 Found 167 hits of kd data for polymerid = 1846,50006599
Found 167 hits of kd data for polymerid = 1846,50006599 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284
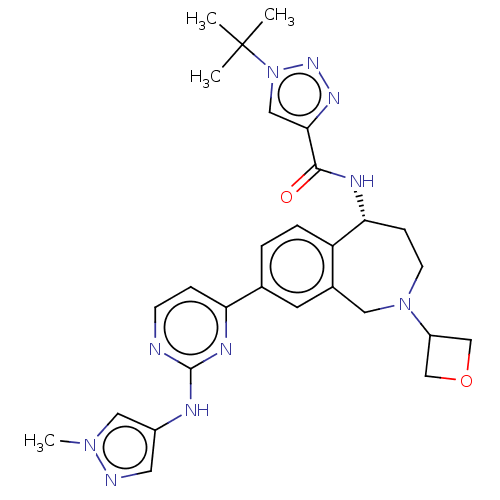
((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244495
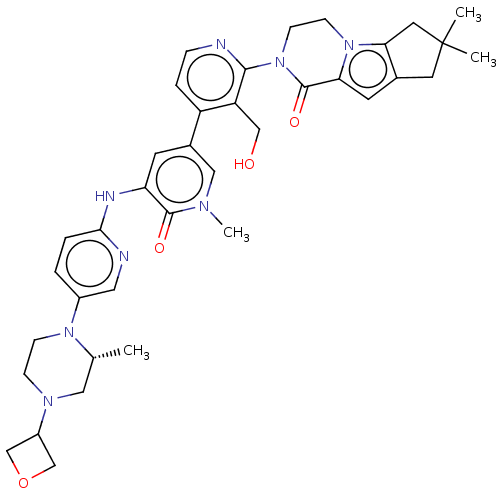
(CHEMBL4066176)Show SMILES C[C@@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244495

(CHEMBL4066176)Show SMILES C[C@@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407
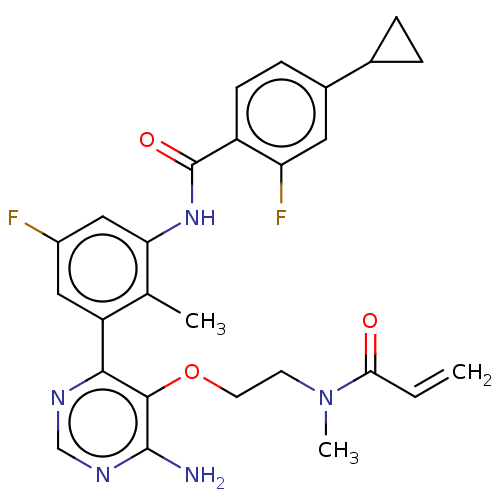
(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553436
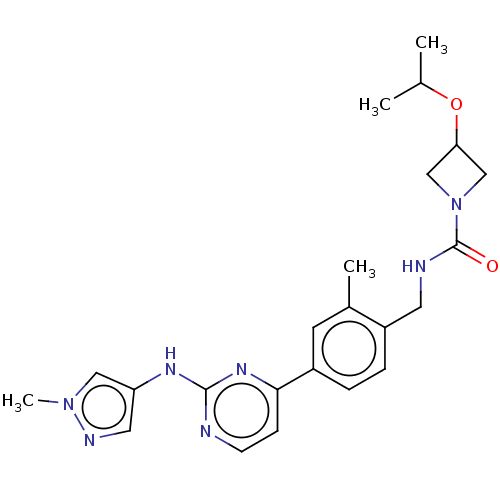
(CHEMBL4744041)Show SMILES CC(C)OC1CN(C1)C(=O)NCc1ccc(cc1C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged recombinant BTK (unknown origin) measured after 1 hr by biotinylated-ligand affinity bead-based qPCR analysis |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407

(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged recombinant BTK (unknown origin) measured after 1 hr by biotinylated-ligand affinity bead-based qPCR analysis |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596438
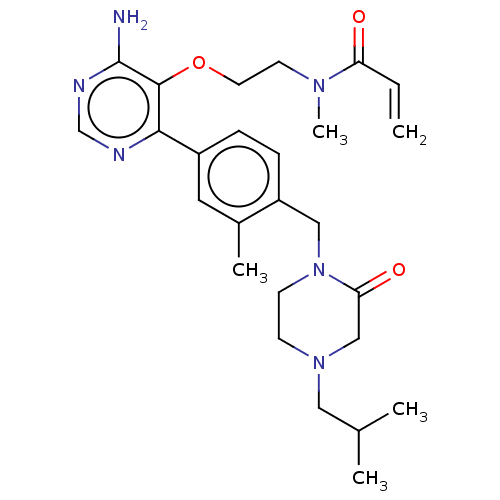
(CHEMBL5188786)Show SMILES CC(C)CN1CCN(Cc2ccc(cc2C)-c2ncnc(N)c2OCCN(C)C(=O)C=C)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PCBioAssay
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50557487
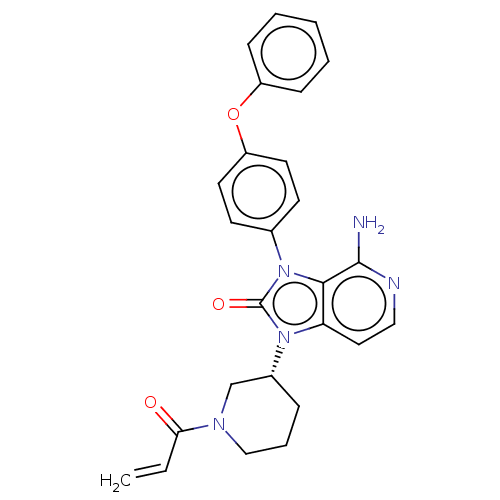
(Tolebrutinib)Show SMILES Nc1nccc2n([C@@H]3CCCN(C3)C(=O)C=C)c(=O)n(-c3ccc(Oc4ccccc4)cc3)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for full-length BTK |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36516
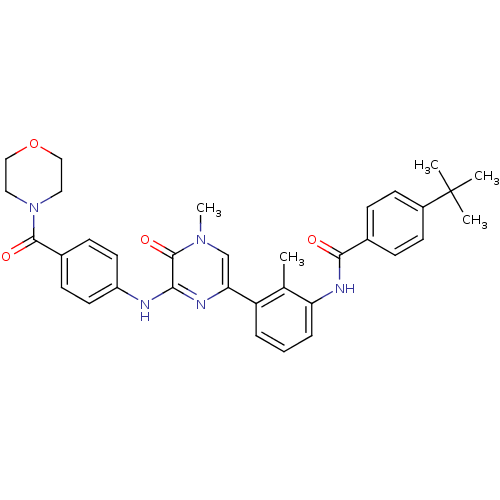
(4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(7-6-8-28(22)37-31(40)23-9-13-25(14-10-23)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-15-11-24(12-16-26)32(41)39-17-19-43-20-18-39/h6-16,21H,17-20H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | 1.5 | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged recombinant BTK (unknown origin) measured after 1 hr by biotinylated-ligand affinity bead-based qPCR analysis |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068574
