 Found 249 hits of kd data for polymerid = 1325,1526
Found 249 hits of kd data for polymerid = 1325,1526 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM50460772
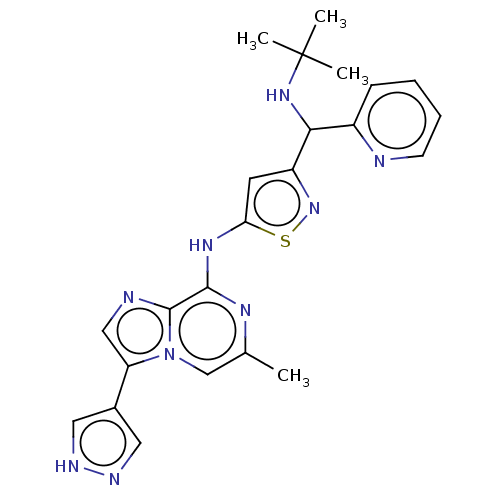
(CHEMBL4225923)Show SMILES Cc1cn2c(cnc2c(Nc2cc(ns2)C(NC(C)(C)C)c2ccccn2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C23H25N9S/c1-14-13-32-18(15-10-26-27-11-15)12-25-22(32)21(28-14)29-19-9-17(31-33-19)20(30-23(2,3)4)16-7-5-6-8-24-16/h5-13,20,30H,1-4H3,(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A (unknown origin) after 45 mins by fluorescence-based thermal stability shift assay |
Bioorg Med Chem Lett 28: 1397-1403 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.037
BindingDB Entry DOI: 10.7270/Q2K35X91 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50460773
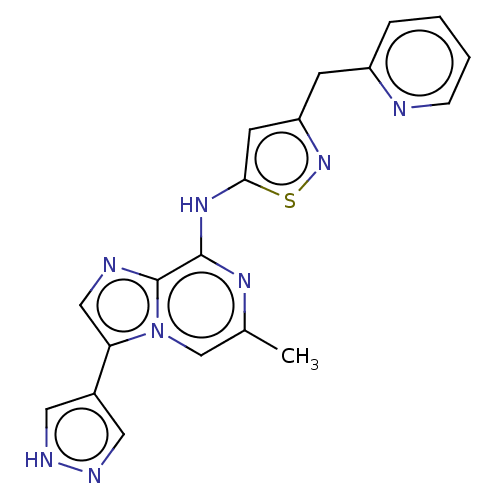
(CHEMBL4227082)Show SMILES Cc1cn2c(cnc2c(Nc2cc(Cc3ccccn3)ns2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H16N8S/c1-12-11-27-16(13-8-22-23-9-13)10-21-19(27)18(24-12)25-17-7-15(26-28-17)6-14-4-2-3-5-20-14/h2-5,7-11H,6H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A (unknown origin) after 45 mins by fluorescence-based thermal stability shift assay |
Bioorg Med Chem Lett 28: 1397-1403 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.037
BindingDB Entry DOI: 10.7270/Q2K35X91 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109208
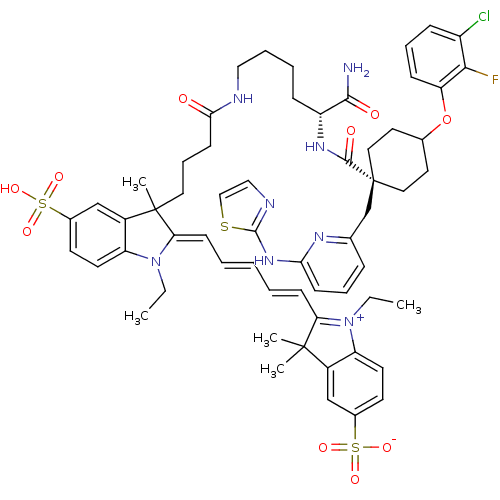
(2-[(1E,3E)-5-[(2E)-3-(3-{[(5R)-5-carbamoyl-5-{[4- ...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@]2(Cc3cccc(Nc4nccs4)n3)CCC(CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:42.43,38.40,wD:42.44,c:9,(-16.27,-52.94,;-14.86,-52.31,;-14.7,-50.78,;-15.85,-49.75,;-17.35,-50.07,;-18.44,-48.98,;-19.93,-49.38,;-21.02,-48.29,;-22.51,-48.69,;-23.6,-47.6,;-25.12,-47.84,;-25.82,-49.22,;-27.36,-49.22,;-25.82,-46.47,;-27.3,-46.07,;-27.7,-44.59,;-26.61,-43.5,;-25.13,-43.89,;-24.73,-45.38,;-23.36,-46.08,;-22.27,-44.99,;-21.87,-46.48,;-27.01,-42.01,;-27.41,-40.52,;-25.52,-41.61,;-28.5,-42.41,;-15.22,-48.34,;-15.22,-46.8,;-16.56,-47.57,;-16.56,-46.03,;-17.89,-45.26,;-17.89,-43.72,;-16.56,-42.95,;-19.22,-42.95,;-19.22,-41.41,;-20.56,-40.64,;-20.56,-39.1,;-21.89,-38.33,;-21.89,-36.79,;-23.22,-36.02,;-23.22,-34.48,;-21.89,-33.71,;-24.56,-33.71,;-24.56,-35.25,;-25.89,-36.02,;-25.89,-37.56,;-27.23,-38.34,;-28.56,-37.57,;-28.56,-36.03,;-29.89,-35.26,;-29.89,-33.72,;-31.14,-32.81,;-30.66,-31.35,;-29.12,-31.35,;-28.65,-32.81,;-27.22,-35.26,;-23.22,-32.94,;-23.22,-31.4,;-24.56,-30.63,;-25.89,-31.4,;-25.89,-32.94,;-24.56,-29.09,;-25.89,-28.32,;-27.22,-29.09,;-28.56,-28.32,;-28.56,-26.78,;-27.22,-26.01,;-27.22,-24.47,;-25.89,-26.78,;-24.56,-26.01,;-20.56,-36.02,;-19.22,-36.79,;-20.56,-34.48,;-13.69,-48.51,;-12.55,-47.48,;-11.08,-47.95,;-10.76,-49.46,;-11.91,-50.49,;-13.37,-50.01,;-9.94,-46.92,;-8.85,-45.83,;-11.03,-45.83,;-8.85,-48.01,)| Show InChI InChI=1S/C60H70ClFN8O10S3/c1-6-69-47-26-24-41(82(74,75)76)36-43(47)58(3,4)50(69)20-9-8-10-21-51-59(5,44-37-42(83(77,78)79)25-27-48(44)70(51)7-2)30-15-23-53(71)64-33-12-11-18-46(55(63)72)67-56(73)60(38-39-16-13-22-52(66-39)68-57-65-34-35-81-57)31-28-40(29-32-60)80-49-19-14-17-45(61)54(49)62/h8-10,13-14,16-17,19-22,24-27,34-37,40,46H,6-7,11-12,15,18,23,28-33,38H2,1-5H3,(H6-,63,64,65,66,67,68,71,72,73,74,75,76,77,78,79)/t40?,46-,59?,60-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109206
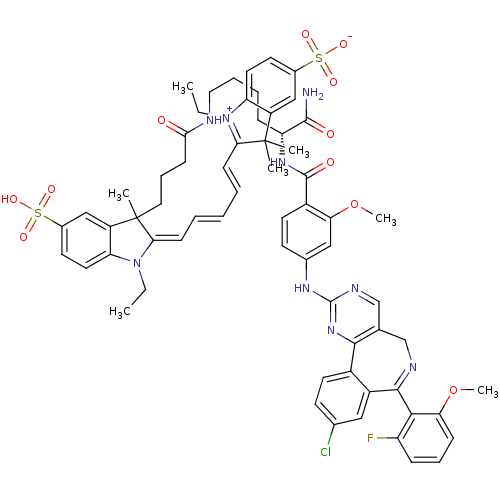
(2-[(1E,3E)-5-[(2E)-3-(3-{[(5R)-5-carbamoyl-5-[(4- ...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)c2ccc(Nc3ncc4CN=C(c5cc(Cl)ccc5-c4n3)c3c(F)cccc3OC)cc2OC)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,c:9,54| Show InChI InChI=1S/C65H69ClFN9O11S2/c1-8-75-51-29-25-42(88(80,81)82)35-47(51)64(3,4)55(75)20-11-10-12-21-56-65(5,48-36-43(89(83,84)85)26-30-52(48)76(56)9-2)31-16-22-57(77)69-32-14-13-18-50(61(68)78)73-62(79)45-28-24-41(34-54(45)87-7)72-63-71-38-39-37-70-60(58-49(67)17-15-19-53(58)86-6)46-33-40(66)23-27-44(46)59(39)74-63/h10-12,15,17,19-21,23-30,33-36,38,50H,8-9,13-14,16,18,22,31-32,37H2,1-7H3,(H6-,68,69,71,72,73,74,77,78,79,80,81,82,83,84,85)/t50-,65?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.273 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106623
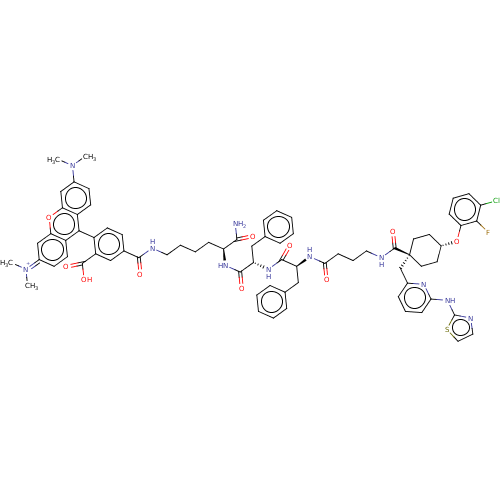
(CHEMBL3600871)Show SMILES CN(C)c1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCC[C@H](NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3ccccc3)NC(=O)CCCNC(=O)[C@@]3(Cc4cccc(Nc5nccs5)n4)CC[C@@H](CC3)Oc3cccc(Cl)c3F)C(N)=O)c3ccc(cc3oc2c1)=[N+](C)C |r,wU:56.58,39.41,wD:72.80,28.29,24.24,(-6.4,.92,;-5.34,1.54,;-5.34,2.77,;-4,.77,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.84,;1.35,-5.38,;.03,-6.16,;-1.31,-5.4,;-1.32,-3.86,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;.04,-7.7,;-1.02,-8.33,;1.38,-8.46,;1.39,-10,;2.74,-10.76,;2.75,-12.3,;4.09,-13.06,;4.1,-14.6,;5.44,-15.36,;5.46,-16.9,;4.4,-17.53,;6.8,-17.66,;8.12,-16.88,;8.11,-15.34,;9.43,-14.55,;9.41,-13.01,;8.07,-12.26,;6.75,-13.04,;6.77,-14.58,;6.81,-19.2,;8.15,-19.96,;9.21,-19.34,;8.17,-21.5,;6.84,-22.28,;5.5,-21.52,;4.17,-22.3,;2.83,-21.53,;2.82,-19.99,;4.15,-19.22,;5.49,-19.98,;9.51,-22.26,;9.52,-23.8,;8.46,-24.43,;10.86,-24.56,;10.88,-26.1,;12.22,-26.86,;12.23,-28.4,;13.57,-29.16,;14.63,-28.54,;13.58,-30.7,;14.93,-31.46,;16.25,-30.68,;16.24,-29.14,;17.56,-28.36,;18.91,-29.11,;18.92,-30.65,;20.27,-31.41,;21.59,-30.62,;21.71,-29.1,;23.21,-28.76,;24,-30.08,;22.99,-31.24,;17.6,-31.44,;13.64,-32.22,;12.31,-33,;10.97,-32.24,;10.95,-30.7,;12.28,-29.92,;9.64,-33.03,;9.66,-34.57,;11,-35.32,;11.02,-36.86,;9.7,-37.65,;8.35,-36.9,;7.29,-37.52,;8.33,-35.36,;7.26,-34.75,;2.77,-15.38,;2.78,-16.61,;1.7,-14.77,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;5.33,1.54,;6.4,.93,;5.33,2.78,)| Show