

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50413891 (VESTIPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.0302 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity to human recombinant NK1 receptor expressed in CHO cells assessed as dissociation constant | J Med Chem 52: 3238-47 (2009) Article DOI: 10.1021/jm900023b BindingDB Entry DOI: 10.7270/Q2BP0425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002462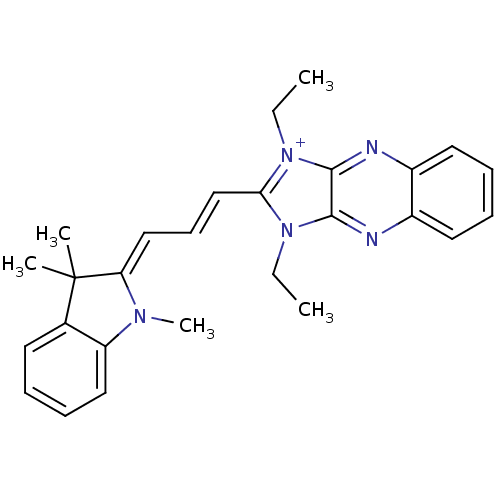 (1,3-Diethyl-2-[3-(1,3,3-trimethyl-1,3-dihydro-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Scatchard analysis at concentration of the 3 uM :Apparent affinity constant (Kd) against tachykinin receptor 1 | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002462 (1,3-Diethyl-2-[3-(1,3,3-trimethyl-1,3-dihydro-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Scatchard analysis at concentration of the 1 uM :Apparent affinity constant (Kd) against tachykinin receptor 1 | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50002462 (1,3-Diethyl-2-[3-(1,3,3-trimethyl-1,3-dihydro-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Scatchard analysis at concentration of the 10 uM :Apparent affinity constant (Kd) against tachykinin receptor 1 | J Med Chem 34: 1751-3 (1991) BindingDB Entry DOI: 10.7270/Q2JQ11MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50346328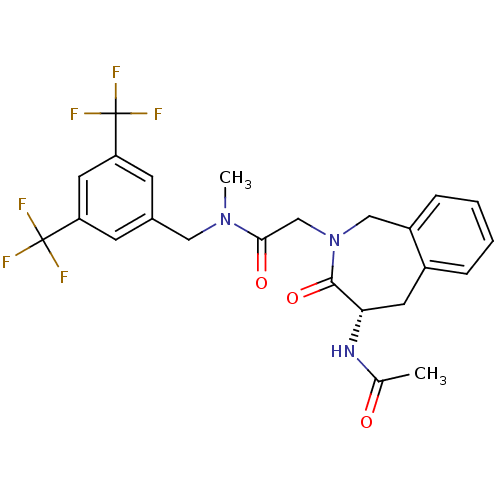 ((S)-2-(4-acetamido-3-oxo-4,5-dihydro-1H-benzo[c]az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Antagonist activity at human NK1 receptor expressed in CHO-K1 cells assessed as inhibition of substance P-induced calcium-dependent aequorine lumines... | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.37 | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG Curated by ChEMBL | Assay Description Competitive inhibition of wild type human NK1 receptor expressed in HEK293 cells assessed as decrease in SP1-induced [3H]IP accumulation after 20 min... | J Med Chem 55: 5061-76 (2012) Article DOI: 10.1021/jm2017072 BindingDB Entry DOI: 10.7270/Q2959JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50346329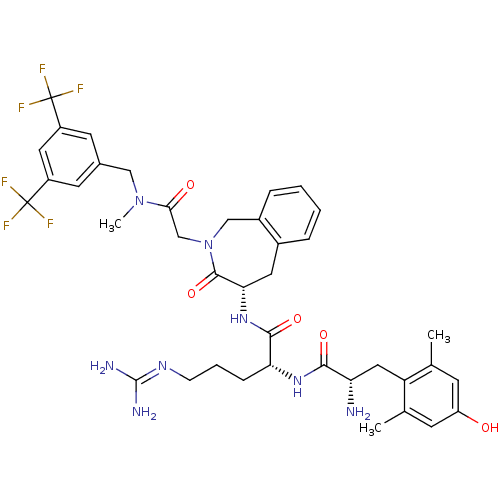 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Antagonist activity at human NK1 receptor expressed in CHO-K1 cells assessed as inhibition of substance P-induced calcium-dependent aequorine lumines... | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50346326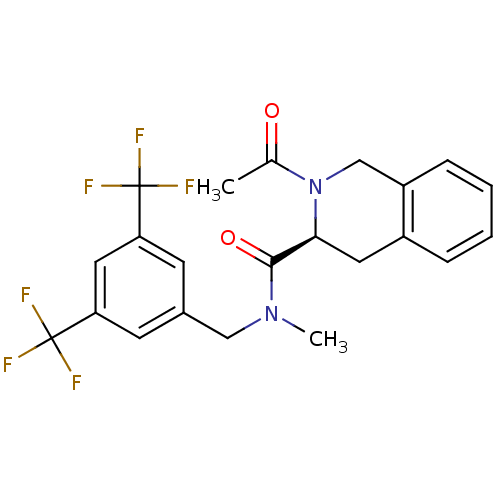 ((S)-2-acetyl-N-(3,5-bis(trifluoromethyl)benzyl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Antagonist activity at human NK1 receptor expressed in CHO-K1 cells assessed as inhibition of substance P-induced calcium-dependent aequorine lumines... | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289765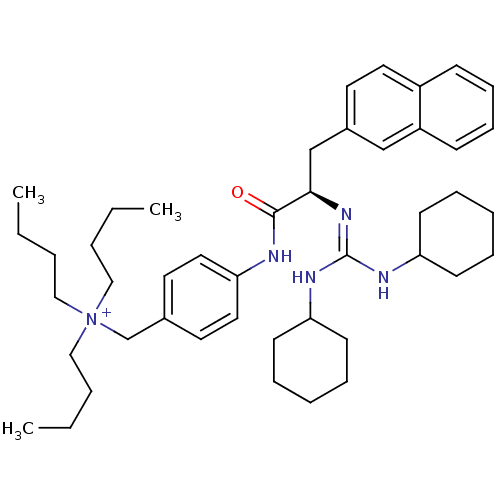 (CHEMBL292816 | Tributyl-{4-[(R)-2-(N',N''-dicycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289769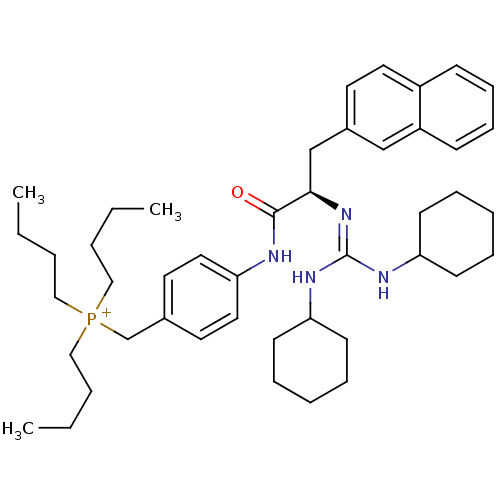 (CHEMBL555236 | Tributyl-{4-[(R)-2-(N',N''-dicycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289768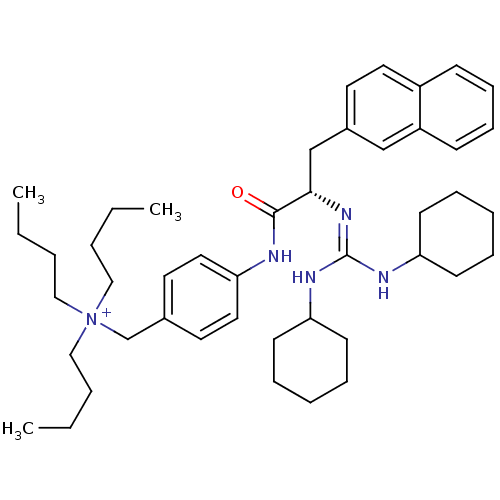 (CHEMBL294452 | Tributyl-{4-[(S)-2-(N',N''-dicycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50346327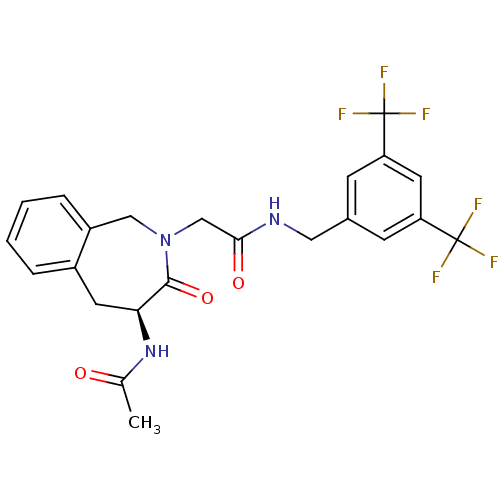 ((S)-2-(4-acetamido-3-oxo-4,5-dihydro-1H-benzo[c]az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Antagonist activity at human NK1 receptor expressed in CHO-K1 cells assessed as inhibition of substance P-induced calcium-dependent aequorine lumines... | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289776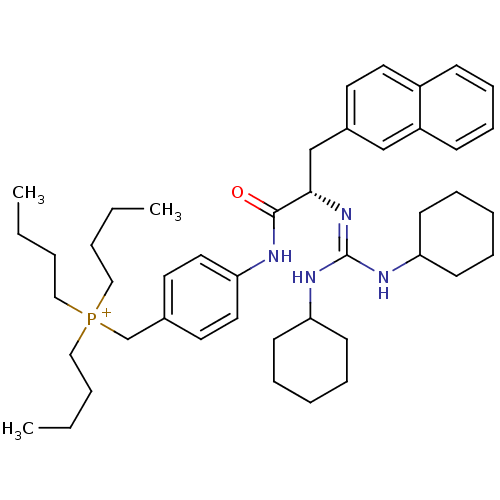 (CHEMBL539631 | Tributyl-{4-[(S)-2-(N',N''-dicycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289775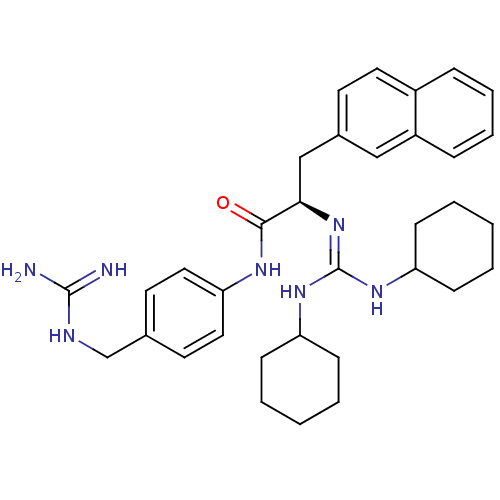 ((R)-2-(N',N''-Dicyclohexyl-guanidino)-N-(4-guanidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289778 ((S)-2-(N',N''-Dicyclohexyl-guanidino)-N-(4-guanidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289766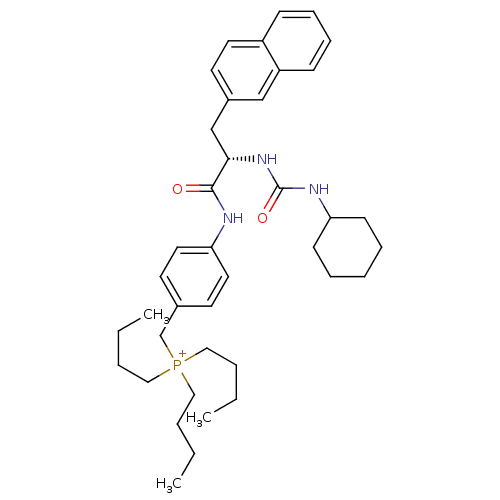 (CHEMBL555235 | Tributyl-{4-[(S)-2-(3-cyclohexyl-ur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289770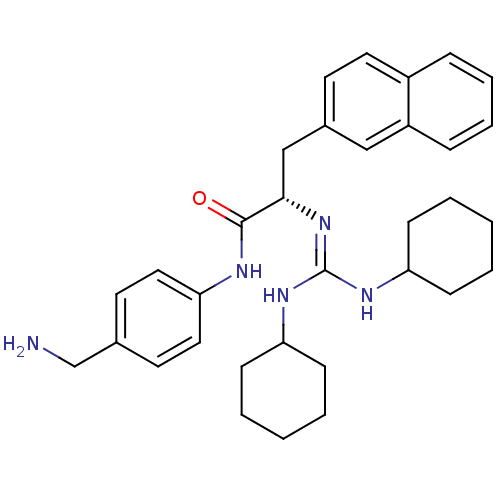 ((S)-N-(4-Aminomethyl-phenyl)-2-(N',N''-dicyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289763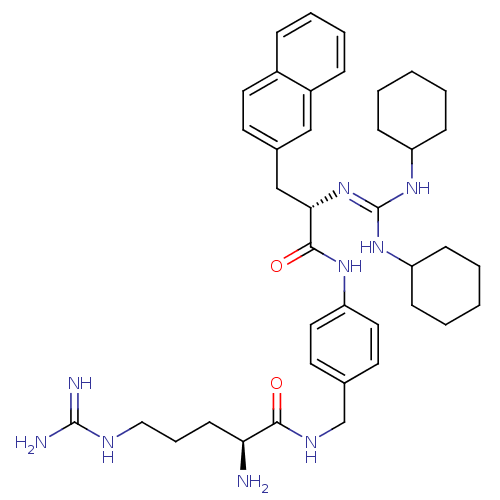 (1N-{4-[1-cyclohexylamino(cyclohexylimino)methylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289777 ((S)-2-(N',N''-Dicyclohexyl-guanidino)-N-[4-(N',N''...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50289772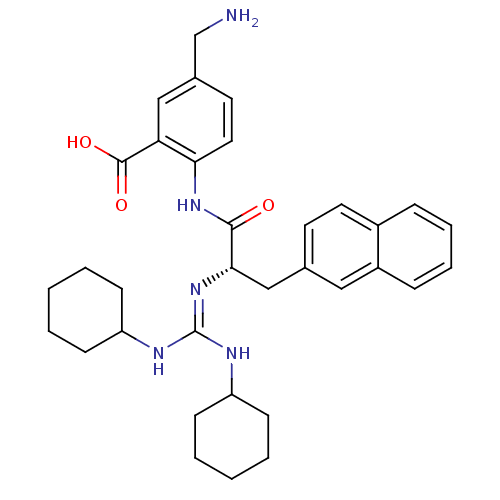 (5-Aminomethyl-2-[(S)-2-(N',N''-dicyclohexyl-guanid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1, activity expressed as Kd | Bioorg Med Chem Lett 7: 1921-1926 (1997) Article DOI: 10.1016/S0960-894X(97)00328-4 BindingDB Entry DOI: 10.7270/Q2XG9R44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||