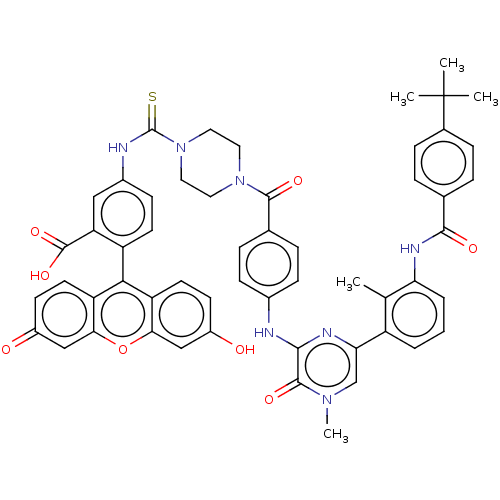
(CHEMBL3402355)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCN(CC2)C(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(-8.97,-12.95,;-7.9,-12.33,;-7.9,-10.79,;-9.23,-10.02,;-9.23,-8.47,;-8.16,-7.86,;-10.56,-7.7,;-10.56,-6.16,;-11.89,-5.39,;-13.23,-6.16,;-13.23,-7.7,;-11.9,-8.47,;-14.56,-5.38,;-14.56,-4.15,;-15.63,-6,;-15.62,-4.77,;-6.57,-10.02,;-5.23,-10.8,;-5.24,-12.34,;-6.57,-13.1,;-6.57,-14.64,;-7.91,-15.41,;-7.91,-16.95,;-8.97,-17.57,;-6.57,-17.72,;-6.57,-18.96,;-5.24,-16.95,;-3.9,-17.72,;-2.57,-16.95,;-1.23,-17.71,;.1,-16.94,;.09,-15.4,;-1.24,-14.63,;-2.57,-15.41,;1.42,-14.62,;2.49,-15.23,;1.42,-13.08,;2.74,-12.3,;2.73,-10.76,;1.39,-10,;.07,-10.78,;.08,-12.32,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;-5.24,-15.41,)| Show InChI InChI=1S/C55H49N7O8S/c1-31-39(7-6-8-44(31)59-50(65)32-9-13-34(14-10-32)55(2,3)4)45-30-60(5)52(67)49(58-45)56-35-15-11-33(12-16-35)51(66)61-23-25-62(26-24-61)54(71)57-36-17-20-40(43(27-36)53(68)69)48-41-21-18-37(63)28-46(41)70-47-29-38(64)19-22-42(47)48/h6-22,27-30,63H,23-26H2,1-5H3,(H,56,58)(H,57,71)(H,59,65)(H,68,69) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50175583
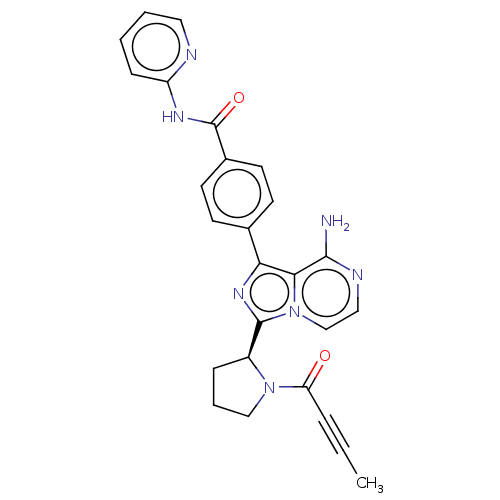
(ACP-196 | Acalabrutinib | US10239883, Example 6 | ...)Show SMILES [H][C@]1(CCCN1C(=O)C#CC)c1nc(-c2ccc(cc2)C(=O)Nc2ccccn2)c2c(N)nccn12 |r| Show InChI InChI=1S/C26H23N7O2/c1-2-6-21(34)32-15-5-7-19(32)25-31-22(23-24(27)29-14-16-33(23)25)17-9-11-18(12-10-17)26(35)30-20-8-3-4-13-28-20/h3-4,8-14,16,19H,5,7,15H2,1H3,(H2,27,29)(H,28,30,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588042

(CHEMBL5205957)Show SMILES Fc1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@@H]2CCCN(C2)c2ncnc3[nH]ccc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068595
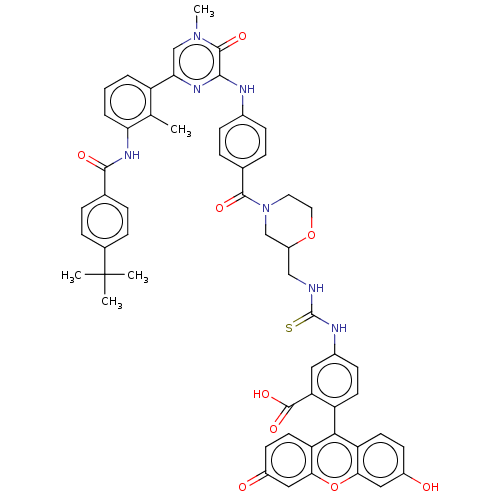