InChI InChI=1S/C75H79ClFN11O10S/c1-87(2)50-27-30-54-62(43-50)98-63-44-51(88(3)4)28-31-55(63)66(54)53-29-26-48(42-56(53)72(94)95)69(91)79-36-12-11-22-58(68(78)90)84-71(93)60(41-47-18-9-6-10-19-47)85-70(92)59(40-46-16-7-5-8-17-46)83-65(89)25-15-37-80-73(96)75(45-49-20-13-24-64(82-49)86-74-81-38-39-99-74)34-32-52(33-35-75)97-61-23-14-21-57(76)67(61)77/h5-10,13-14,16-21,23-24,26-31,38-39,42-44,52,58-60H,11-12,15,22,25,32-37,40-41,45H2,1-4H3,(H8-,78,79,80,81,82,83,84,85,86,89,90,91,92,93,94,95,96)/p+1/t52-,58-,59-,60-,75-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a |
Heidelberg University
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged human partial length AURKA expressed in mammalian expression system by qPCR method |
J Med Chem 61: 4851-4859 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00140
BindingDB Entry DOI: 10.7270/Q25H7JVT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106624

(CHEMBL3600870)Show SMILES CN(C)c1ccc2C(C3C=CC(C=C3Oc2c1)=[N+](C)C)c1ccc(cc1C(O)=O)C(=O)NCCCC[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(cc1)N=[N+]=[N-])NC(=O)CCCCCNC(=O)[C@@]1(Cc2cccc(Nc3nccs3)n2)CC[C@@H](CC1)Oc1cccc(Cl)c1F)C(N)=O |r,wU:106.110,40.44,62.66,128.132,84.88,wD:73.77,95.99,51.55,36.39,144.154,c:9,12,(27.66,36,;26.6,35.38,;26.6,34.15,;25.26,36.15,;23.93,35.38,;22.6,36.15,;22.59,37.7,;21.26,38.45,;21.26,39.99,;19.92,40.76,;19.92,42.3,;21.25,43.07,;22.59,42.3,;22.59,40.77,;23.92,40,;23.92,38.48,;25.26,37.69,;21.25,44.61,;22.31,45.23,;20.18,45.22,;19.93,37.68,;18.6,38.44,;17.27,37.67,;17.27,36.13,;18.6,35.37,;19.93,36.14,;21.27,35.37,;22.16,35.88,;21.27,34.14,;15.93,35.37,;15.93,34.13,;14.6,36.14,;13.27,35.37,;11.93,36.14,;10.6,35.37,;9.26,36.14,;7.93,35.37,;6.6,36.14,;5.26,35.37,;5.26,34.14,;3.93,36.14,;3.93,37.68,;5.27,38.45,;5.27,39.99,;6.61,40.75,;6.61,42.29,;5.54,42.91,;7.68,42.91,;2.59,35.37,;1.26,36.14,;1.26,37.37,;-.07,35.37,;-.08,33.83,;-1.41,33.06,;-1.42,31.52,;-2.75,30.76,;-2.76,29.22,;-1.69,28.6,;-3.82,28.6,;-1.41,36.14,;-2.74,35.37,;-2.74,34.14,;-4.08,36.14,;-4.07,37.68,;-2.74,38.45,;-2.73,39.99,;-1.4,40.76,;-1.39,42.3,;-2.46,42.92,;-.33,42.91,;-5.41,35.37,;-6.74,36.14,;-6.74,37.37,;-8.08,35.37,;-8.08,33.83,;-9.42,33.07,;-9.42,31.53,;-10.76,30.76,;-10.76,29.22,;-9.7,28.6,;-11.65,28.71,;-9.41,36.14,;-10.75,35.38,;-10.75,34.14,;-12.08,36.15,;-12.08,37.69,;-13.42,38.45,;-13.42,39.99,;-14.75,40.76,;-14.76,42.3,;-13.69,42.92,;-15.82,42.92,;-13.41,35.38,;-14.75,36.15,;-14.75,37.38,;-16.08,35.38,;-16.08,33.84,;-14.75,33.07,;-14.74,31.53,;-13.41,30.76,;-13.41,29.22,;-14.47,28.6,;-12.52,28.71,;-17.42,36.15,;-18.75,35.38,;-18.75,34.15,;-20.08,36.15,;-20.09,37.69,;-21.42,38.46,;-21.42,40,;-22.75,40.77,;-24.09,40,;-24.09,38.46,;-22.76,37.69,;-25.42,40.77,;-25.41,42.31,;-25.41,43.54,;-21.42,35.38,;-22.75,36.15,;-22.75,37.18,;-24.09,35.38,;-25.42,36.15,;-26.75,35.38,;-28.09,36.15,;-29.42,35.38,;-30.76,36.15,;-32.09,35.38,;-32.09,34.15,;-33.42,36.15,;-34.76,35.38,;-34.76,33.84,;-33.43,33.08,;-33.42,31.54,;-34.76,30.76,;-36.09,31.53,;-37.42,30.76,;-38.76,31.52,;-38.9,33.04,;-40.41,33.36,;-41.18,32.03,;-40.14,30.88,;-36.09,33.07,;-34.76,36.88,;-34.76,38.42,;-33.42,39.19,;-32.09,38.42,;-32.09,36.88,;-33.43,40.73,;-34.76,41.5,;-36.09,40.72,;-37.43,41.49,;-37.43,43.03,;-36.1,43.8,;-36.11,45.03,;-34.77,43.04,;-33.7,43.66,;7.93,33.83,;6.87,33.21,;9,33.21,)| Show InChI InChI=1S/C104H147ClFN37O15S/c1-142(2)63-35-38-67-80(56-63)158-81-57-64(143(3)4)36-39-68(81)84(67)66-37-32-60(55-69(66)95(154)155)87(146)121-44-9-7-20-71(86(107)145)132-88(147)72(21-12-46-123-97(108)109)133-89(148)73(22-13-47-124-98(110)111)134-90(149)74(23-14-48-125-99(112)113)135-91(150)75(24-15-49-126-100(114)115)136-92(151)76(25-16-50-127-101(116)117)137-93(152)77(26-17-51-128-102(118)119)138-94(153)78(54-59-30-33-61(34-31-59)140-141-120)131-83(144)29-6-5-8-45-122-96(156)104(58-62-18-10-28-82(130-62)139-103-129-52-53-159-103)42-40-65(41-43-104)157-79-27-11-19-70(105)85(79)106/h10-11,18-19,27-28,30-39,52-53,55-57,65,67,71-78,84H,5-9,12-17,20-26,29,40-51,54,58H2,1-4H3,(H37-,107,108,109,110,111,112,113,114,115,116,117,118,119,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,144,145,146,147,148,149,150,151,152,153,154,155,156)/p+1/t65-,67?,71-,72+,73+,74+,75+,76+,77+,78+,84?,104-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50038423
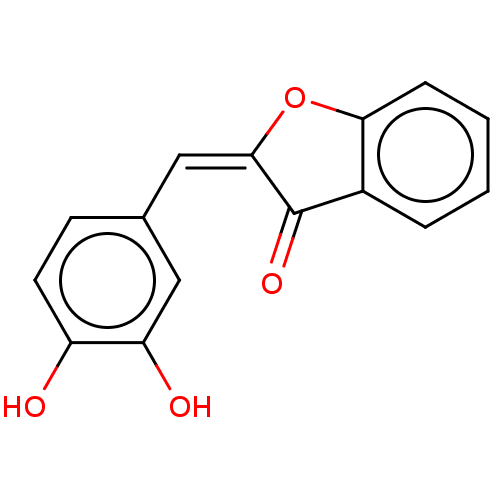
(CHEMBL3361128)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Binding affinity to AURKA (unknown origin) |
Eur J Med Chem 89: 310-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.10.044
BindingDB Entry DOI: 10.7270/Q2JQ12NJ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106631
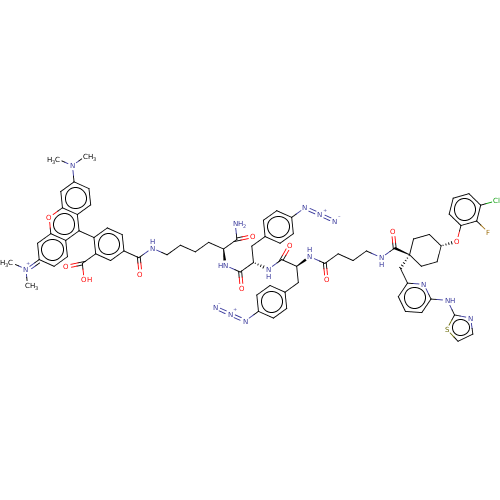
