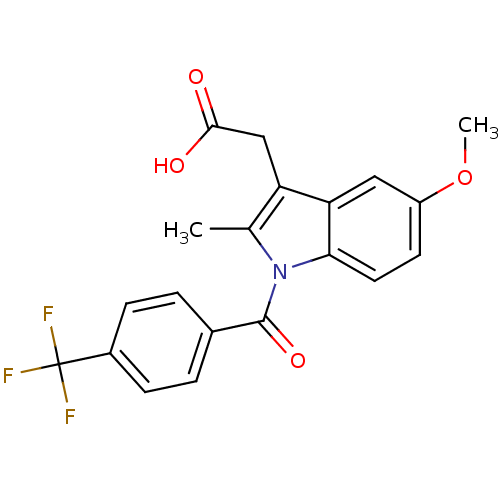 (CHEMBL2323474 | US9346803, Table 2, Compound 9: 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427629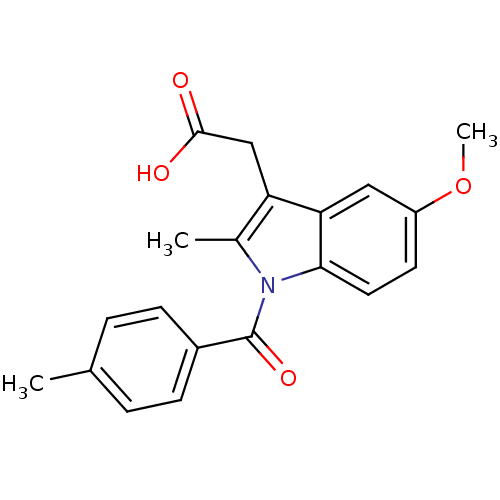 (CHEMBL179587 | US9346803, Table 2, Compound 7: 2-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427625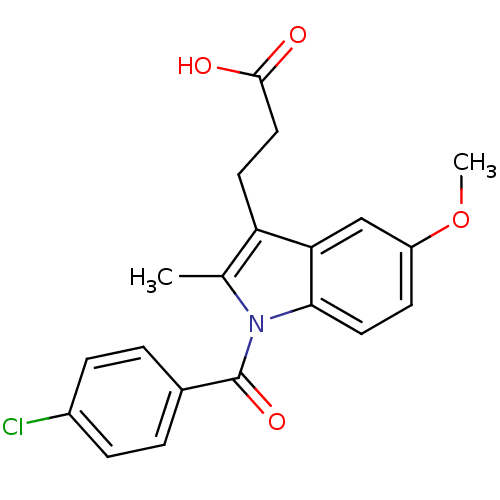 (CHEMBL178687 | US9346803, Table 2, Compound 6: 3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427626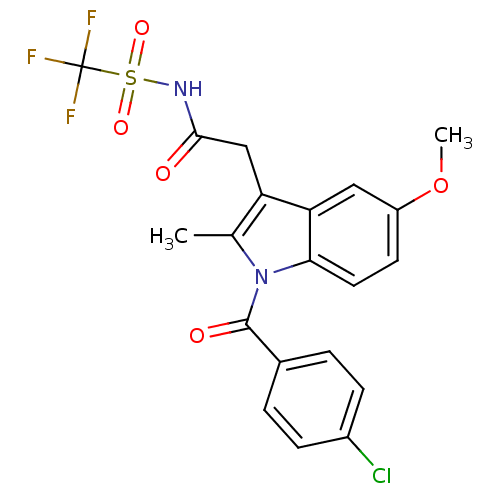 (CHEMBL2323481 | US9346803, Table 2, Compound 5: 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50241828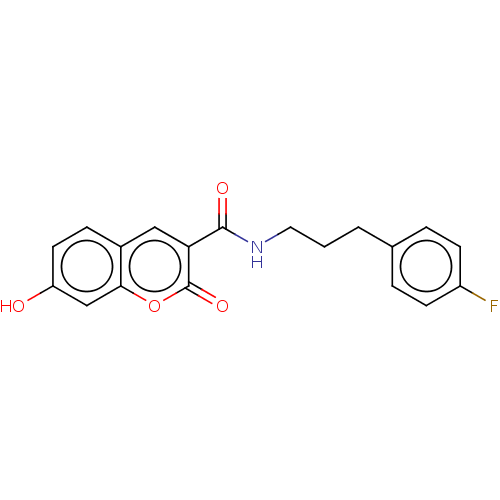 (CHEMBL4089817) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C4 using S-tetralol as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50595596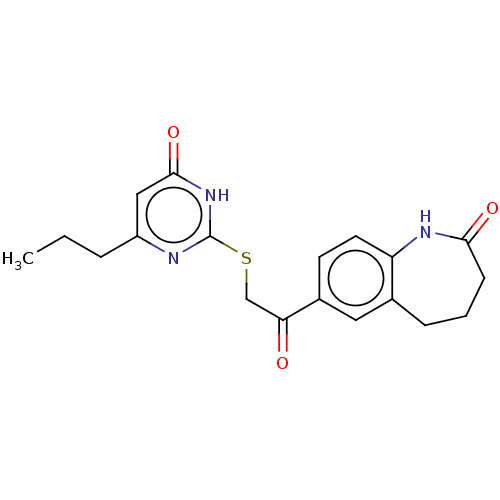 (CHEMBL5203670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00175 BindingDB Entry DOI: 10.7270/Q2DB85WJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50595592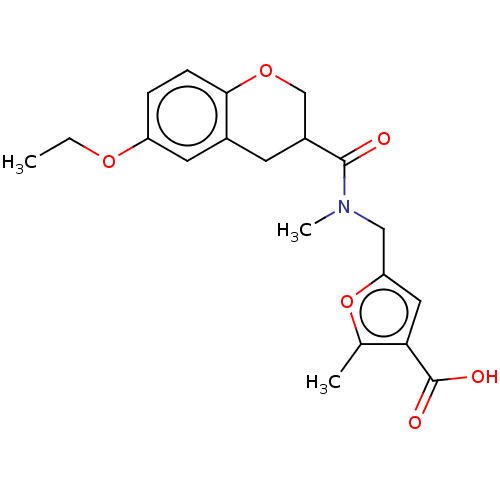 (CHEMBL5179823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00175 BindingDB Entry DOI: 10.7270/Q2DB85WJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50241817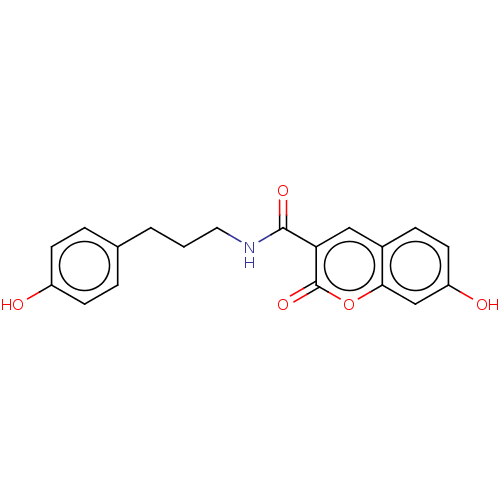 (CHEMBL4081954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C4 using S-tetralol as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50029207 ((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged AKR1C4 expressed in Escherichia coli using S-tetralol as substrate by fluorometry | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396689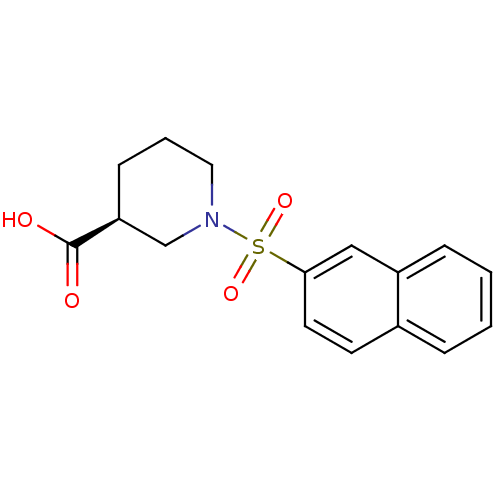 (CHEMBL2172077) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427624 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427620 (CHEMBL2323507 | US9346803, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50240376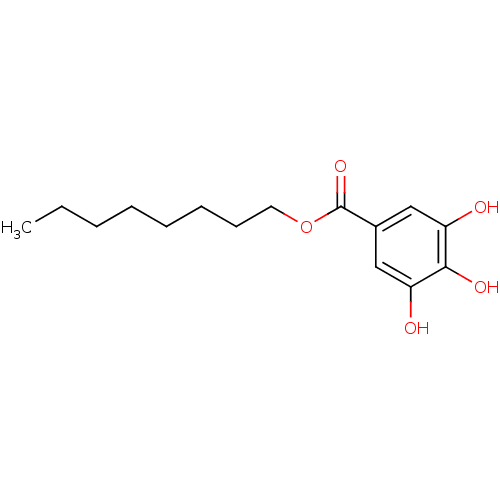 (3,4,5-Trihydroxy-benzoic acid octyl ester | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His-tagged human AKR1C4 expressed in Escherichia coli BL21 (Condon Plus) competent cells using 9,10 -Phenanthrenequinone as ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50546234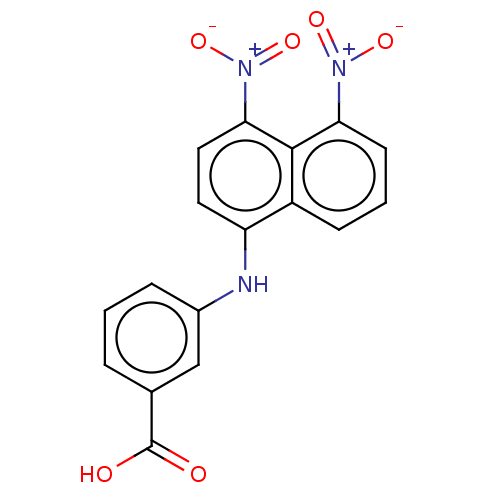 (CHEMBL4758386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AKR1C4 using S-tetralol as substrate in presence of NADP by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM220123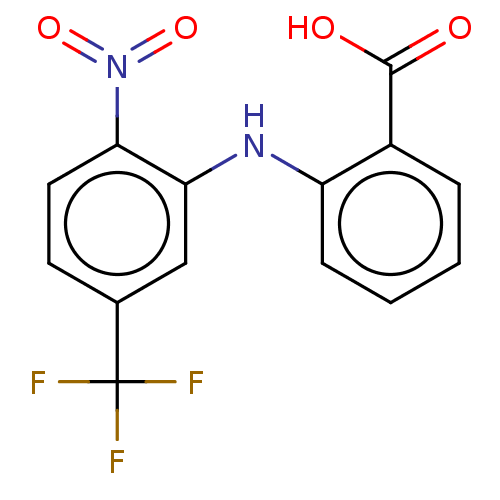 (US9271961, BMT 5-119) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50385687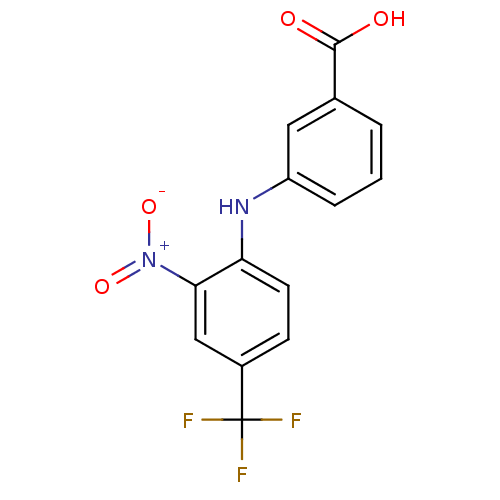 (CHEMBL2041555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM220115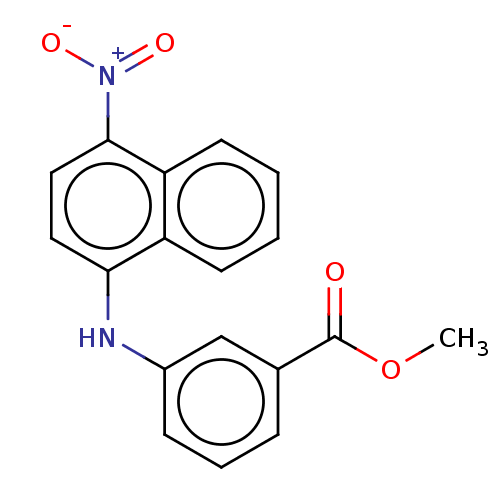 (US9271961, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50382163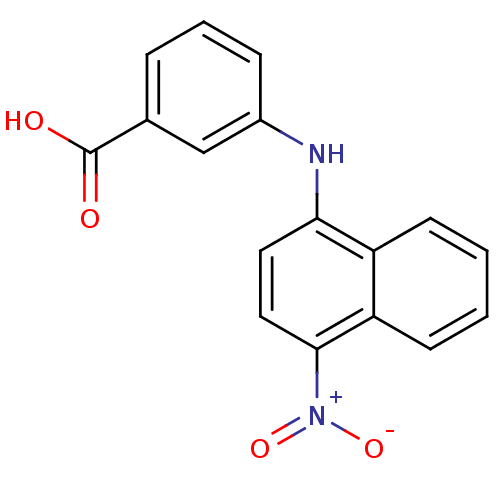 (CHEMBL2023820) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assay | Bioorg Med Chem Lett 22: 3492-7 (2012) Article DOI: 10.1016/j.bmcl.2012.03.085 