(CHEMBL3402356)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOC(CNC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)C2)n1 |(-1.21,-23.53,;-1.21,-22.3,;-2.54,-21.53,;-3.88,-22.29,;-5.21,-21.52,;-5.21,-20.29,;-6.55,-22.28,;-7.88,-21.51,;-9.22,-22.28,;-9.22,-23.82,;-7.89,-24.59,;-6.55,-23.82,;-10.56,-24.59,;-11.62,-23.97,;-10.56,-25.82,;-11.62,-25.2,;-2.54,-19.99,;-1.21,-19.22,;.13,-19.99,;.12,-21.53,;1.46,-22.3,;1.46,-23.84,;2.79,-24.61,;2.79,-25.84,;4.12,-23.84,;5.19,-24.46,;4.12,-22.3,;5.46,-21.53,;5.46,-19.99,;6.79,-19.21,;6.78,-17.67,;5.44,-16.91,;4.11,-17.68,;4.12,-19.22,;5.44,-15.37,;6.5,-14.75,;4.1,-14.6,;2.77,-15.38,;1.43,-14.62,;1.42,-13.08,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;4.09,-13.06,;2.79,-21.53,)| Show InChI InChI=1S/C56H51N7O9S/c1-31-40(7-6-8-45(31)61-51(66)32-9-13-34(14-10-32)56(2,3)4)46-30-62(5)53(68)50(60-46)58-35-15-11-33(12-16-35)52(67)63-23-24-71-39(29-63)28-57-55(73)59-36-17-20-41(44(25-36)54(69)70)49-42-21-18-37(64)26-47(42)72-48-27-38(65)19-22-43(48)49/h6-22,25-27,30,39,64H,23-24,28-29H2,1-5H3,(H,58,60)(H,61,66)(H,69,70)(H2,57,59,73) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM250082

(US9447106, 27b (peak 2) | US9556188, Compound 27a)Show SMILES NC(=O)c1c2NCC[C@@H](C3CCN(CC3)C(=O)C=C)n2nc1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N5O3/c1-2-23(33)31-16-13-18(14-17-31)22-12-15-29-27-24(26(28)34)25(30-32(22)27)19-8-10-21(11-9-19)35-20-6-4-3-5-7-20/h2-11,18,22,29H,1,12-17H2,(H2,28,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588036
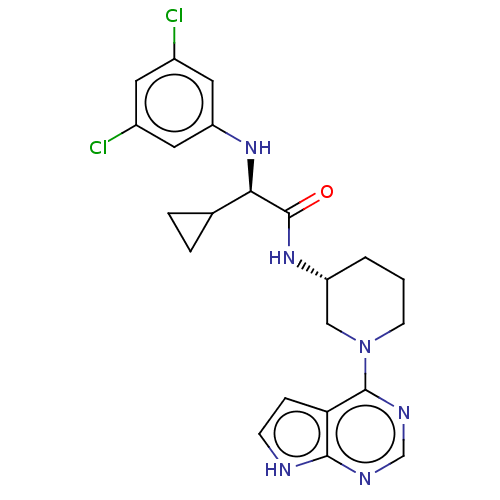
(CHEMBL5175032)Show SMILES Clc1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@@H]2CCCN(C2)c2ncnc3[nH]ccc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068543
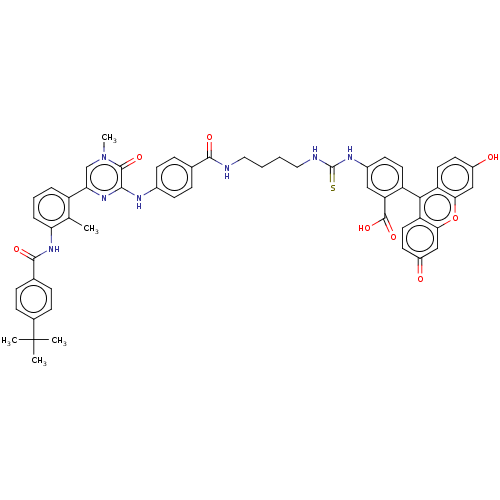
(CHEMBL3402353)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(15.83,-15.08,;14.75,-14.48,;14.73,-12.94,;16.05,-12.15,;16.02,-10.61,;14.95,-10.01,;17.35,-9.81,;17.32,-8.27,;18.64,-7.48,;19.99,-8.23,;20.01,-9.77,;18.69,-10.56,;21.31,-7.44,;21.29,-6.21,;22.39,-8.04,;22.37,-6.81,;13.38,-12.19,;12.06,-12.98,;12.09,-14.52,;13.43,-15.27,;13.45,-16.81,;14.8,-17.56,;14.82,-19.1,;15.89,-19.71,;13.5,-19.89,;13.51,-21.12,;12.15,-19.14,;10.83,-19.93,;9.48,-19.17,;8.16,-19.96,;6.81,-19.2,;6.8,-17.66,;8.12,-16.88,;9.47,-17.63,;5.46,-16.9,;4.4,-17.53,;5.44,-15.36,;4.1,-14.6,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;12.13,-17.6,)| Show InChI InChI=1S/C55H51N7O8S/c1-31-39(9-8-10-44(31)61-51(66)33-11-15-34(16-12-33)55(2,3)4)45-30-62(5)52(67)49(60-45)58-35-17-13-32(14-18-35)50(65)56-25-6-7-26-57-54(71)59-36-19-22-40(43(27-36)53(68)69)48-41-23-20-37(63)28-46(41)70-47-29-38(64)21-24-42(47)48/h8-24,27-30,63H,6-7,25-26H2,1-5H3,(H,56,65)(H,58,60)(H,61,66)(H,68,69)(H2,57,59,71) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588031
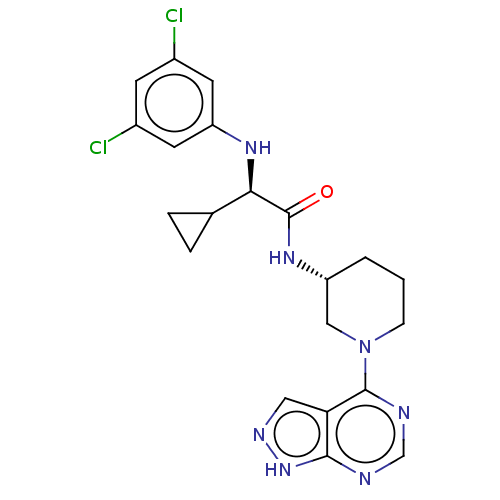
(CHEMBL5207862)Show SMILES Clc1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@@H]2CCCN(C2)c2ncnc3[nH]ncc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068541
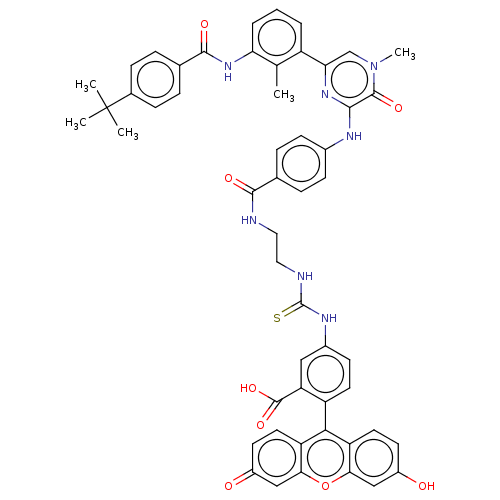
(CHEMBL3400825)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(14.47,-12.78,;13.4,-12.18,;13.37,-10.64,;14.7,-9.85,;14.67,-8.31,;13.59,-7.71,;15.99,-7.51,;15.97,-5.97,;17.29,-5.18,;18.63,-5.93,;18.66,-7.47,;17.34,-8.26,;19.96,-5.14,;19.94,-3.91,;21.03,-5.74,;21.01,-4.51,;12.03,-9.89,;10.71,-10.68,;10.73,-12.22,;12.08,-12.97,;12.1,-14.51,;13.44,-15.26,;13.46,-16.8,;14.54,-17.41,;12.14,-17.59,;12.16,-18.82,;10.8,-16.84,;9.47,-17.63,;8.13,-16.87,;6.8,-17.66,;5.46,-16.9,;5.44,-15.36,;6.77,-14.58,;8.11,-15.33,;4.1,-14.6,;3.04,-15.23,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;10.78,-15.3,)| Show InChI InChI=1S/C53H47N7O8S/c1-29-37(7-6-8-42(29)59-49(64)31-9-13-32(14-10-31)53(2,3)4)43-28-60(5)50(65)47(58-43)56-33-15-11-30(12-16-33)48(63)54-23-24-55-52(69)57-34-17-20-38(41(25-34)51(66)67)46-39-21-18-35(61)26-44(39)68-45-27-36(62)19-22-40(45)46/h6-22,25-28,61H,23-24H2,1-5H3,(H,54,63)(H,56,58)(H,59,64)(H,66,67)(H2,55,57,69) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068542
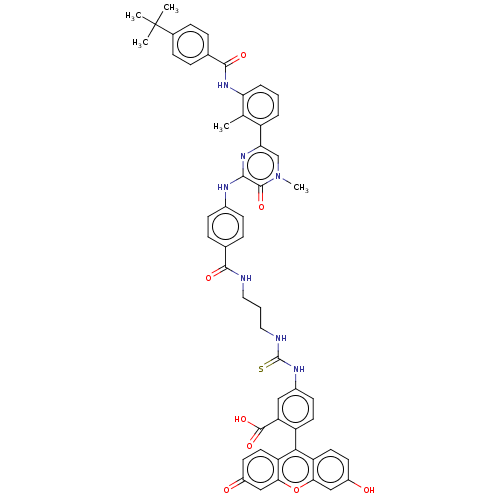
(CHEMBL3400826)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(-1.18,-23.55,;-1.18,-22.32,;-2.52,-21.55,;-3.85,-22.32,;-5.18,-21.55,;-5.18,-20.32,;-6.52,-22.32,;-7.85,-21.55,;-9.19,-22.32,;-9.18,-23.86,;-7.85,-24.63,;-6.52,-23.86,;-10.52,-24.64,;-11.59,-24.02,;-10.51,-25.87,;-11.58,-25.26,;-2.52,-20.01,;-1.19,-19.24,;.15,-20,;.15,-21.54,;1.49,-22.31,;1.49,-23.85,;2.83,-24.61,;2.83,-25.85,;4.16,-23.84,;5.23,-24.45,;4.16,-22.3,;5.49,-21.52,;5.48,-19.98,;6.81,-19.2,;6.8,-17.66,;5.46,-16.9,;4.13,-17.68,;4.14,-19.22,;5.44,-15.36,;6.51,-14.74,;4.1,-14.6,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;2.82,-21.54,)| Show InChI InChI=1S/C54H49N7O8S/c1-30-38(8-6-9-43(30)60-50(65)32-10-14-33(15-11-32)54(2,3)4)44-29-61(5)51(66)48(59-44)57-34-16-12-31(13-17-34)49(64)55-24-7-25-56-53(70)58-35-18-21-39(42(26-35)52(67)68)47-40-22-19-36(62)27-45(40)69-46-28-37(63)20-23-41(46)47/h6,8-23,26-29,62H,7,24-25H2,1-5H3,(H,55,64)(H,57,59)(H,60,65)(H,67,68)(H2,56,58,70) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068544
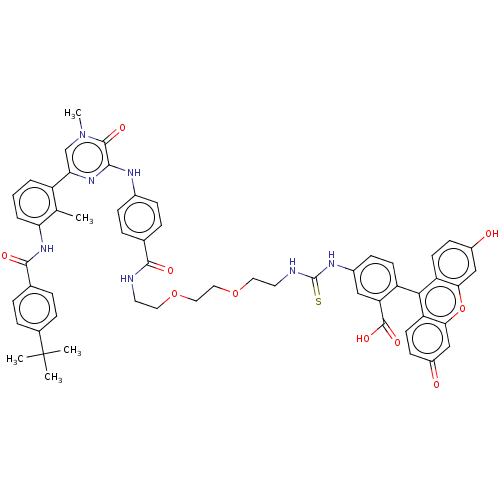
(CHEMBL3402354)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCOCCOCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(18.54,-19.68,;17.46,-19.08,;17.44,-17.54,;18.76,-16.75,;18.73,-15.21,;17.66,-14.61,;20.05,-14.41,;20.03,-12.87,;21.35,-12.08,;22.7,-12.83,;22.72,-14.37,;21.4,-15.16,;24.02,-12.04,;24,-10.81,;25.1,-12.64,;25.08,-11.41,;16.09,-16.79,;14.77,-17.58,;14.8,-19.12,;16.14,-19.87,;16.16,-21.41,;17.51,-22.16,;17.53,-23.7,;18.6,-24.31,;16.2,-24.49,;16.22,-25.72,;14.86,-23.74,;13.53,-24.53,;12.19,-23.77,;10.87,-24.56,;9.52,-23.8,;9.51,-22.26,;10.83,-21.48,;12.17,-22.23,;8.17,-21.5,;7.11,-22.13,;8.15,-19.96,;6.81,-19.2,;6.8,-17.66,;5.46,-16.9,;5.44,-15.36,;4.1,-14.6,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;14.84,-22.2,)| Show InChI InChI=1S/C57H55N7O10S/c1-33-41(7-6-8-46(33)63-53(68)35-9-13-36(14-10-35)57(2,3)4)47-32-64(5)54(69)51(62-47)60-37-15-11-34(12-16-37)52(67)58-23-25-72-27-28-73-26-24-59-56(75)61-38-17-20-42(45(29-38)55(70)71)50-43-21-18-39(65)30-48(43)74-49-31-40(66)19-22-44(49)50/h6-22,29-32,65H,23-28H2,1-5H3,(H,58,67)(H,60,62)(H,63,68)(H,70,71)(H2,59,61,75) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
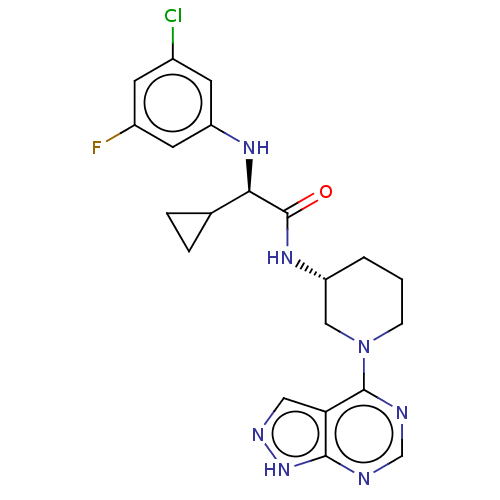
(Homo sapiens (Human)) | BDBM50588040
(CHEMBL5196736)Show SMILES Fc1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@@H]2CCCN(C2)c2ncnc3[nH]ncc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
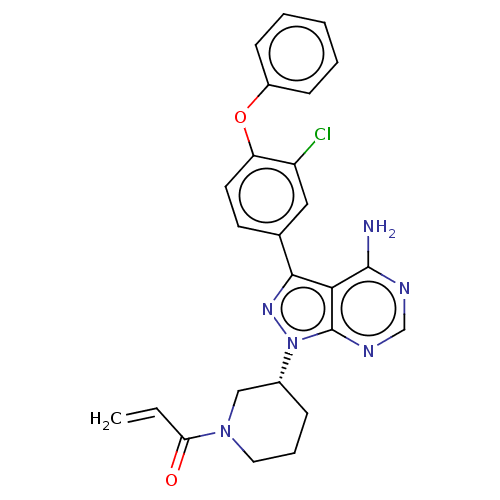
(Homo sapiens (Human)) | BDBM76973
(US9694011, Example 2 (Compound 18))Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)c(Cl)c3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C25H23ClN6O2/c1-2-21(33)31-12-6-7-17(14-31)32-25-22(24(27)28-15-29-25)23(30-32)16-10-11-20(19(26)13-16)34-18-8-4-3-5-9-18/h2-5,8-11,13,15,17H,1,6-7,12,14H2,(H2,27,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 32 |
Jiangsu Medolution Ltd
US Patent
| Assay Description
Kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase and infected with T7 ph... |
US Patent US9694011 (2017)
BindingDB Entry DOI: 10.7270/Q20K26Q4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
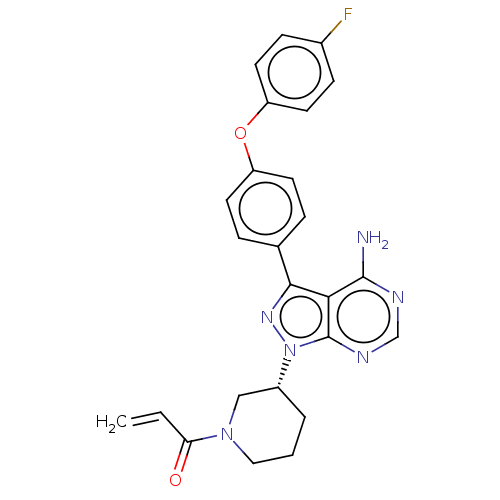
(Homo sapiens (Human)) | BDBM76963
(US9694011, Example 1 (Compound 17))Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccc(F)cc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C25H23FN6O2/c1-2-21(33)31-13-3-4-18(14-31)32-25-22(24(27)28-15-29-25)23(30-32)16-5-9-19(10-6-16)34-20-11-7-17(26)8-12-20/h2,5-12,15,18H,1,3-4,13-14H2,(H2,27,28,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 32 |
Jiangsu Medolution Ltd
US Patent
| Assay Description
Kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase and infected with T7 ph... |
US Patent US9694011 (2017)
BindingDB Entry DOI: 10.7270/Q20K26Q4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM194087

(US20230364079, Example Tirabrutinib | US9199997, 9...)Show SMILES CC#CC(=O)N1CC[C@H](C1)n1c2ncnc(N)c2n(-c2ccc(Oc3ccccc3)cc2)c1=O |r| Show InChI InChI=1S/C25H22N6O3/c1-2-6-21(32)29-14-13-18(15-29)31-24-22(23(26)27-16-28-24)30(25(31)33)17-9-11-20(12-10-17)34-19-7-4-3-5-8-19/h3-5,7-12,16,18H,13-15H2,1H3,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged recombinant BTK (unknown origin) measured after 1 hr by biotinylated-ligand affinity bead-based qPCR analysis |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
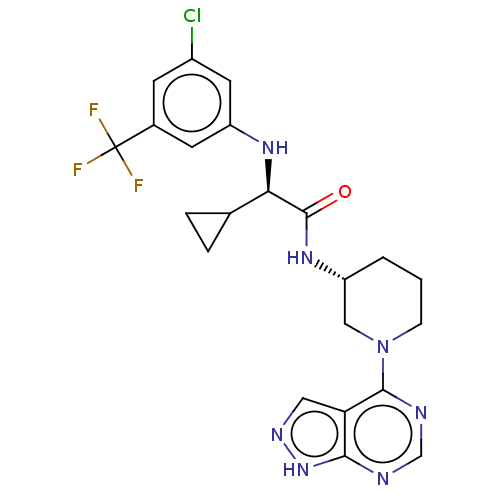
(Homo sapiens (Human)) | BDBM50588041
(CHEMBL5179394)Show SMILES FC(F)(F)c1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@@H]2CCCN(C2)c2ncnc3[nH]ncc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged recombinant BTK (unknown origin) measured after 1 hr by biotinylated-ligand affinity bead-based qPCR analysis |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50175583

(ACP-196 | Acalabrutinib | US10239883, Example 6 | ...)Show SMILES [H][C@]1(CCCN1C(=O)C#CC)c1nc(-c2ccc(cc2)C(=O)Nc2ccccn2)c2c(N)nccn12 |r| Show InChI InChI=1S/C26H23N7O2/c1-2-6-21(34)32-15-5-7-19(32)25-31-22(23-24(27)29-14-16-33(23)25)17-9-11-18(12-10-17)26(35)30-20-8-3-4-13-28-20/h3-4,8-14,16,19H,5,7,15H2,1H3,(H2,27,29)(H,28,30,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to DNA-tagged recombinant BTK (unknown origin) measured after 1 hr by biotinylated-ligand affinity bead-based qPCR analysis |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM482158
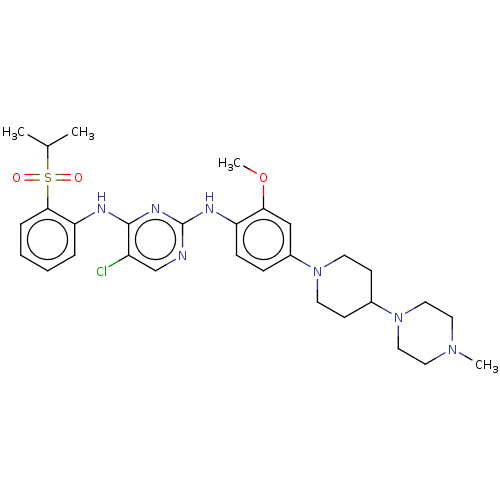
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588043
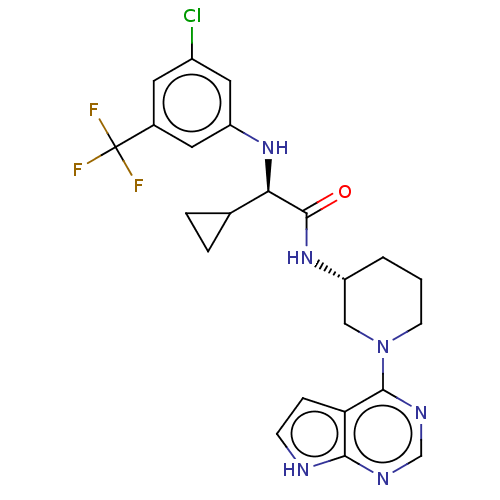
(CHEMBL5182743)Show SMILES FC(F)(F)c1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@@H]2CCCN(C2)c2ncnc3[nH]ccc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM473173
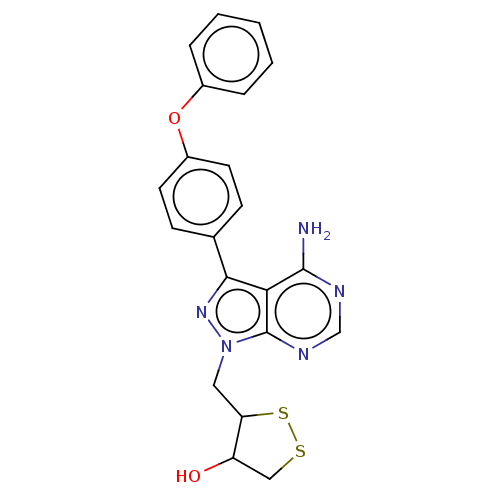
(US10844038, Example 42 | US10844038, Example 45 | ...)Show SMILES Nc1ncnc2n(CC3SSCC3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
Kd: For most assays, kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase an... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM473176
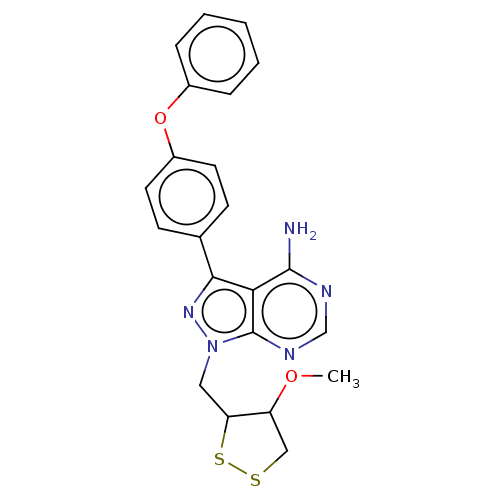
(US10844038, Example 37 | US10844038, Example 38 | ...)Show SMILES COC1CSSC1Cn1nc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C22H21N5O2S2/c1-28-17-12-30-31-18(17)11-27-22-19(21(23)24-13-25-22)20(26-27)14-7-9-16(10-8-14)29-15-5-3-2-4-6-15/h2-10,13,17-18H,11-12H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
Kd: For most assays, kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase an... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM473176

(US10844038, Example 37 | US10844038, Example 38 | ...)Show SMILES COC1CSSC1Cn1nc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C22H21N5O2S2/c1-28-17-12-30-31-18(17)11-27-22-19(21(23)24-13-25-22)20(26-27)14-7-9-16(10-8-14)29-15-5-3-2-4-6-15/h2-10,13,17-18H,11-12H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
Kd: For most assays, kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase an... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM473173

(US10844038, Example 42 | US10844038, Example 45 | ...)Show SMILES Nc1ncnc2n(CC3SSCC3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
Kd: For most assays, kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase an... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50308060
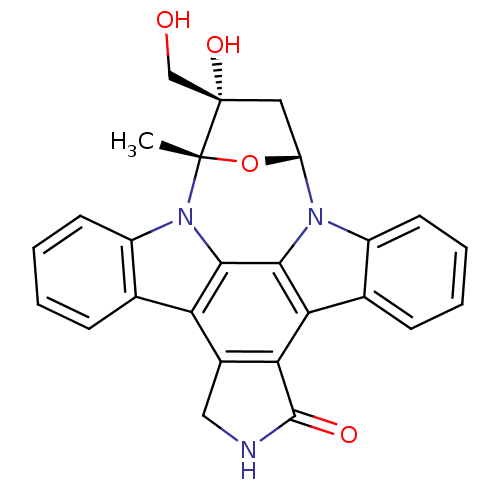
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50355504
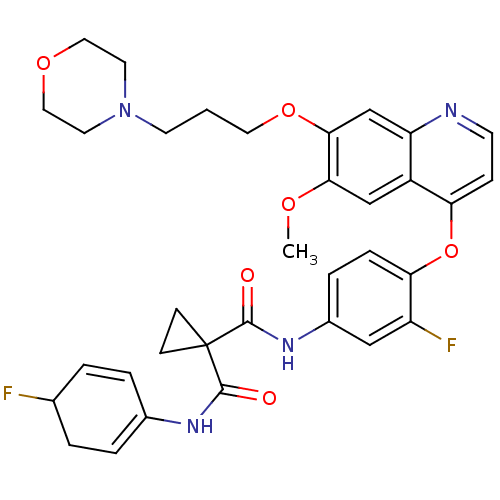
(CHEMBL1908393)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)NC4=CCC(F)C=C4)cc3F)ccnc2cc1OCCCN1CCOCC1 |c:26,t:21| Show InChI InChI=1S/C34H36F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3,5-9,12,19-22H,2,4,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50355499
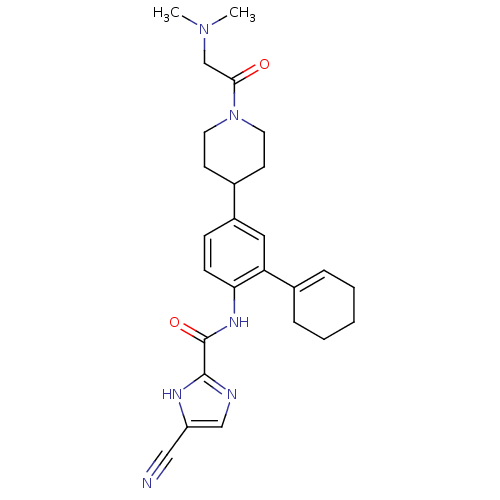
(CHEMBL1908395 | CHEMBL1908842)Show SMILES CN(C)CC(=O)N1CCC(CC1)c1ccc(NC(=O)c2ncc([nH]2)C#N)c(c1)C1=CCCCC1 |t:31| Show InChI InChI=1S/C26H32N6O2/c1-31(2)17-24(33)32-12-10-18(11-13-32)20-8-9-23(22(14-20)19-6-4-3-5-7-19)30-26(34)25-28-16-21(15-27)29-25/h6,8-9,14,16,18H,3-5,7,10-13,17H2,1-2H3,(H,28,29)(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50161957
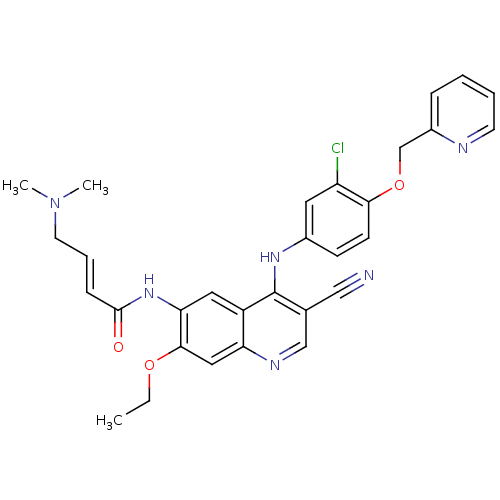
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM60665
(BDBM50249542 | US9145414, R406 | US9212178, R406)Show SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)Nc4n3)n2)cc(OC)c1OC Show InChI InChI=1S/C22H23FN6O5/c1-22(2)20(30)28-19-13(34-22)6-7-16(27-19)26-18-12(23)10-24-21(29-18)25-11-8-14(31-3)17(33-5)15(9-11)32-4/h6-10H,1-5H3,(H3,24,25,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM31096

(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for full-length BTK |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data