(CHEMBL3600653)Show SMILES CN(C)c1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCC[C@H](NC(=O)[C@H](Cc3ccc(cc3)N=[N+]=[N-])NC(=O)[C@H](Cc3ccc(cc3)N=[N+]=[N-])NC(=O)CCCNC(=O)[C@@]3(Cc4cccc(Nc5nccs5)n4)CC[C@@H](CC3)Oc3cccc(Cl)c3F)C(N)=O)c3ccc(cc3oc2c1)=[N+](C)C |r,wU:62.64,42.44,wD:78.86,28.29,24.24,(-6.4,.92,;-5.34,1.54,;-5.34,2.77,;-4,.77,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.84,;1.35,-5.38,;.03,-6.16,;-1.31,-5.4,;-1.32,-3.86,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;.04,-7.7,;-1.02,-8.33,;1.38,-8.46,;1.39,-10,;2.74,-10.76,;2.75,-12.3,;4.09,-13.06,;4.1,-14.6,;5.44,-15.36,;5.46,-16.9,;4.4,-17.53,;6.8,-17.66,;8.12,-16.88,;8.11,-15.34,;9.43,-14.55,;9.41,-13.01,;8.07,-12.26,;6.75,-13.04,;6.77,-14.58,;8.05,-10.72,;6.71,-9.96,;5.63,-9.36,;6.81,-19.2,;8.15,-19.96,;9.21,-19.34,;8.17,-21.5,;6.84,-22.28,;5.5,-21.52,;4.17,-22.3,;2.83,-21.53,;2.82,-19.99,;4.15,-19.22,;5.49,-19.98,;1.49,-19.23,;1.48,-17.69,;1.48,-16.45,;9.51,-22.26,;9.52,-23.8,;8.46,-24.43,;10.86,-24.56,;10.88,-26.1,;12.22,-26.86,;12.23,-28.4,;13.57,-29.16,;14.63,-28.54,;13.58,-30.7,;14.93,-31.46,;16.25,-30.68,;16.24,-29.14,;17.56,-28.36,;18.91,-29.11,;18.92,-30.65,;20.27,-31.41,;21.59,-30.62,;21.71,-29.1,;23.21,-28.76,;24,-30.08,;22.99,-31.24,;17.6,-31.44,;13.64,-32.22,;12.31,-33,;10.97,-32.24,;10.95,-30.7,;12.28,-29.92,;9.64,-33.03,;9.66,-34.57,;11,-35.32,;11.02,-36.86,;9.7,-37.65,;8.35,-36.9,;7.29,-37.52,;8.33,-35.36,;7.26,-34.75,;2.77,-15.38,;2.78,-16.61,;1.7,-14.77,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;5.33,1.54,;6.4,.93,;5.33,2.78,)| Show InChI InChI=1S/C75H77ClFN17O10S/c1-93(2)50-25-28-54-62(41-50)104-63-42-51(94(3)4)26-29-55(63)66(54)53-27-20-46(40-56(53)72(100)101)69(97)81-34-6-5-12-58(68(78)96)86-71(99)60(39-45-18-23-48(24-19-45)90-92-80)87-70(98)59(38-44-16-21-47(22-17-44)89-91-79)85-65(95)15-9-35-82-73(102)75(43-49-10-7-14-64(84-49)88-74-83-36-37-105-74)32-30-52(31-33-75)103-61-13-8-11-57(76)67(61)77/h7-8,10-11,13-14,16-29,36-37,40-42,52,58-60H,5-6,9,12,15,30-35,38-39,43H2,1-4H3,(H8-,78,81,82,83,84,85,86,87,88,95,96,97,98,99,100,101,102)/p+1/t52-,58-,59-,60-,75-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106630
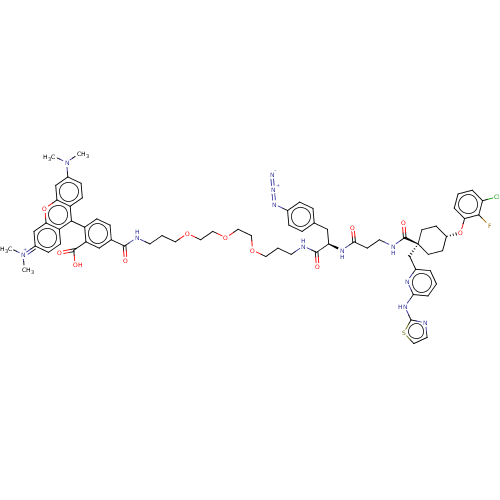
(CHEMBL3600760)Show SMILES CN(C)c1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCOCCOCCOCCCNC(=O)[C@@H](Cc3ccc(cc3)N=[N+]=[N-])NC(=O)CCNC(=O)[C@@]3(Cc4cccc(Nc5nccs5)n4)CC[C@@H](CC3)Oc3cccc(Cl)c3F)c3ccc(cc3oc2c1)=[N+](C)C |r,wU:55.56,36.37,wD:71.78,(-6.4,.92,;-5.34,1.54,;-5.34,2.77,;-4,.77,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.84,;1.35,-5.38,;.03,-6.16,;-1.31,-5.4,;-1.32,-3.86,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;.04,-7.7,;-1.02,-8.33,;1.38,-8.46,;1.39,-10,;2.74,-10.76,;2.75,-12.3,;4.09,-13.06,;4.1,-14.6,;5.44,-15.36,;5.46,-16.9,;6.8,-17.66,;6.81,-19.2,;8.15,-19.96,;8.17,-21.5,;9.51,-22.26,;9.52,-23.8,;10.86,-24.56,;10.88,-26.1,;9.81,-26.73,;12.22,-26.86,;13.54,-26.08,;13.53,-24.54,;14.85,-23.75,;14.83,-22.21,;13.49,-21.46,;12.17,-22.24,;12.18,-23.78,;13.47,-19.92,;12.12,-19.16,;11.05,-18.56,;12.23,-28.4,;13.57,-29.16,;14.63,-28.54,;13.58,-30.7,;14.93,-31.46,;14.94,-33,;16.28,-33.76,;17.34,-33.14,;16.29,-35.3,;17.64,-36.06,;18.96,-35.28,;18.95,-33.74,;20.27,-32.96,;21.62,-33.71,;21.63,-35.25,;22.97,-36.01,;24.3,-35.22,;24.42,-33.7,;25.92,-33.36,;26.71,-34.68,;25.7,-35.84,;20.3,-36.04,;16.34,-36.82,;15.02,-37.6,;13.68,-36.84,;13.66,-35.3,;14.99,-34.52,;12.35,-37.63,;12.37,-39.17,;13.71,-39.92,;13.73,-41.46,;12.4,-42.25,;11.06,-41.5,;10,-42.12,;11.04,-39.96,;9.97,-39.35,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;5.33,1.54,;6.4,.93,;5.33,2.78,)| Show InChI InChI=1S/C69H76ClFN12O11S/c1-82(2)48-18-21-52-58(41-48)94-59-42-49(83(3)4)19-22-53(59)62(52)51-20-15-45(40-54(51)66(87)88)64(85)73-28-7-32-90-34-36-92-37-35-91-33-8-29-74-65(86)56(39-44-13-16-46(17-14-44)80-81-72)78-61(84)25-30-75-67(89)69(43-47-9-5-12-60(77-47)79-68-76-31-38-95-68)26-23-50(24-27-69)93-57-11-6-10-55(70)63(57)71/h5-6,9-22,31,38,40-42,50,56H,7-8,23-30,32-37,39,43H2,1-4H3,(H5-,73,74,75,76,77,78,79,84,85,86,87,88,89)/p+1/t50-,56-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394776
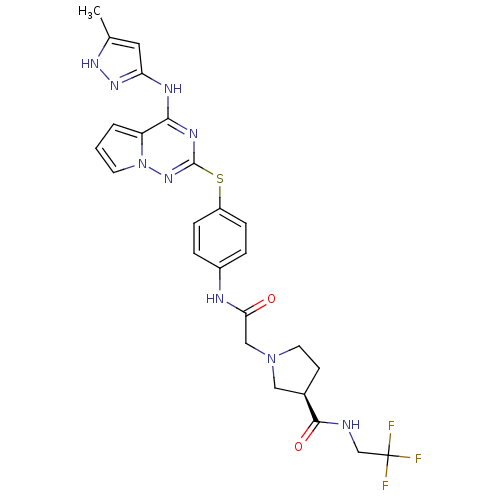
(CHEMBL2163388)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NCC(F)(F)F)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C25H26F3N9O2S/c1-15-11-20(34-33-15)31-22-19-3-2-9-37(19)35-24(32-22)40-18-6-4-17(5-7-18)30-21(38)13-36-10-8-16(12-36)23(39)29-14-25(26,27)28/h2-7,9,11,16H,8,10,12-14H2,1H3,(H,29,39)(H,30,38)(H2,31,32,33,34,35)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106625

(CHEMBL3600869)Show SMILES CCN1\C(=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)[C@@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)CCNC(=O)[C@@]2(Cc3cccc(Nc4nccs4)n3)CC[C@@H](CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:139.142,73.75,106.108,95.97,51.53,wD:120.123,62.64,40.42,36.37,84.86,155.164,c:7,(33.39,-23.49,;32.18,-23.73,;31.69,-25.19,;32.56,-26.41,;34.1,-26.39,;34.88,-27.72,;36.42,-27.71,;37.21,-29.03,;38.71,-29.16,;39.71,-27.99,;39.3,-26.83,;39.07,-30.65,;40.43,-31.38,;40.46,-32.94,;39.15,-33.74,;37.78,-32.99,;37.76,-31.45,;36.6,-30.45,;36.26,-31.63,;35.78,-29.54,;39.2,-35.27,;40.28,-35.86,;38.15,-35.92,;39.24,-36.51,;31.68,-27.66,;30.85,-28.57,;33.18,-27.94,;33.72,-29.38,;32.73,-30.57,;33.27,-32.01,;34.48,-32.22,;32.28,-33.2,;32.82,-34.64,;31.83,-35.83,;32.37,-37.27,;31.38,-38.46,;31.92,-39.9,;30.93,-41.09,;31.47,-42.53,;32.68,-42.74,;30.48,-43.72,;28.97,-43.46,;27.98,-44.65,;26.46,-44.39,;25.48,-45.58,;23.96,-45.32,;23.53,-44.17,;23.18,-46.27,;31.02,-45.16,;30.03,-46.35,;28.82,-46.14,;30.57,-47.79,;32.09,-48.05,;33.07,-46.86,;34.59,-47.12,;35.57,-45.93,;37.09,-46.19,;37.52,-47.34,;37.87,-45.24,;29.58,-48.98,;30.12,-50.42,;31.33,-50.63,;29.14,-51.61,;27.62,-51.35,;26.63,-52.54,;25.12,-52.28,;24.13,-53.47,;22.62,-53.21,;22.19,-52.06,;21.83,-54.16,;29.67,-53.05,;28.69,-54.24,;27.47,-54.03,;29.22,-55.68,;30.74,-55.94,;31.27,-57.39,;32.79,-57.65,;33.32,-59.1,;34.84,-59.36,;35.62,-58.41,;35.19