BindingDB Entry DOI: 10.7270/Q24M95JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50029207 ((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His-tagged human AKR1C4 expressed in Escherichia coli BL21 (Condon Plus) competent cells using 9,10 -Phenanthrenequinone as ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396647 (CHEMBL2172083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543402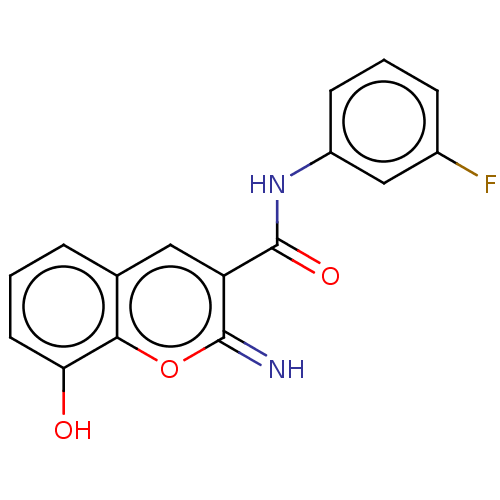 (CHEMBL4634960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543397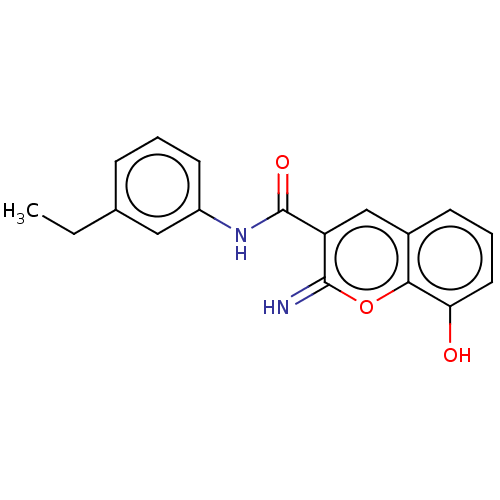 (CHEMBL4646593) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543399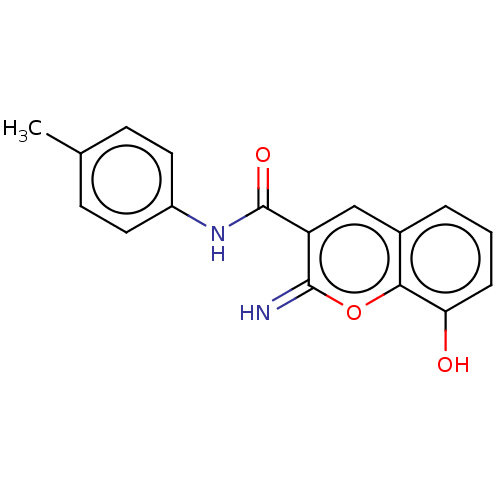 (CHEMBL4648793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543400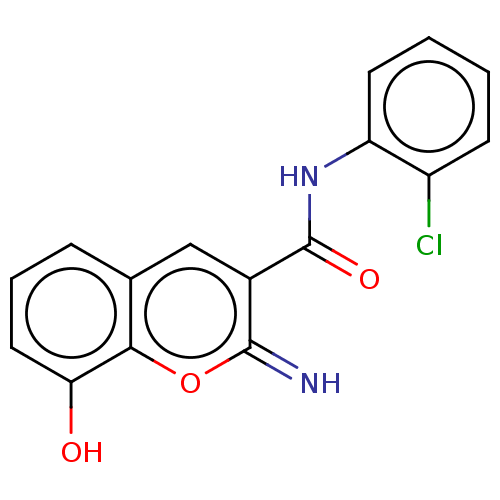 (CHEMBL4640248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543401 (CHEMBL4644092) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543404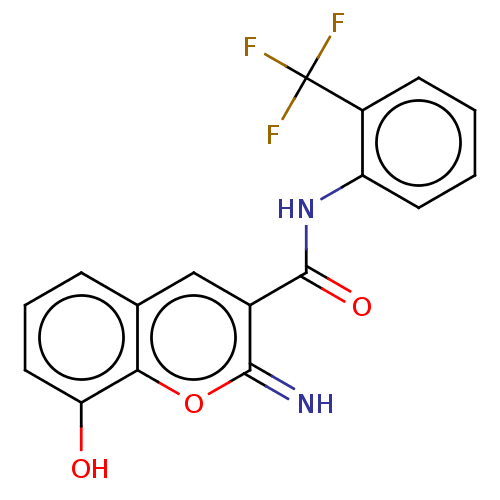 (CHEMBL4640154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50543405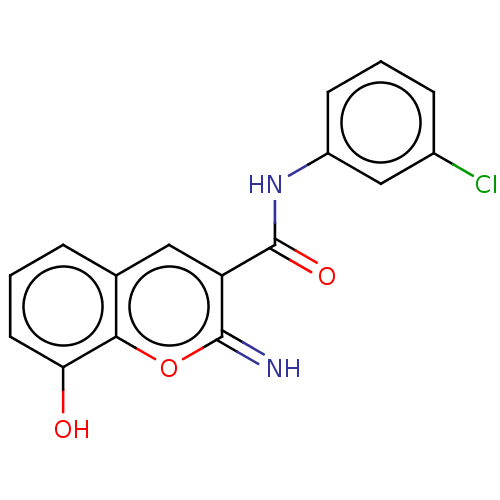 (CHEMBL4639417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427619 (CHEMBL2323511 | US9346803, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50509728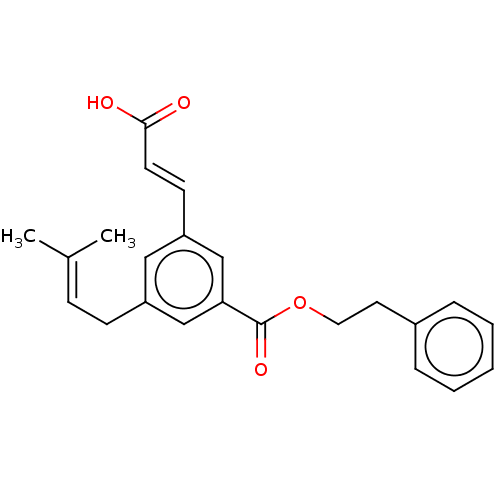 (CHEMBL4545749) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Tech University Health Sciences Center Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C4 expressed in Escherichia coli BL21 (D3) using S-tetralol as substrate in presence of NADP+ by UV-spectrophotom... | J Med Chem 62: 3590-3616 (2019) Article DOI: 10.1021/acs.jmedchem.9b00090 BindingDB Entry DOI: 10.7270/Q2QF8X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50546235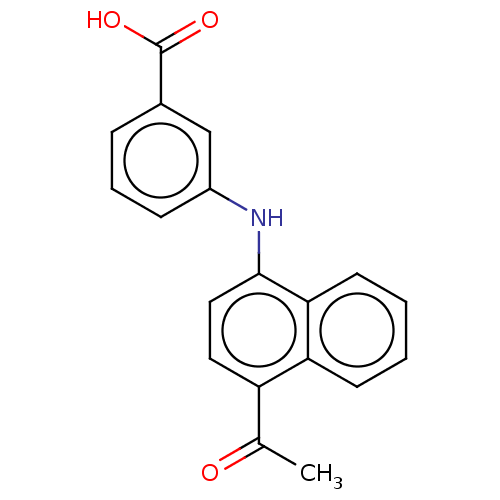 (CHEMBL4795494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AKR1C4 using S-tetralol as substrate in presence of NADP by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50024764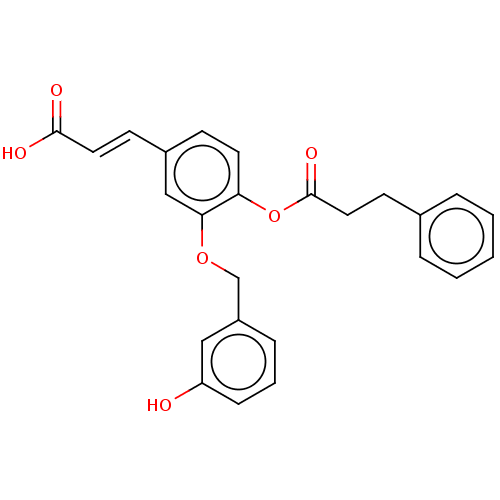 (CHEMBL3337721) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AKR1C4 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay | Bioorg Med Chem 22: 5220-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.007 BindingDB Entry DOI: 10.7270/Q28P623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50390657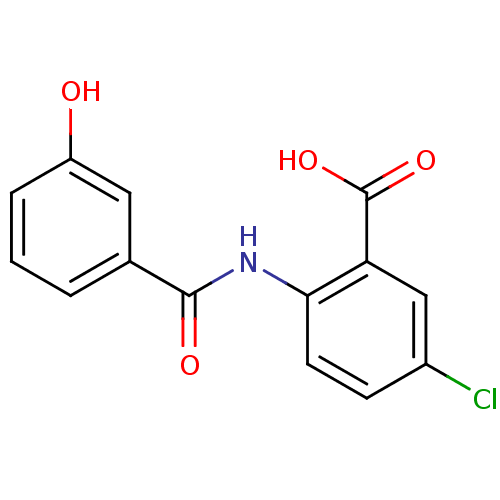 (CHEMBL2070002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 using S-tetralol as substrate by fluorimetry | Bioorg Med Chem Lett 22: 5948-51 (2012) Article DOI: 10.1016/j.bmcl.2012.07.062 BindingDB Entry DOI: 10.7270/Q2BK1DFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50385688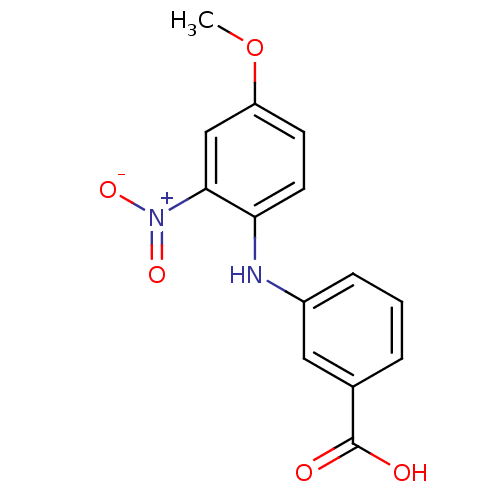 (CHEMBL2041556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM220124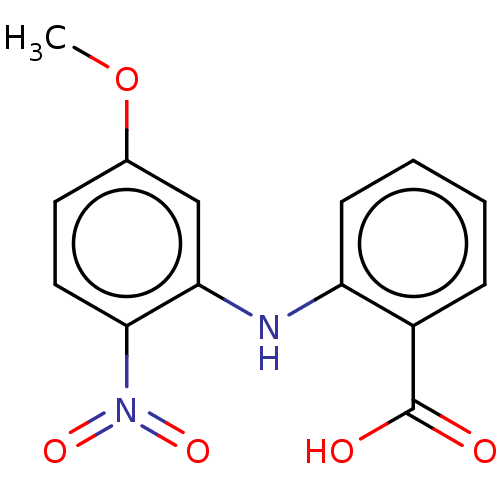 (US9271961, BMT 4-90) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50384946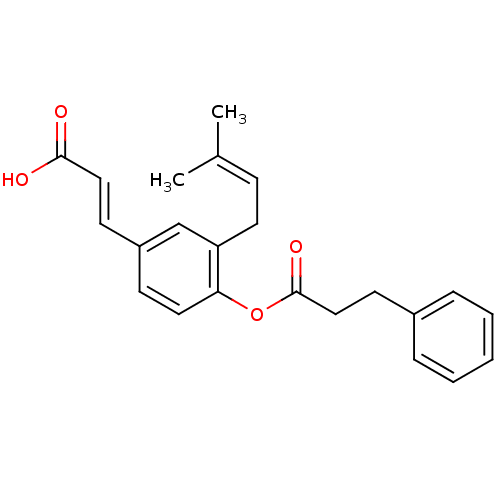 (CHEMBL511708) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Tech University Health Sciences Center Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C4 expressed in Escherichia coli BL21 (D3) using S-tetralol as substrate in presence of NADP+ by UV-spectrophotom... | J Med Chem 62: 3590-3616 (2019) Article DOI: 10.1021/acs.jmedchem.9b00090 BindingDB Entry DOI: 10.7270/Q2QF8X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50337282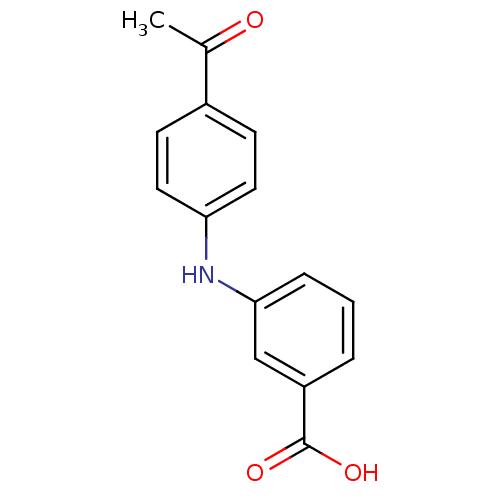 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM220122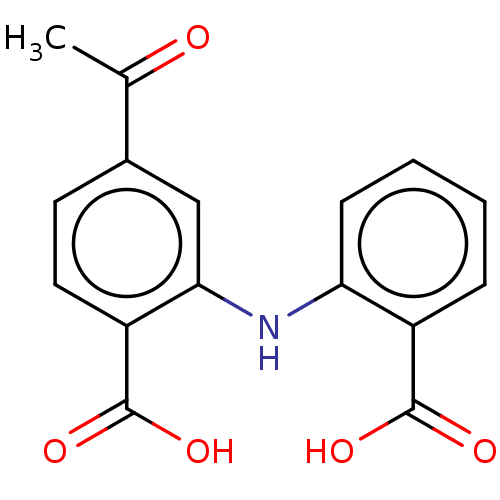 (US9271961, BMT 3-224) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50385686 (CHEMBL2041554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396669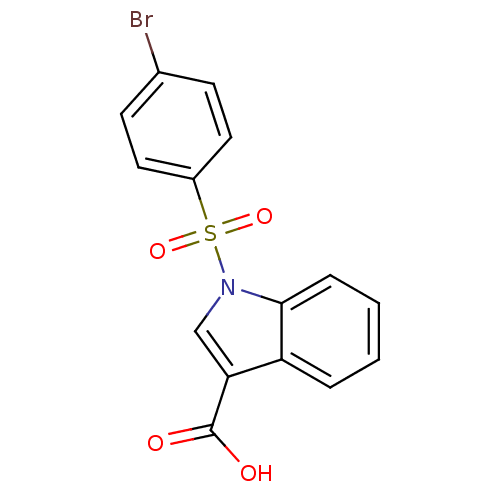 (CHEMBL2172108) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396670 (CHEMBL2172107) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396671 (CHEMBL2172106) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |