 Found 14 hits of kd data for polymerid = 10127,1044,254,3620
Found 14 hits of kd data for polymerid = 10127,1044,254,3620 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50599186
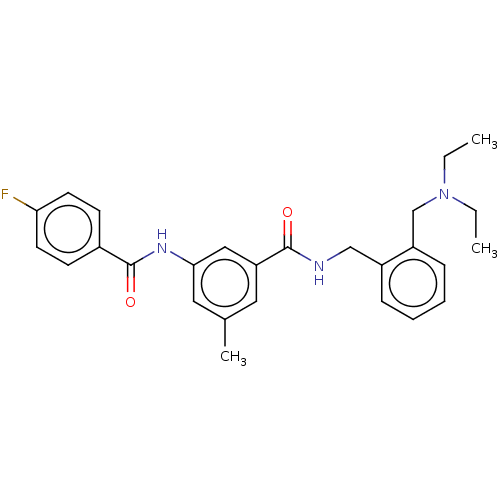
(CHEMBL5201089)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(C)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599167
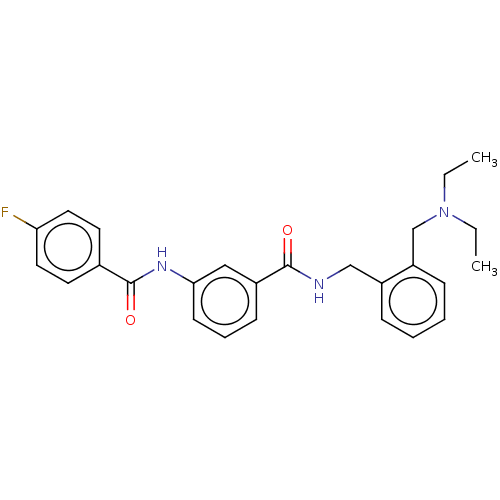
(CHEMBL5195228)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50574799
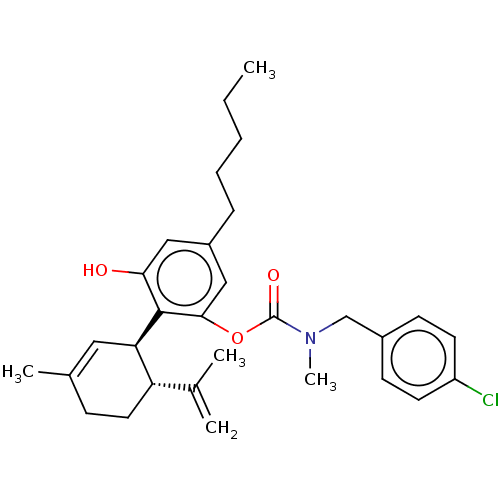
(CHEMBL4870785)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(OC(=O)N(C)Cc2ccc(Cl)cc2)c1 |r,t:11| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Pseudo-irreversible inhibition of equine serum BuChE assessed as binding affinity in an equilibrium state using butyrylthiocholine iodide as substrat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113735
BindingDB Entry DOI: 10.7270/Q2H41W7X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50574801
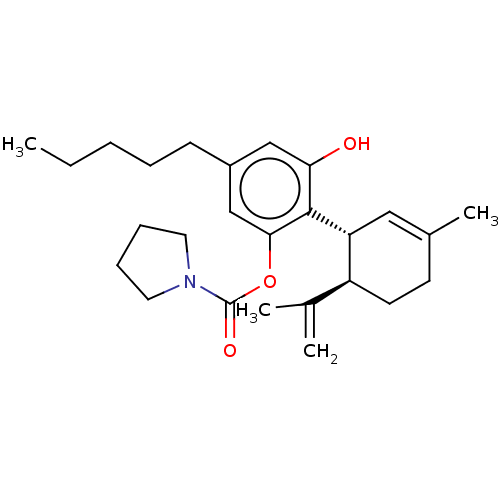
(CHEMBL4847128)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(OC(=O)N2CCCC2)c1 |r,t:11| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Pseudo-irreversible inhibition of equine serum BuChE assessed as binding affinity in an equilibrium state using butyrylthiocholine iodide as substrat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113735
BindingDB Entry DOI: 10.7270/Q2H41W7X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM11682

(2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Pseudo-irreversible inhibition of equine serum BuChE assessed as binding affinity in an equilibrium state using butyrylthiocholine iodide as substrat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113735
BindingDB Entry DOI: 10.7270/Q2H41W7X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91725

(Carbacylamidophosphate, 2a)Show InChI InChI=1S/C7H5BrCl2NO2P/c8-6-3-1-5(2-4-6)7(12)11-14(9,10)13/h1-4H,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+5 | 2.10E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91726
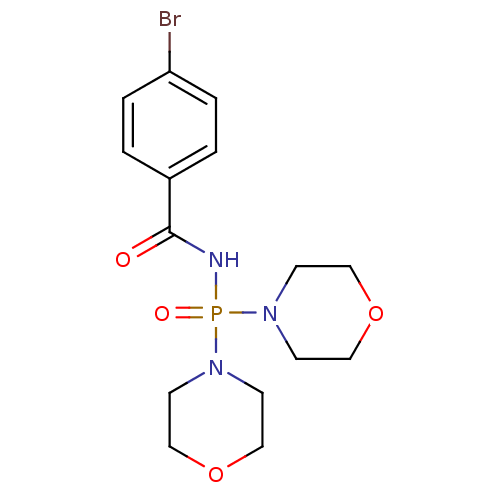
(Carbacylamidophosphate, 2b)Show InChI InChI=1S/C15H21BrN3O4P/c16-14-3-1-13(2-4-14)15(20)17-24(21,18-5-9-22-10-6-18)19-7-11-23-12-8-19/h1-4H,5-12H2,(H,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.24E+6 | 4.37E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91724
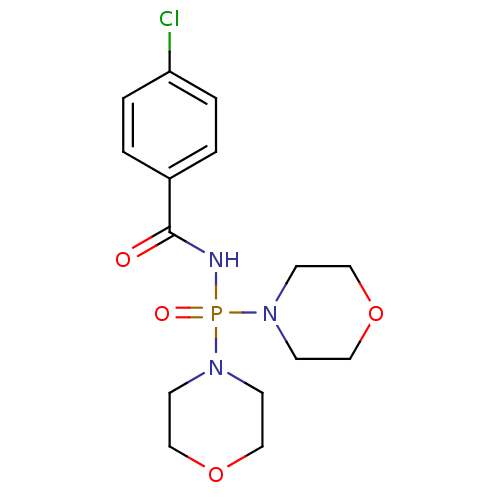
(Carbacylamidophosphate, 1b)Show InChI InChI=1S/C15H21ClN3O4P/c16-14-3-1-13(2-4-14)15(20)17-24(21,18-5-9-22-10-6-18)19-7-11-23-12-8-19/h1-4H,5-12H2,(H,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.98E+6 | 5.06E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50241982
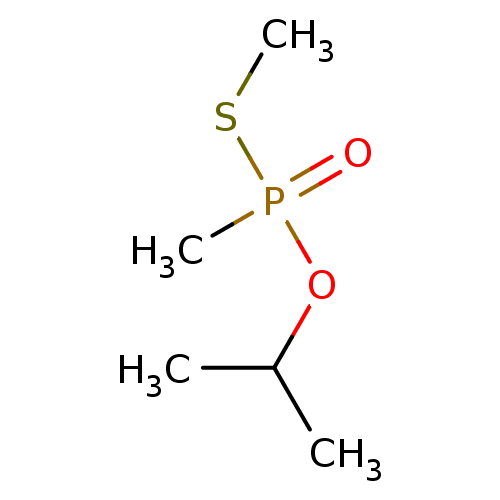
(CHEMBL4093201)Show InChI InChI=1S/C5H13O2PS/c1-5(2)7-8(3,6)9-4/h5H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.30E+6 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Binding affinity to human BChE using butyrylthiocholine as substrate measured for 30 secs by Ellman's method |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91729

(Carbacylamidophosphate, 4a)Show InChI InChI=1S/C8H8Cl2NO2P/c1-6-2-4-7(5-3-6)8(12)11-14(9,10)13/h2-5H,1H3,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+6 | 8.90E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91727

(Carbacylamidophosphate, 3a)Show InChI InChI=1S/C7H6Cl2NO2P/c8-13(9,12)10-7(11)6-4-2-1-3-5-6/h1-5H,(H,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+6 | 1.80E+7 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91730

(Carbacylamidophosphate, 4b)Show InChI InChI=1S/C16H24N3O4P/c1-14-2-4-15(5-3-14)16(20)17-24(21,18-6-10-22-11-7-18)19-8-12-23-13-9-19/h2-5H,6-13H2,1H3,(H,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+7 | 3.36E+7 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91723
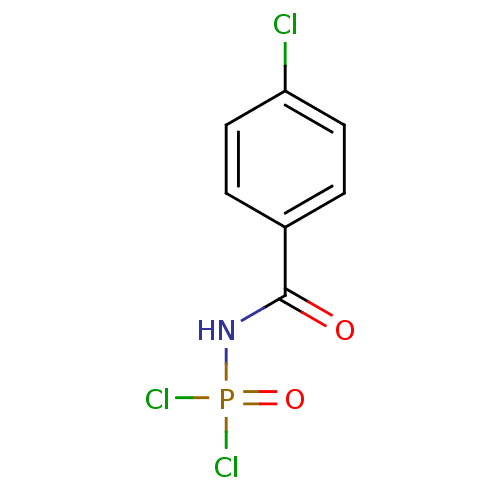
(Carbacylamidophosphate, 1a)Show InChI InChI=1S/C7H5Cl3NO2P/c8-6-3-1-5(2-4-6)7(12)11-14(9,10)13/h1-4H,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.20E+5 | 4.30E+7 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91728

(Carbacylamidophosphate, 3b)Show InChI InChI=1S/C15H22N3O4P/c19-15(14-4-2-1-3-5-14)16-23(20,17-6-10-21-11-7-17)18-8-12-22-13-9-18/h1-5H,6-13H2,(H,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.56E+7 | 7.68E+7 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data