,-60.32,;28.24,-56.87,;28.77,-58.31,;29.98,-58.52,;27.79,-59.5,;26.27,-59.24,;25.29,-60.43,;23.77,-60.17,;22.79,-61.36,;21.27,-61.1,;20.84,-59.95,;20.48,-62.05,;28.32,-60.94,;27.34,-62.13,;26.12,-61.92,;27.87,-63.57,;29.39,-63.83,;30.37,-62.64,;31.89,-62.9,;32.87,-61.71,;34.39,-61.97,;34.82,-63.12,;35.05,-61.18,;26.89,-64.76,;25.37,-64.5,;24.94,-63.34,;24.39,-65.68,;24.92,-67.13,;26.44,-67.38,;27.42,-66.19,;28.94,-66.45,;29.48,-67.89,;28.5,-69.08,;26.98,-68.82,;31,-68.14,;31.98,-66.95,;32.76,-66,;22.87,-65.43,;21.88,-66.61,;22.31,-67.77,;20.37,-66.35,;19.83,-64.91,;18.31,-64.65,;17.78,-63.21,;16.26,-62.95,;15.27,-64.14,;15.81,-65.58,;17.33,-65.84,;13.76,-63.87,;13.23,-62.43,;12.8,-61.27,;19.38,-67.54,;17.86,-67.28,;17.51,-66.32,;16.88,-68.47,;15.36,-68.21,;14.38,-69.39,;12.86,-69.13,;12.43,-67.98,;11.88,-70.32,;10.36,-70.06,;9.82,-68.62,;10.81,-67.43,;10.27,-65.99,;8.76,-65.73,;7.77,-66.91,;6.25,-66.66,;5.27,-67.84,;3.75,-67.73,;3.18,-69.16,;4.37,-70.14,;5.67,-69.31,;8.31,-68.36,;13.38,-70.54,;13.91,-71.98,;12.93,-73.17,;11.41,-72.91,;10.88,-71.46,;13.47,-74.61,;14.99,-74.87,;15.97,-73.68,;17.49,-73.93,;18.03,-75.37,;17.05,-76.56,;17.48,-77.71,;15.53,-76.31,;14.75,-77.26,;33.44,-40.16,;33.86,-41.31,;34.22,-39.21,;30.22,-27.19,;28.89,-27.97,;27.55,-27.18,;27.55,-25.65,;28.9,-24.86,;30.23,-25.65,;26.21,-27.94,;26.2,-29.17,;25.15,-27.32,;25.14,-28.55,)| Show InChI InChI=1S/C115H161ClFN41O19S3/c1-6-157-87-44-42-73(179(171,172)173)64-75(87)113(3,4)90(157)30-12-31-91-114(5,76-65-74(180(174,175)176)43-45-88(76)158(91)7-2)49-13-33-93(159)133-52-9-8-22-78(96(118)161)144-97(162)79(23-14-53-135-106(119)120)145-98(163)80(24-15-54-136-107(121)122)146-99(164)81(25-16-55-137-108(123)124)147-100(165)82(26-17-56-138-109(125)126)148-101(166)83(27-18-57-139-110(127)128)149-102(167)84(28-19-58-140-111(129)130)150-104(169)86(63-68-36-40-70(41-37-68)154-156-132)151-103(168)85(62-67-34-38-69(39-35-67)153-155-131)143-94(160)48-59-134-105(170)115(66-71-20-10-32-92(142-71)152-112-141-60-61-178-112)50-46-72(47-51-115)177-89-29-11-21-77(116)95(89)117/h10-12,20-21,29-32,34-45,60-61,64-65,72,78-86H,6-9,13-19,22-28,33,46-59,62-63,66H2,1-5H3,(H39-,118,119,120,121,122,123,124,125,126,127,128,129,130,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176)/p+1/t72-,78-,79-,80-,81-,82-,83-,84-,85-,86-,114?,115-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394789
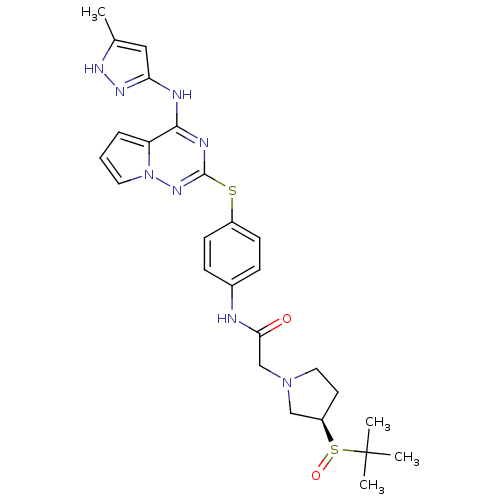
(CHEMBL2163400)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)S(=O)C(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H32N8O2S2/c1-17-14-22(31-30-17)28-24-21-6-5-12-34(21)32-25(29-24)37-19-9-7-18(8-10-19)27-23(35)16-33-13-11-20(15-33)38(36)26(2,3)4/h5-10,12,14,20H,11,13,15-16H2,1-4H3,(H,27,35)(H2,28,29,30,31,32)/t20-,38?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for AURKA kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394783
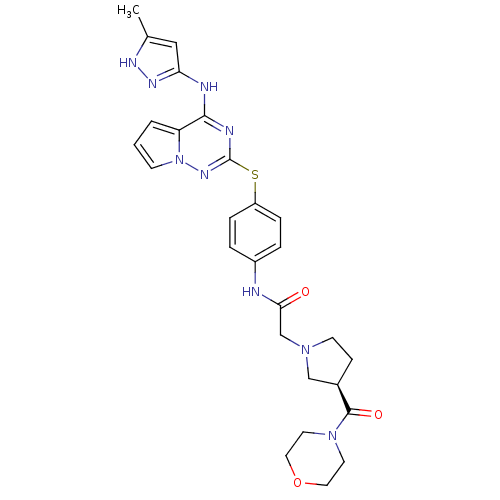
(CHEMBL2163390)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCOCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O3S/c1-18-15-23(32-31-18)29-25-22-3-2-9-36(22)33-27(30-25)40-21-6-4-20(5-7-21)28-24(37)17-34-10-8-19(16-34)26(38)35-11-13-39-14-12-35/h2-7,9,15,19H,8,10-14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394788
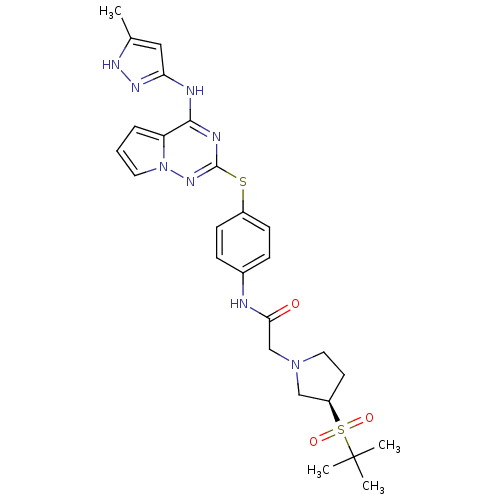
(CHEMBL2163401)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)S(=O)(=O)C(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H32N8O3S2/c1-17-14-22(31-30-17)28-24-21-6-5-12-34(21)32-25(29-24)38-19-9-7-18(8-10-19)27-23(35)16-33-13-11-20(15-33)39(36,37)26(2,3)4/h5-10,12,14,20H,11,13,15-16H2,1-4H3,(H,27,35)(H2,28,29,30,31,32)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394782
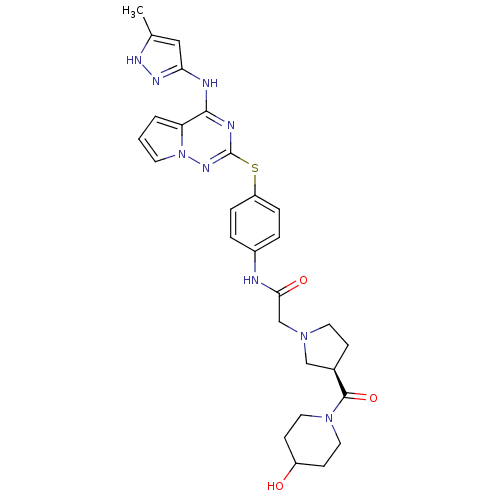
(CHEMBL2163391)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCC(O)CC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H33N9O3S/c1-18-15-24(33-32-18)30-26-23-3-2-11-37(23)34-28(31-26)41-22-6-4-20(5-7-22)29-25(39)17-35-12-8-19(16-35)27(40)36-13-9-21(38)10-14-36/h2-7,11,15,19,21,38H,8-10,12-14,16-17H2,1H3,(H,29,39)(H2,30,31,32,33,34)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PCBioAssay
| n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2NC5ZHH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for AURKA kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394778
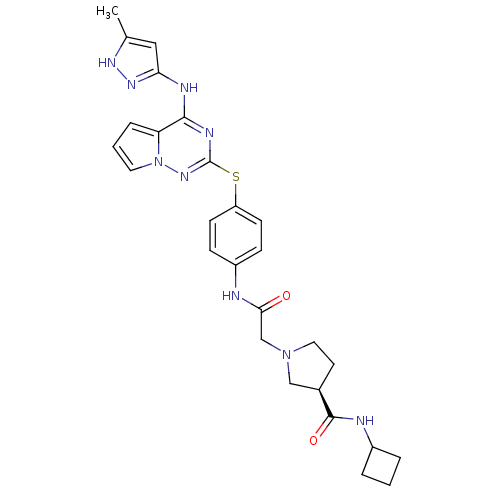
(CHEMBL2163395)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NC4CCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-17-14-23(33-32-17)30-25-22-6-3-12-36(22)34-27(31-25)39-21-9-7-20(8-10-21)28-24(37)16-35-13-11-18(15-35)26(38)29-19-4-2-5-19/h3,6-10,12,14,18-19H,2,4-5,11,13,15-16H2,1H3,(H,28,37)(H,29,38)(H2,30,31,32,33,34)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394799
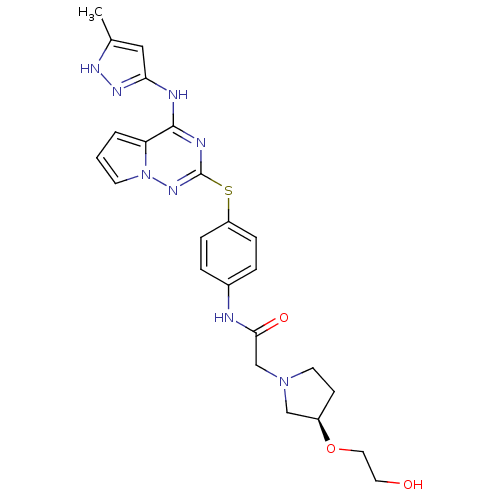
(CHEMBL2163407)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)OCCO)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C24H28N8O3S/c1-16-13-21(29-28-16)26-23-20-3-2-9-32(20)30-24(27-23)36-19-6-4-17(5-7-19)25-22(34)15-31-10-8-18(14-31)35-12-11-33/h2-7,9,13,18,33H,8,10-12,14-15H2,1H3,(H,25,34)(H2,26,27,28,29,30)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352328
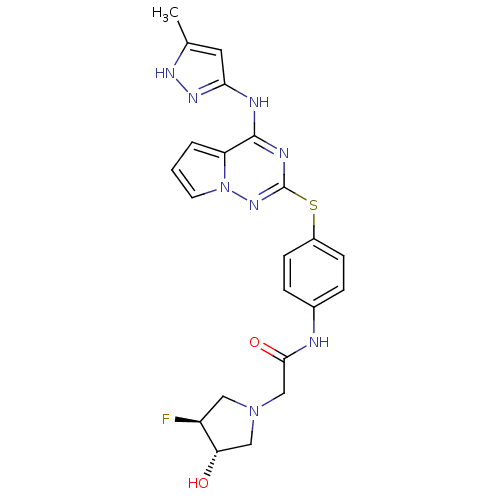
(CHEMBL1822648)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@@H](F)C4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C22H23FN8O2S/c1-13-9-19(28-27-13)25-21-17-3-2-8-31(17)29-22(26-21)34-15-6-4-14(5-7-15)24-20(33)12-30-10-16(23)18(32)11-30/h2-9,16,18,32H,10-12H2,1H3,(H,24,33)(H2,25,26,27,28,29)/t16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198027
