 Found 32 hits of kd data for polymerid = 114
Found 32 hits of kd data for polymerid = 114 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8466
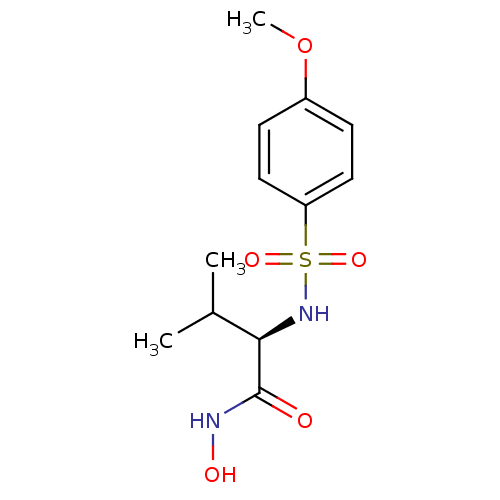
((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50096645
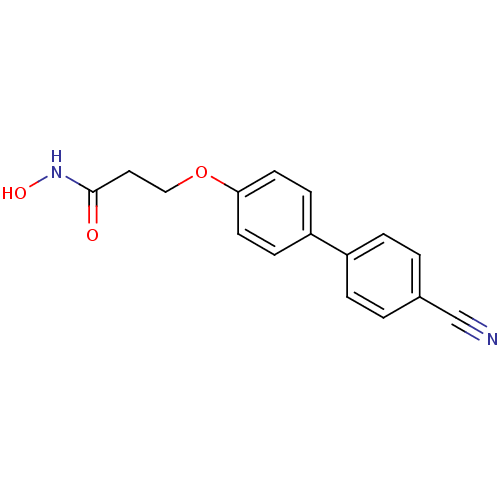
(3-(4'-Cyano-biphenyl-4-yloxy)-N-hydroxy-propionami...)Show InChI InChI=1S/C16H14N2O3/c17-11-12-1-3-13(4-2-12)14-5-7-15(8-6-14)21-10-9-16(19)18-20/h1-8,20H,9-10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50096645

(3-(4'-Cyano-biphenyl-4-yloxy)-N-hydroxy-propionami...)Show InChI InChI=1S/C16H14N2O3/c17-11-12-1-3-13(4-2-12)14-5-7-15(8-6-14)21-10-9-16(19)18-20/h1-8,20H,9-10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50305115
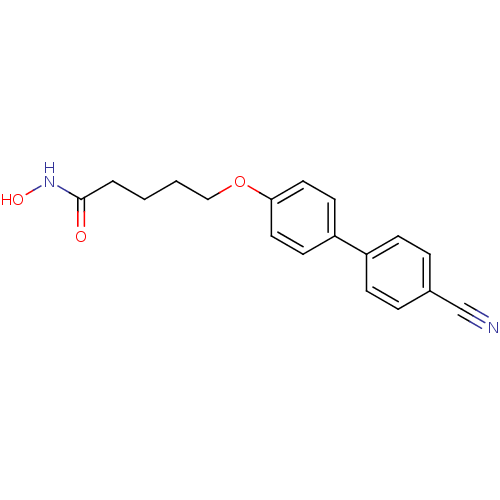
(5-(4'-cyanobiphenyl-4-yloxy)-N-hydroxypentanamide ...)Show InChI InChI=1S/C18H18N2O3/c19-13-14-4-6-15(7-5-14)16-8-10-17(11-9-16)23-12-2-1-3-18(21)20-22/h4-11,22H,1-3,12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50305115

(5-(4'-cyanobiphenyl-4-yloxy)-N-hydroxypentanamide ...)Show InChI InChI=1S/C18H18N2O3/c19-13-14-4-6-15(7-5-14)16-8-10-17(11-9-16)23-12-2-1-3-18(21)20-22/h4-11,22H,1-3,12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121943
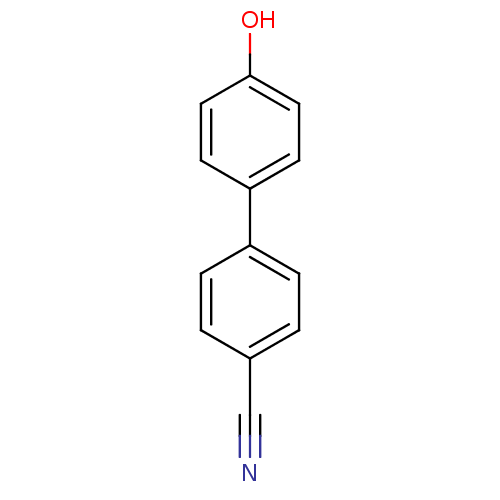
(4'-Hydroxy-biphenyl-4-carbonitrile | CHEMBL114523)Show InChI InChI=1S/C13H9NO/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h1-8,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121943

(4'-Hydroxy-biphenyl-4-carbonitrile | CHEMBL114523)Show InChI InChI=1S/C13H9NO/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h1-8,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149241
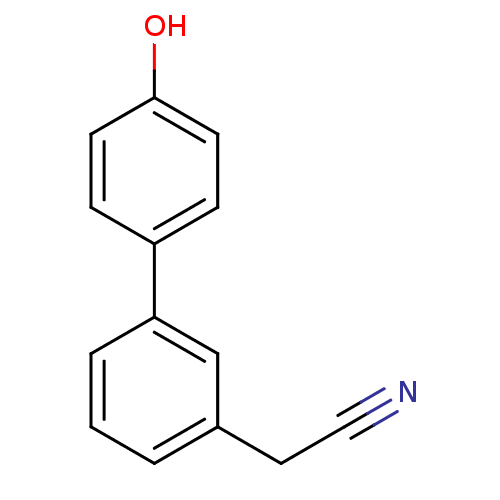
((4'-Hydroxy-biphenyl-3-yl)-acetonitrile | CHEMBL11...)Show InChI InChI=1S/C14H11NO/c15-9-8-11-2-1-3-13(10-11)12-4-6-14(16)7-5-12/h1-7,10,16H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015152
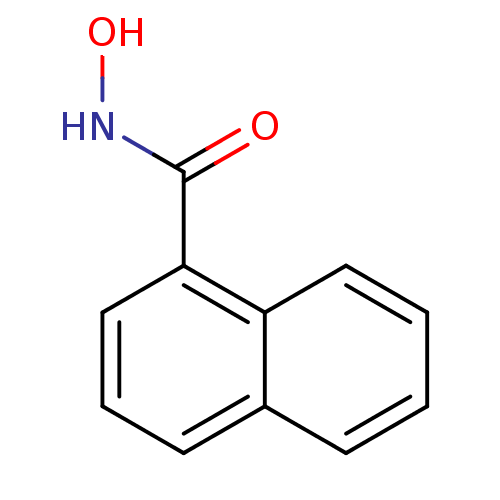
(CHEMBL115468 | N-hydroxy-1-naphthamide | Naphthale...)Show InChI InChI=1S/C11H9NO2/c13-11(12-14)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,14H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015152

(CHEMBL115468 | N-hydroxy-1-naphthamide | Naphthale...)Show InChI InChI=1S/C11H9NO2/c13-11(12-14)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,14H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121958

(4,4'-Biphenyldiol | 4,4'-Dihydroxybiphenyl | 4,4'-...)Show InChI InChI=1S/C12H10O2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8,13-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121957
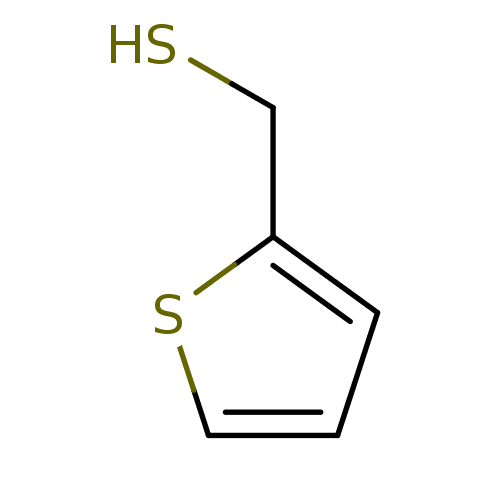
(CHEMBL152603 | Thiophen-2-yl-methanethiol)Show InChI InChI=1S/C5H6S2/c6-4-5-2-1-3-7-5/h1-3,6H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121958

(4,4'-Biphenyldiol | 4,4'-Dihydroxybiphenyl | 4,4'-...)Show InChI InChI=1S/C12H10O2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8,13-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121955
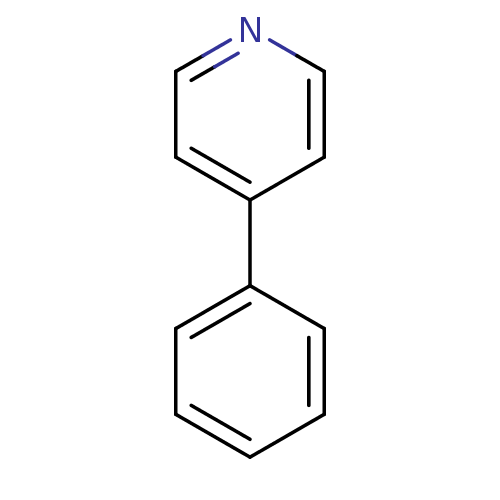
(4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...)Show InChI InChI=1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149238
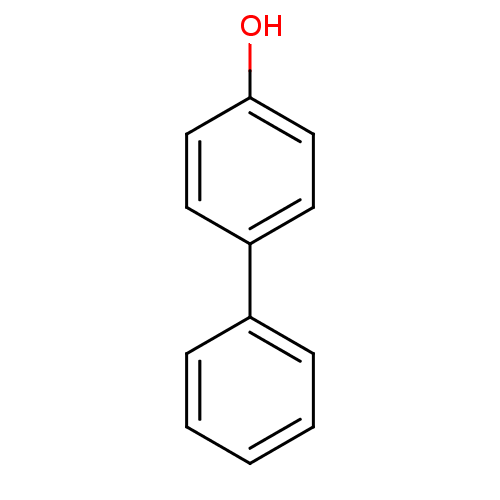
(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human MMP3 catalytic domain (81 to 256 residues) expressed in Escherichia coli BL21 (DE3) pLysS by 15N-HSQC-NMR spectroscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149238

(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121955

(4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...)Show InChI InChI=1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121953
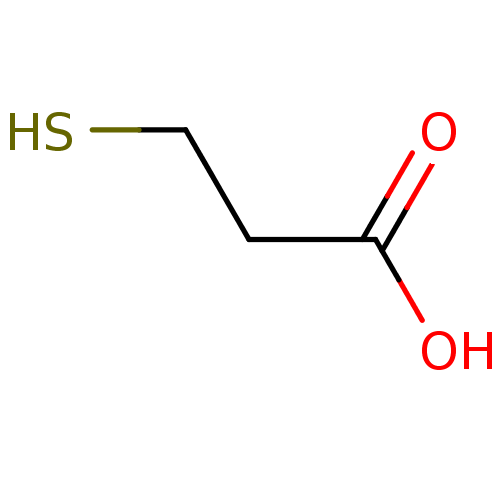
(2-mercaptoethanecarboxylic acid | 3-mercaptopropan...)Show InChI InChI=1S/C3H6O2S/c4-3(5)1-2-6/h6H,1-2H2,(H,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 7.00E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857
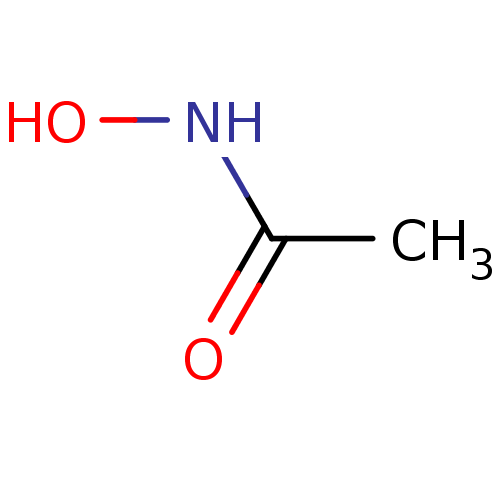
(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human MMP3 catalytic domain (81 to 256 residues) expressed in Escherichia coli BL21 (DE3) pLysS by 15N-HSQC-NMR spectroscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857

(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857

(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50056900
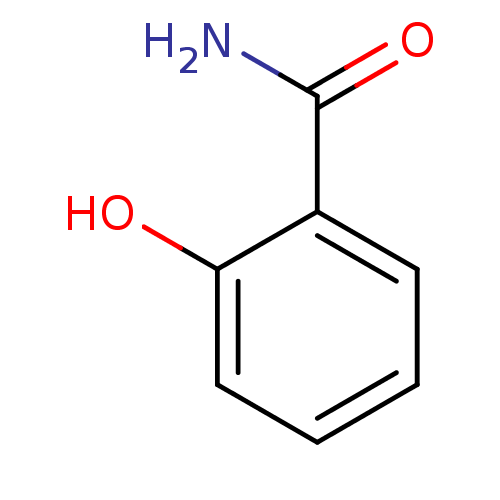
(2-Carbamoylphenol | 2-Carboxamidophenol | 2-Hydrox...)Show InChI InChI=1S/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121960

(4,4,4-Trifluoro-1-phenyl-butane-1,3-dione | 4,4,4-...)Show InChI InChI=1S/C10H7F3O2/c11-10(12,13)9(15)6-8(14)7-4-2-1-3-5-7/h1-5H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121952
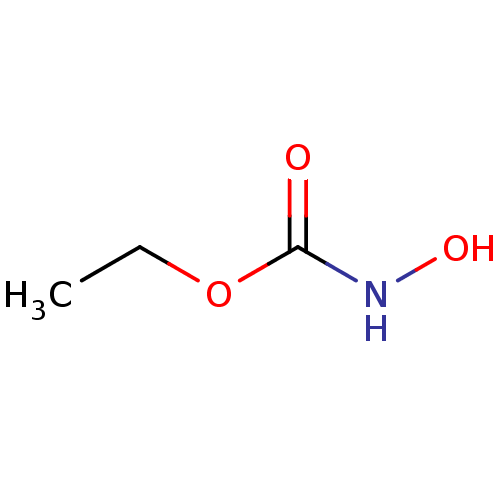
(CHEMBL153081 | Ethyl hydroxycarbamate)Show InChI InChI=1S/C3H7NO3/c1-2-7-3(5)4-6/h6H,2H2,1H3,(H,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50017811
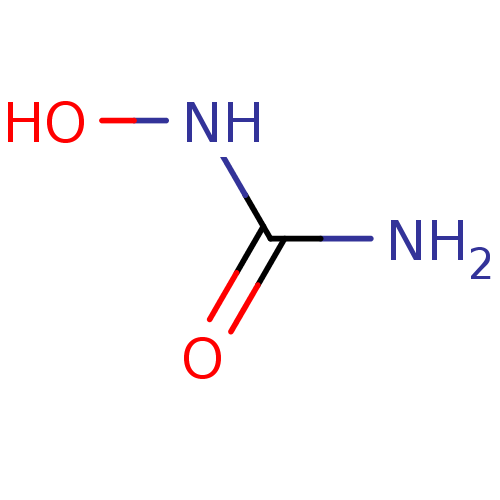
(CHEMBL467 | HU | US10155732, Compound HU | hydroxy...)Show InChI InChI=1S/CH4N2O2/c2-1(4)3-5/h5H,(H3,2,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50026891

(1-Pyridin-2-yl-ethanone | CHEMBL11945)Show InChI InChI=1S/C7H7NO/c1-6(9)7-4-2-3-5-8-7/h2-5H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM26193
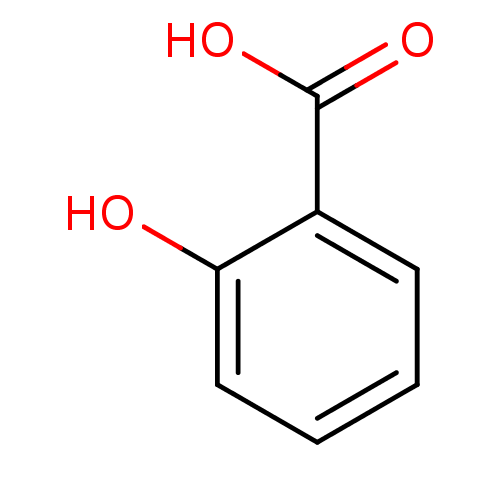
(2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...)Show InChI InChI=1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121956
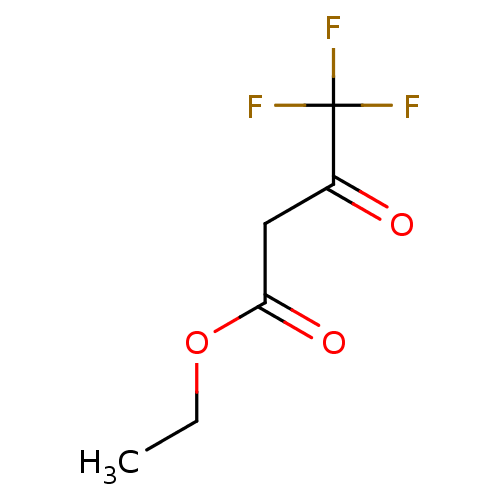
(4,4,4-Trifluoro-3-oxo-butyric acid ethyl ester | C...)Show InChI InChI=1S/C6H7F3O3/c1-2-12-5(11)3-4(10)6(7,8)9/h2-3H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data