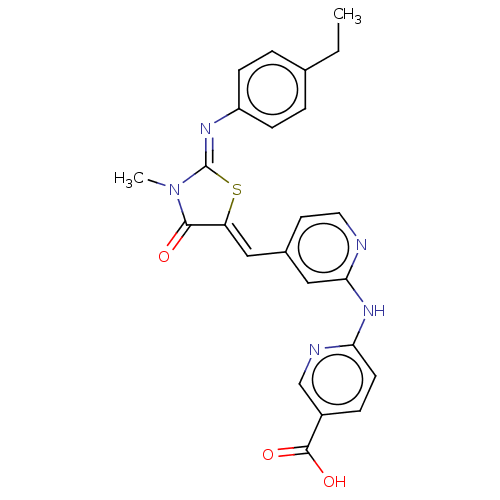
(CHEMBL3921246)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(cn3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C24H21N5O3S/c1-3-15-4-7-18(8-5-15)27-24-29(2)22(30)19(33-24)12-16-10-11-25-21(13-16)28-20-9-6-17(14-26-20)23(31)32/h4-14H,3H2,1-2H3,(H,31,32)(H,25,26,28)/b19-12-,27-24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Binding affinity to human Aurora A kinase expressed in Escherichia coli BL21 cells incubated for 1 hr by active site directed binding competition ass... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352325
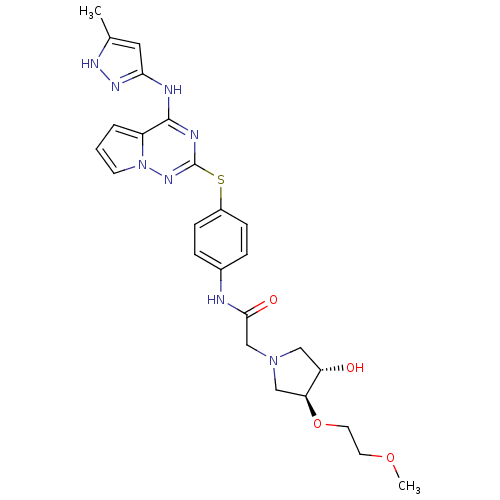
(CHEMBL1822651)Show SMILES COCCO[C@H]1CN(CC(=O)Nc2ccc(Sc3nc(Nc4cc(C)[nH]n4)c4cccn4n3)cc2)C[C@@H]1O |r| Show InChI InChI=1S/C25H30N8O4S/c1-16-12-22(30-29-16)27-24-19-4-3-9-33(19)31-25(28-24)38-18-7-5-17(6-8-18)26-23(35)15-32-13-20(34)21(14-32)37-11-10-36-2/h3-9,12,20-21,34H,10-11,13-15H2,1-2H3,(H,26,35)(H2,27,28,29,30,31)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394795

(CHEMBL2163411)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)OCCCN4CCOCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C29H37N9O3S/c1-21-18-26(34-33-21)31-28-25-4-2-11-38(25)35-29(32-28)42-24-7-5-22(6-8-24)30-27(39)20-37-12-9-23(19-37)41-15-3-10-36-13-16-40-17-14-36/h2,4-8,11,18,23H,3,9-10,12-17,19-20H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109207
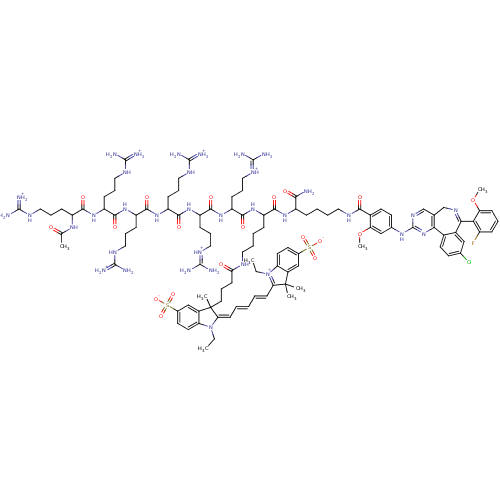
(Compound II: Ac-D-Arg6-D-Lys(PromoFluor 647)-D-Lys...)Show SMILES [#6]-[#6]-[#7]-1\[#6](=[#6]\[#6]=[#6]\[#6]=[#6]\[#6]2=[#7+](-[#6]-[#6])-c3ccc(cc3C2([#6])[#6])S([#8-])(=O)=O)C([#6])([#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7+]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c2ccc(-[#7]-c3ncc4-[#6]-[#7]=[#6](-c5cc(Cl)ccc5-c4n3)-c3c(F)cccc3-[#8]-[#6])cc2-[#8]-[#6])-[#6](-[#7])=O)c2cc(ccc-12)S([#8-])(=O)=O |c:9,132| Show InChI InChI=1S/C109H155ClFN35O19S2/c1-9-145-82-45-41-66(166(158,159)160)58-71(82)108(4,5)86(145)36-12-11-13-37-87-109(6,72-59-67(167(161,162)163)42-46-83(72)146(87)10-2)47-19-38-88(148)125-48-16-15-28-76(95(152)137-74(92(112)149)27-14-17-49-126-93(150)69-44-40-65(57-85(69)165-8)136-107-134-61-63-60-133-91(89-73(111)26-18-35-84(89)164-7)70-56-64(110)39-43-68(70)90(63)144-107)138-96(153)78(31-22-52-129-103(117)118)140-98(155)80(33-24-54-131-105(121)122)142-100(157)81(34-25-55-132-106(123)124)143-99(156)79(32-23-53-130-104(119)120)141-97(154)77(30-21-51-128-102(115)116)139-94(151)75(135-62(3)147)29-20-50-127-101(113)114/h11-13,18,26,35-37,39-46,56-59,61,74-81H,9-10,14-17,19-25,27-34,38,47-55,60H2,1-8H3,(H38-,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,134,135,136,137,138,139,140,141,142,143,144,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163)/p+5 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394777
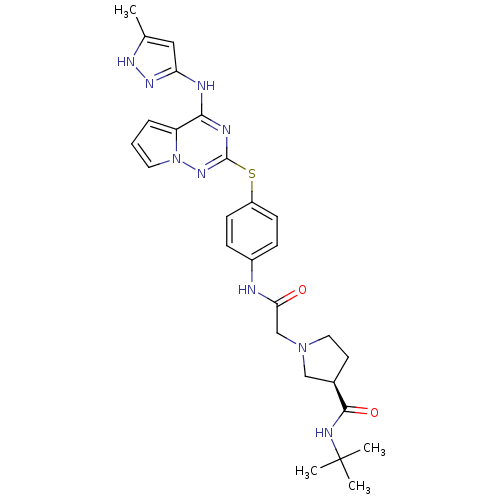
(CHEMBL2163387)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NC(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H33N9O2S/c1-17-14-22(33-32-17)29-24-21-6-5-12-36(21)34-26(30-24)39-20-9-7-19(8-10-20)28-23(37)16-35-13-11-18(15-35)25(38)31-27(2,3)4/h5-10,12,14,18H,11,13,15-16H2,1-4H3,(H,28,37)(H,31,38)(H2,29,30,32,33,34)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106627
(CHEMBL3600764)Show SMILES CCN1\C(=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)CCNC(=O)[C@@]2(Cc3cccc(Nc4nccs4)n3)CC[C@@H](CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:51.53,139.142,106.108,73.75,95.97,wD:84.86,120.123,62.64,40.42,36.37,155.164,c:7,(37.18,-30.09,;37.63,-28.95,;36.68,-27.74,;35.15,-27.8,;34.31,-29.09,;32.77,-29.01,;31.93,-30.3,;30.39,-30.21,;29.44,-31.39,;29.86,-32.87,;31.05,-33.17,;28,-30.86,;26.64,-31.59,;25.33,-30.75,;25.4,-29.21,;26.78,-28.49,;28.07,-29.33,;29.55,-28.92,;30.77,-28.74,;28.75,-27.98,;24.09,-28.4,;23,-28.98,;24.13,-27.17,;23.05,-27.74,;34.6,-26.41,;34.29,-25.22,;33.55,-27.51,;32.05,-27.18,;31.59,-25.71,;30.08,-25.37,;29.25,-26.27,;29.62,-23.9,;28.12,-23.56,;27.66,-22.09,;26.16,-21.75,;25.7,-20.28,;24.2,-19.94,;23.74,-18.47,;22.24,-18.14,;21.4,-19.04,;21.78,-16.67,;22.82,-15.54,;24.32,-15.88,;25.37,-14.74,;26.87,-15.08,;27.92,-13.95,;27.55,-12.78,;29.12,-14.23,;20.27,-16.33,;19.81,-14.86,;20.65,-13.95,;18.31,-14.52,;17.27,-15.65,;15.76,-15.31,;14.72,-16.44,;13.22,-16.1,;12.17,-17.23,;12.53,-18.41,;11.17,-17.01,;17.85,-13.05,;16.35,-12.71,;15.52,-13.62,;15.89,-11.24,;16.94,-10.11,;18.44,-10.45,;19.48,-9.32,;20.99,-9.66,;22.03,-8.53,;21.67,-7.35,;23.23,-8.8,;14.39,-10.9,;13.93,-9.43,;14.76,-8.53,;12.43,-9.1,;11.38,-10.23,;11.85,-11.7,;10.8,-12.84,;11.27,-14.31,;10.22,-15.44,;9.02,-15.17,;10.53,-16.42,;11.97,-7.63,;10.46,-7.29,;9.63,-8.19,;10,-5.82,;11.05,-4.68,;12.55,-5.02,;13.59,-3.88,;15.09,-4.22,;16.13,-3.08,;15.77,-1.91,;17.34,-3.35,;8.5,-5.48,;8.04,-4.01,;8.88,-3.11,;6.54,-3.67,;5.5,-4.81,;5.96,-6.28,;4.92,-7.41,;5.38,-8.88,;4.34,-10.02,;3.14,-9.75,;4.71,-11.19,;6.08,-2.2,;4.58,-1.87,;3.74,-2.77,;4.12,-.4,;5.16,.73,;6.67,.4,;7.12,-1.08,;8.62,-1.42,;9.67,-.29,;9.21,1.18,;7.71,1.52,;11.17,-.64,;11.62,-2.11,;11.98,-3.28,;2.62,-.06,;2.16,1.41,;2.99,2.32,;.65,1.75,;-.39,.62,;-1.89,.96,;-2.94,-.17,;-4.44,.16,;-4.9,1.63,;-3.86,2.76,;-2.35,2.43,;-6.4,1.97,;-6.86,3.44,;-7.22,4.62,;.19,3.22,;-1.31,3.56,;-2,2.8,;-1.77,5.03,;-3.27,5.37,;-3.73,6.84,;-5.23,7.17,;-6.07,6.27,;-5.69,8.64,;-7.2,8.98,;-8.24,7.85,;-9.74,8.18,;-10.78,7.05,;-10.32,5.58,;-8.82,5.25,;-8.36,3.78,;-9.39,2.64,;-9.07,1.15,;-10.41,.39,;-11.55,1.43,;-10.91,2.83,;-7.78,6.38,;-6.18,10.08,;-5.14,11.21,;-3.64,10.88,;-3.18,9.41,;-4.22,8.28,;-2.59,12.01,;-3.06,13.48,;-4.56,13.81,;-5.02,15.28,;-3.98,16.42,;-2.48,16.08,;-1.65,16.99,;-2.02,14.62,;-.81,14.35,;23.16,-21.08,;21.95,-20.81,;23.53,-22.25,;35.78,-25.43,;35.84,-23.89,;37.23,-23.18,;38.52,-24.02,;38.45,-25.57,;37.07,-26.26,;37.32,-21.65,;37.39,-20.42,;38.42,-21.09,;36.29,-20.97,)| Show InChI InChI=1S/C115H161ClFN41O19S3/c1-6-157-87-44-42-73(179(171,172)173)64-75(87)113(3,4)90(157)30-12-31-91-114(5,76-65-74(180(174,175)176)43-45-88(76)158(91)7-2)49-13-33-93(159)133-52-9-8-22-78(96(118)161)144-97(162)79(23-14-53-135-106(119)120)145-98(163)80(24-15-54-136-107(121)122)146-99(164)81(25-16-55-137-108(123)124)147-100(165)82(26-17-56-138-109(125)126)148-101(166)83(27-18-57-139-110(127)128)149-102(167)84(28-19-58-140-111(129)130)150-104(169)86(63-68-36-40-70(41-37-68)154-156-132)151-103(168)85(62-67-34-38-69(39-35-67)153-155-131)143-94(160)48-59-134-105(170)115(66-71-20-10-32-92(142-71)152-112-141-60-61-178-112)50-46-72(47-51-115)177-89-29-11-21-77(116)95(89)117/h10-12,20-21,29-32,34-45,60-61,64-65,72,78-86H,6-9,13-19,22-28,33,46-59,62-63,66H2,1-5H3,(H39-,118,119,120,121,122,123,124,125,126,127,128,129,130,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176)/p+1/t72-,78-,79-,80-,81-,82-,83-,84-,85+,86+,114?,115-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394785
(CHEMBL2163404)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCC(F)(F)C4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H29F2N9O2S/c1-17-13-22(34-33-17)31-24-21-3-2-10-38(21)35-26(32-24)41-20-6-4-19(5-7-20)30-23(39)15-36-11-8-18(14-36)25(40)37-12-9-27(28,29)16-37/h2-7,10,13,18H,8-9,11-12,14-16H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora kinase A catalytic domain by competition binding assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352327
(CHEMBL1822649)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)OC(C)(C)C(F)(F)F)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H29F3N8O3S/c1-15-11-21(34-33-15)31-23-18-5-4-10-37(18)35-24(32-23)41-17-8-6-16(7-9-17)30-22(39)14-36-12-19(38)20(13-36)40-25(2,3)26(27,28)29/h4-11,19-20,38H,12-14H2,1-3H3,(H,30,39)(H2,31,32,33,34,35)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106626
(CHEMBL3600868)Show SMILES CCN1\C(=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)[C@@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)CCNC(=O)[C@@]2(Cc3cccc(Nc4nccs4)n3)CC[C@@H](CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:51.53,106.108,95.97,139.142,36.37,73.75,wD:40.42,62.64,84.86,120.123,155.164,c:7,(5.48,2.13,;6.59,2.66,;7.86,1.8,;7.89,.3,;6.65,-.62,;6.82,-2.15,;5.58,-3.06,;5.75,-4.59,;7.09,-5.36,;8.49,-4.73,;8.62,-3.5,;6.77,-6.86,;7.69,-8.1,;7.05,-9.52,;5.52,-9.67,;4.6,-8.41,;5.25,-7.01,;4.63,-5.61,;4.27,-4.43,;3.82,-6.53,;4.9,-11.08,;4.4,-12.21,;3.67,-11.22,;5.63,-12.07,;9.35,-.17,;10.56,-.4,;8.32,-1.3,;8.76,-2.78,;10.26,-3.13,;10.7,-4.61,;9.85,-5.5,;12.2,-4.96,;12.64,-6.44,;14.13,-6.8,;14.58,-8.27,;16.07,-8.63,;16.52,-10.1,;18.01,-10.46,;18.46,-11.93,;17.61,-12.83,;19.95,-12.29,;21.01,-11.17,;22.51,-11.53,;23.57,-10.41,;25.07,-10.77,;26.13,-9.65,;25.78,-8.47,;27.33,-9.94,;20.4,-13.77,;21.89,-14.12,;22.74,-13.23,;22.34,-15.6,;21.28,-16.72,;21.72,-18.19,;20.67,-19.32,;21.11,-20.79,;20.06,-21.91,;18.86,-21.63,;20.41,-23.09,;23.83,-15.95,;24.28,-17.43,;23.43,-18.32,;25.77,-17.79,;26.83,-16.67,;28.33,-17.03,;29.39,-15.91,;30.89,-16.27,;31.95,-15.15,;31.6,-13.97,;33.15,-15.43,;26.22,-19.26,;27.71,-19.62,;28.56,-18.72,;28.16,-21.09,;27.1,-22.21,;27.54,-23.69,;26.49,-24.81,;26.93,-26.29,;25.88,-27.41,;24.68,-27.13,;26.23,-28.59,;29.65,-21.45,;30.1,-22.92,;29.25,-23.82,;31.59,-23.28,;32.65,-22.16,;32.22,-20.69,;33.28,-19.57,;32.84,-18.09,;33.9,-16.97,;35.1,-17.26,;33.55,-15.79,;32.04,-24.76,;33.53,-25.11,;34.38,-24.22,;33.97,-26.59,;32.92,-27.71,;33.36,-29.18,;32.31,-30.31,;30.81,-29.95,;29.75,-31.08,;30.11,-32.25,;28.55,-30.79,;35.47,-26.94,;35.91,-28.42,;35.07,-29.31,;37.41,-28.77,;38.47,-27.66,;38.04,-26.18,;36.54,-25.82,;36.1,-24.35,;37.16,-23.23,;38.65,-23.59,;39.09,-25.06,;36.71,-21.76,;35.21,-21.4,;34.02,-21.12,;37.85,-30.25,;39.35,-30.61,;40.2,-29.71,;39.79,-32.08,;38.73,-33.2,;37.24,-32.84,;36.8,-31.37,;35.3,-31,;34.24,-32.12,;34.68,-33.6,;36.18,-33.96,;32.75,-31.76,;31.69,-32.88,;30.84,-33.77,;41.29,-32.44,;41.73,-33.91,;40.89,-34.81,;43.23,-34.27,;43.67,-35.75,;45.17,-36.1,;45.61,-37.58,;44.77,-38.47,;47.11,-37.93,;47.55,-39.41,;46.5,-40.53,;46.94,-42,;45.88,-43.12,;44.38,-42.76,;43.94,-41.29,;42.44,-40.93,;42.01,-39.45,;42.95,-38.25,;42.08,-36.98,;40.61,-37.42,;40.56,-38.96,;45,-40.17,;46.64,-36.49,;47.7,-35.37,;49.2,-35.73,;49.64,-37.2,;48.58,-38.32,;50.26,-34.61,;51.75,-34.97,;52.19,-36.44,;53.69,-36.8,;54.75,-35.69,;54.31,-34.21,;55.16,-33.32,;52.81,-33.85,;52.47,-32.67,;15.46,-11.22,;15.81,-12.4,;14.26,-10.93,;10.23,1.08,;11.76,1.25,;12.36,2.69,;11.44,3.92,;9.89,3.73,;9.3,2.3,;13.89,2.89,;14.64,1.91,;15.11,3.05,;14.36,4.03,)| Show InChI InChI=1S/C115H161ClFN41O19S3/c1-6-157-87-44-42-73(179(171,172)173)64-75(87)113(3,4)90(157)30-12-31-91-114(5,76-65-74(180(174,175)176)43-45-88(76)158(91)7-2)49-13-33-93(159)133-52-9-8-22-78(96(118)161)144-97(162)79(23-14-53-135-106(119)120)145-98(163)80(24-15-54-136-107(121)122)146-99(164)81(25-16-55-137-108(123)124)147-100(165)82(26-17-56-138-109(125)126)148-101(166)83(27-18-57-139-110(127)128)149-102(167)84(28-19-58-140-111(129)130)150-104(169)86(63-68-36-40-70(41-37-68)154-156-132)151-103(168)85(62-67-34-38-69(39-35-67)153-155-131)143-94(160)48-59-134-105(170)115(66-71-20-10-32-92(142-71)152-112-141-60-61-178-112)50-46-72(47-51-115)177-89-29-11-21-77(116)95(89)117/h10-12,20-21,29-32,34-45,60-61,64-65,72,78-86H,6-9,13-19,22-28,33,46-59,62-63,66H2,1-5H3,(H39-,118,119,120,121,122,123,124,125,126,127,128,129,130,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176)/p+1/t72-,78-,79+,80+,81+,82+,83+,84+,85-,86-,114?,115-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394781
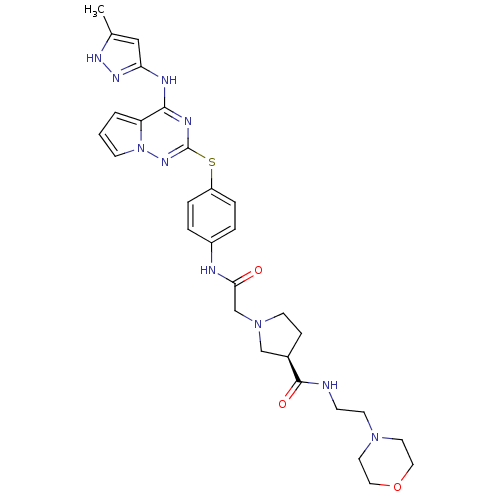
(CHEMBL2163392)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NCCN4CCOCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C29H36N10O3S/c1-20-17-25(35-34-20)32-27-24-3-2-10-39(24)36-29(33-27)43-23-6-4-22(5-7-23)31-26(40)19-38-11-8-21(18-38)28(41)30-9-12-37-13-15-42-16-14-37/h2-7,10,17,21H,8-9,11-16,18-19H2,1H3,(H,30,41)(H,31,40)(H2,32,33,34,35,36)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394786
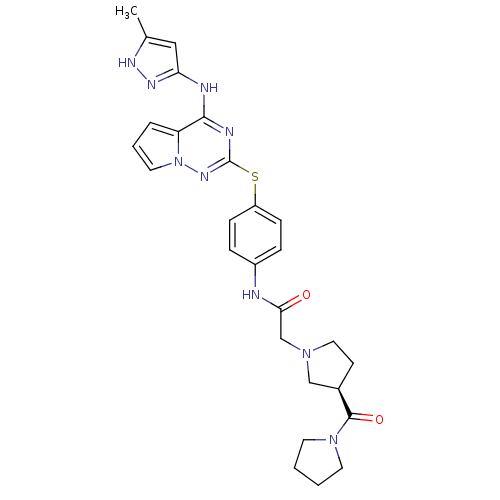
(CHEMBL2163403)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-18-15-23(32-31-18)29-25-22-5-4-13-36(22)33-27(30-25)39-21-8-6-20(7-9-21)28-24(37)17-34-14-10-19(16-34)26(38)35-11-2-3-12-35/h4-9,13,15,19H,2-3,10-12,14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
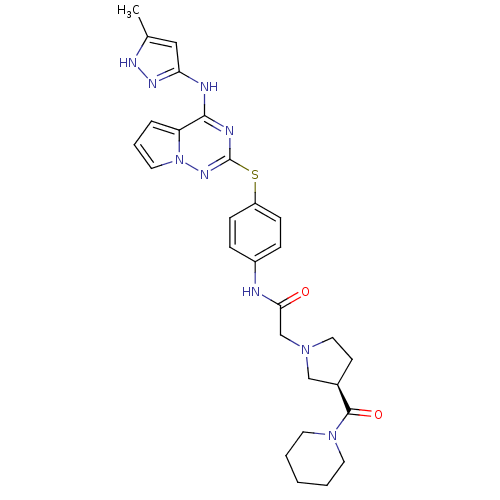
(Homo sapiens (Human)) | BDBM50394784
(CHEMBL2163389)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H33N9O2S/c1-19-16-24(33-32-19)30-26-23-6-5-14-37(23)34-28(31-26)40-22-9-7-21(8-10-22)29-25(38)18-35-15-11-20(17-35)27(39)36-12-3-2-4-13-36/h5-10,14,16,20H,2-4,11-13,15,17-18H2,1H3,(H,29,38)(H2,30,31,32,33,34)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for AURKA kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PCBioAssay
| n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2NC5ZHH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50106628
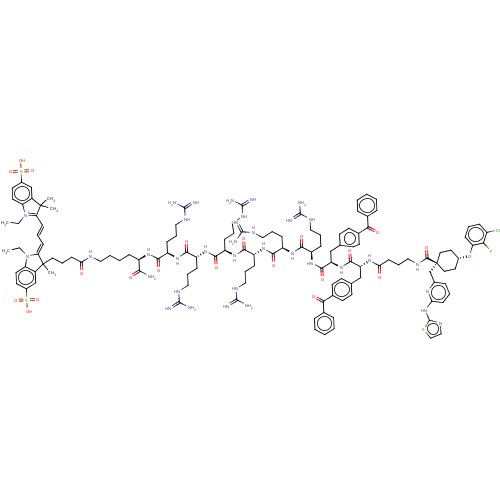
(CHEMBL3600763)Show SMILES CCN1\C(=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)CCCNC(=O)[C@@]2(Cc3cccc(Nc4nccs4)n3)CC[C@@H](CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:150.155,40.42,106.108,62.64,36.37,84.86,wD:125.129,95.97,73.75,51.53,166.177,c:7,(-13.89,58.17,;-14.9,58.86,;-14.79,60.39,;-13.5,61.2,;-12.08,60.61,;-10.85,61.55,;-9.43,60.96,;-8.21,61.9,;-8.25,63.44,;-9.51,64.32,;-10.63,63.79,;-6.8,63.95,;-6.21,65.37,;-4.66,65.56,;-3.74,64.33,;-4.34,62.89,;-5.88,62.72,;-6.76,61.47,;-5.55,61.26,;-7.58,60.55,;-2.21,64.53,;-.99,64.69,;-1.74,65.66,;-1.47,63.55,;-13.83,62.67,;-14.25,63.82,;-12.34,62.35,;-11.29,63.47,;-11.74,64.95,;-10.69,66.07,;-9.49,65.8,;-11.14,67.55,;-10.09,68.67,;-10.54,70.15,;-9.49,71.27,;-9.94,72.75,;-8.89,73.87,;-9.34,75.35,;-8.29,76.47,;-7.09,76.19,;-8.74,77.94,;-10.24,78.29,;-10.69,79.77,;-12.19,80.12,;-12.64,81.59,;-14.14,81.94,;-14.98,81.04,;-14.44,82.92,;-7.69,79.07,;-8.14,80.54,;-9.34,80.82,;-7.09,81.67,;-5.59,81.33,;-4.54,82.46,;-3.04,82.11,;-1.99,83.24,;-.49,82.9,;-.13,81.72,;.35,83.8,;-7.54,83.14,;-6.49,84.27,;-5.29,83.99,;-6.94,85.74,;-8.44,86.09,;-9.49,84.96,;-10.99,85.3,;-12.04,84.17,;-13.54,84.52,;-13.9,85.69,;-14.24,83.77,;-5.89,86.87,;-6.34,88.34,;-7.54,88.62,;-5.29,89.47,;-3.79,89.13,;-2.74,90.25,;-1.24,89.91,;-.19,91.04,;1.31,90.7,;1.67,89.52,;2.15,91.6,;-5.74,90.94,;-4.69,92.07,;-3.49,91.79,;-5.14,93.54,;-6.64,93.89,;-7.09,95.37,;-8.59,95.72,;-9.04,97.19,;-10.54,97.54,;-11.38,96.64,;-10.89,98.72,;-4.09,94.67,;-4.54,96.14,;-5.74,96.42,;-3.49,97.27,;-1.99,96.93,;-.94,98.05,;.56,97.71,;1.61,98.84,;3.11,98.49,;3.47,97.32,;3.95,99.4,;-3.94,98.74,;-2.89,99.87,;-1.69,99.59,;-3.34,101.34,;-4.84,101.69,;-5.29,103.16,;-4.24,104.29,;-4.69,105.76,;-6.19,106.11,;-7.24,104.98,;-6.79,103.51,;-6.64,107.58,;-5.81,108.48,;-8.15,107.92,;-8.6,109.39,;-10.1,109.73,;-11.15,108.6,;-10.69,107.13,;-9.19,106.79,;-2.29,102.47,;-2.74,103.94,;-3.74,104.17,;-1.69,105.07,;-.19,104.72,;.86,105.85,;.41,107.32,;1.46,108.45,;2.96,108.1,;3.41,106.63,;2.36,105.51,;4.01,109.23,;3.66,110.4,;5.51,108.88,;5.96,107.4,;7.46,107.05,;8.51,108.17,;8.07,109.65,;6.57,110,;-2.14,106.54,;-1.09,107.67,;-.09,107.44,;-1.54,109.14,;-.49,110.27,;-.94,111.74,;.11,112.87,;-.34,114.34,;-1.54,114.62,;.71,115.47,;.26,116.94,;-1.24,117.29,;-2.29,116.16,;-3.79,116.5,;-4.24,117.98,;-3.19,119.1,;-3.64,120.58,;-5.14,120.92,;-5.72,122.34,;-7.26,122.2,;-7.6,120.7,;-6.28,119.91,;-1.69,118.76,;1.72,116.6,;3.22,116.26,;3.67,114.78,;2.62,113.66,;1.12,114,;5.17,114.44,;6.22,115.56,;5.77,117.03,;6.82,118.16,;8.32,117.81,;8.77,116.34,;9.97,116.06,;7.72,115.21,;8.08,114.03,;-7.39,73.53,;-7.03,72.35,;-6.55,74.43,;-15.36,62.8,;-16.28,64.04,;-17.82,63.84,;-18.42,62.43,;-17.49,61.18,;-15.96,61.39,;-18.76,65.06,;-19.98,64.9,;-19.51,66.04,;-18.29,66.2,)| Show InChI InChI=1S/C130H173ClFN35O21S3/c1-6-166-100-56-54-86(190(182,183)184)75-88(100)128(3,4)103(166)41-18-42-104-129(5,89-76-87(191(185,186)187)55-57-101(89)167(104)7-2)60-19-44-106(168)146-63-15-14-33-91(111(133)172)157-112(173)92(34-20-65-148-121(134)135)158-113(174)93(35-21-66-149-122(136)137)159-114(175)94(36-22-67-150-123(138)139)160-115(176)95(37-23-68-151-124(140)141)161-116(177)96(38-24-69-152-125(142)143)162-117(178)97(39-25-70-153-126(144)145)163-119(180)99(74-79-48-52-83(53-49-79)110(171)81-29-12-9-13-30-81)164-118(179)98(73-78-46-50-82(51-47-78)109(170)80-27-10-8-11-28-80)156-107(169)45-26-64-147-120(181)130(77-84-31-16-43-105(155-84)165-127-154-71-72-189-127)61-58-85(59-62-130)188-102-40-17-32-90(131)108(102)132/h8-13,16-18,27-32,40-43,46-57,71-72,75-76,85,91-99H,6-7,14-15,19-26,33-39,44-45,58-70,73-74,77H2,1-5H3,(H39-,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,168,169,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187)/p+1/t85-,91-,92-,93-,94-,95-,96-,97-,98-,99-,129?,130-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Binding affinity to purified recombinant full-length Aurora A (unknown origin) by fluorescence polarisation/anisotropy based equilibrium binding assa... |
Bioorg Med Chem Lett 25: 3290-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.060
BindingDB Entry DOI: 10.7270/Q2C53NMT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for AURKA kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352298
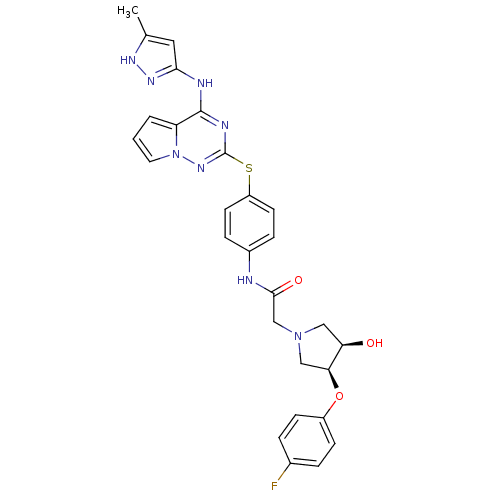
(CHEMBL1822661)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@@H](O)[C@H](C4)Oc4ccc(F)cc4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H27FN8O3S/c1-17-13-25(34-33-17)31-27-22-3-2-12-37(22)35-28(32-27)41-21-10-6-19(7-11-21)30-26(39)16-36-14-23(38)24(15-36)40-20-8-4-18(29)5-9-20/h2-13,23-24,38H,14-16H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t23-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352300
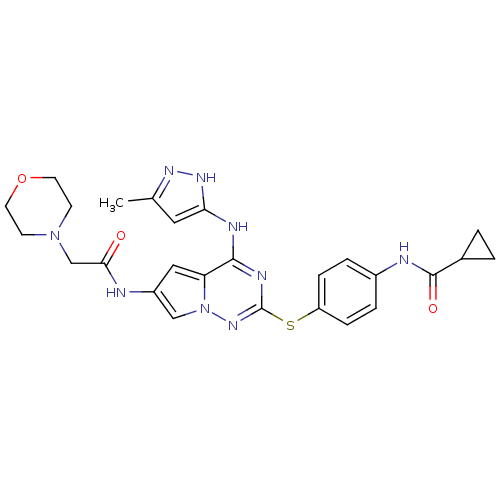
(CHEMBL1822487)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)C4CC4)cc3)nn3cc(NC(=O)CN4CCOCC4)cc23)[nH]n1 Show InChI InChI=1S/C26H29N9O3S/c1-16-12-22(32-31-16)29-24-21-13-19(27-23(36)15-34-8-10-38-11-9-34)14-35(21)33-26(30-24)39-20-6-4-18(5-7-20)28-25(37)17-2-3-17/h4-7,12-14,17H,2-3,8-11,15H2,1H3,(H,27,36)(H,28,37)(H2,29,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352303
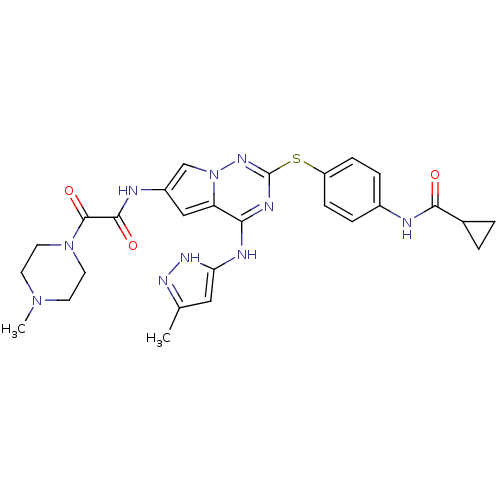
(CHEMBL1822490)Show SMILES CN1CCN(CC1)C(=O)C(=O)Nc1cc2c(Nc3cc(C)n[nH]3)nc(Sc3ccc(NC(=O)C4CC4)cc3)nn2c1 Show InChI InChI=1S/C27H30N10O3S/c1-16-13-22(33-32-16)30-23-21-14-19(29-25(39)26(40)36-11-9-35(2)10-12-36)15-37(21)34-27(31-23)41-20-7-5-18(6-8-20)28-24(38)17-3-4-17/h5-8,13-15,17H,3-4,9-12H2,1-2H3,(H,28,38)(H,29,39)(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352323
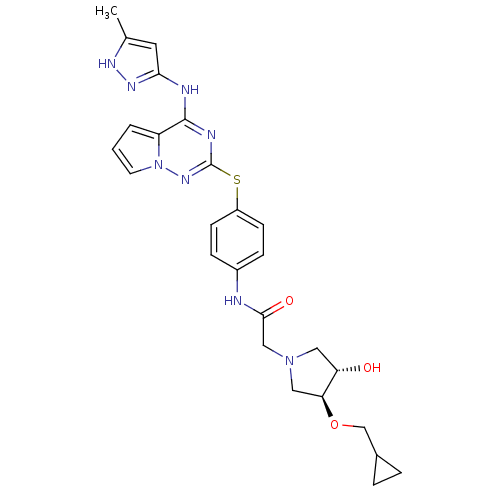
(CHEMBL1822653)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)OCC4CC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H30N8O3S/c1-16-11-23(31-30-16)28-25-20-3-2-10-34(20)32-26(29-25)38-19-8-6-18(7-9-19)27-24(36)14-33-12-21(35)22(13-33)37-15-17-4-5-17/h2-3,6-11,17,21-22,35H,4-5,12-15H2,1H3,(H,27,36)(H2,28,29,30,31,32)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50401421
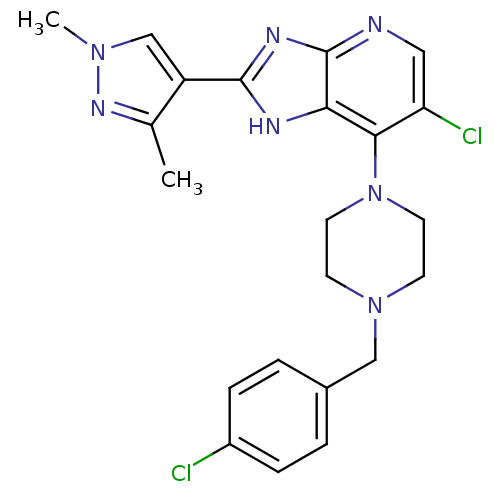
(CHEMBL2207503)Show SMILES Cc1nn(C)cc1-c1nc2ncc(Cl)c(N3CCN(Cc4ccc(Cl)cc4)CC3)c2[nH]1 Show InChI InChI=1S/C22H23Cl2N7/c1-14-17(13-29(2)28-14)21-26-19-20(18(24)11-25-22(19)27-21)31-9-7-30(8-10-31)12-15-3-5-16(23)6-4-15/h3-6,11,13H,7-10,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase |
J Med Chem 55: 8721-34 (2012)
Article DOI: 10.1021/jm300952s
BindingDB Entry DOI: 10.7270/Q22J6D2W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM247371
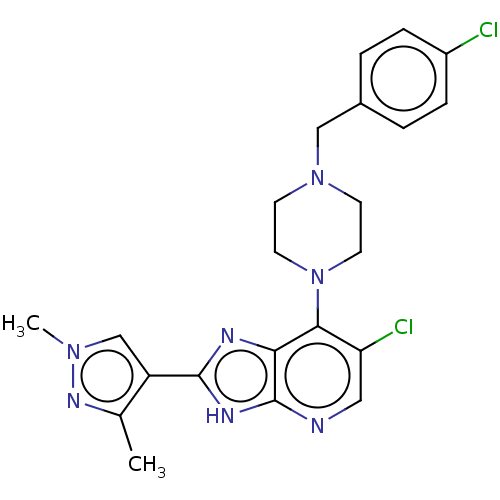
(US9447092, 1)Show SMILES Cc1nn(C)cc1-c1nc2c(N3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)cnc2[nH]1 Show InChI InChI=1S/C22H23Cl2N7/c1-14-17(13-29(2)28-14)21-26-19-20(18(24)11-25-22(19)27-21)31-9-7-30(8-10-31)12-15-3-5-16(23)6-4-15/h3-6,11,13H,7-10,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352324
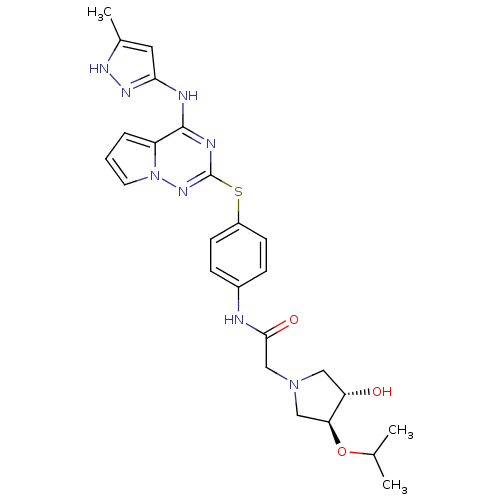
(CHEMBL1822652)Show SMILES CC(C)O[C@H]1CN(CC(=O)Nc2ccc(Sc3nc(Nc4cc(C)[nH]n4)c4cccn4n3)cc2)C[C@@H]1O |r| Show InChI InChI=1S/C25H30N8O3S/c1-15(2)36-21-13-32(12-20(21)34)14-23(35)26-17-6-8-18(9-7-17)37-25-28-24(19-5-4-10-33(19)31-25)27-22-11-16(3)29-30-22/h4-11,15,20-21,34H,12-14H2,1-3H3,(H,26,35)(H2,27,28,29,30,31)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352326
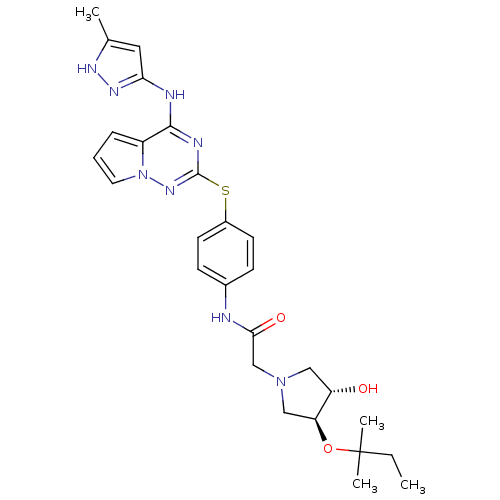
(CHEMBL1822650)Show SMILES CCC(C)(C)O[C@H]1CN(CC(=O)Nc2ccc(Sc3nc(Nc4cc(C)[nH]n4)c4cccn4n3)cc2)C[C@@H]1O |r| Show InChI InChI=1S/C27H34N8O3S/c1-5-27(3,4)38-22-15-34(14-21(22)36)16-24(37)28-18-8-10-19(11-9-18)39-26-30-25(20-7-6-12-35(20)33-26)29-23-13-17(2)31-32-23/h6-13,21-22,36H,5,14-16H2,1-4H3,(H,28,37)(H2,29,30,31,32,33)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352307
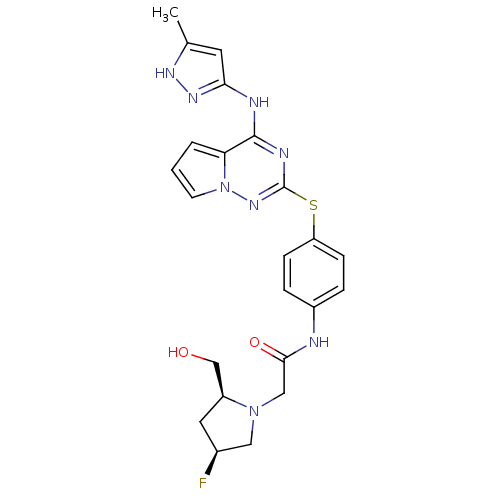
(CHEMBL1822640)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@@H](F)C[C@H]4CO)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C23H25FN8O2S/c1-14-9-20(29-28-14)26-22-19-3-2-8-32(19)30-23(27-22)35-18-6-4-16(5-7-18)25-21(34)12-31-11-15(24)10-17(31)13-33/h2-9,15,17,33H,10-13H2,1H3,(H,25,34)(H2,26,27,28,29,30)/t15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352309
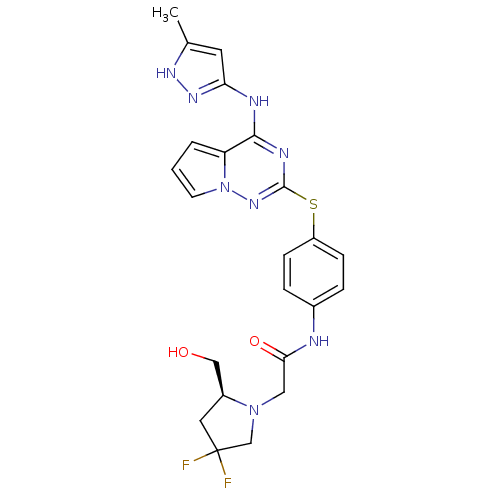
(CHEMBL1822638)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC(F)(F)C[C@H]4CO)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C23H24F2N8O2S/c1-14-9-19(30-29-14)27-21-18-3-2-8-33(18)31-22(28-21)36-17-6-4-15(5-7-17)26-20(35)11-32-13-23(24,25)10-16(32)12-34/h2-9,16,34H,10-13H2,1H3,(H,26,35)(H2,27,28,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A kinase catalytic domain by competitive binding assay |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394786

(CHEMBL2163403)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-18-15-23(32-31-18)29-25-22-5-4-13-36(22)33-27(30-25)39-21-8-6-20(7-9-21)28-24(37)17-34-14-10-19(16-34)26(38)35-11-2-3-12-35/h4-9,13,15,19H,2-3,10-12,14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora kinase A catalytic domain by competition binding assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data