 Found 131 hits of kd data for polymerid = 3972,50000055
Found 131 hits of kd data for polymerid = 3972,50000055 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075366
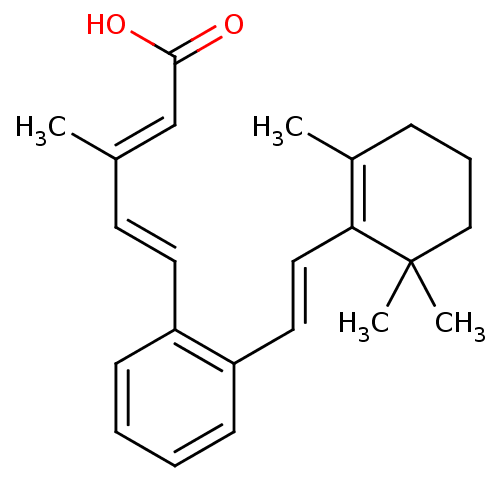
((2E,4E)-3-Methyl-5-{2-[(E)-2-(2,6,6-trimethyl-cycl...)Show SMILES C\C(\C=C\c1ccccc1\C=C\C1=C(C)CCCC1(C)C)=C/C(O)=O |c:13| Show InChI InChI=1S/C23H28O2/c1-17(16-22(24)25)11-12-19-9-5-6-10-20(19)13-14-21-18(2)8-7-15-23(21,3)4/h5-6,9-14,16H,7-8,15H2,1-4H3,(H,24,25)/b12-11+,14-13+,17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-RA binding to retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 9: 589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2J38RQ5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075367

((2E,4E)-3-Methyl-5-{2-[(E)-2-(2,6,6-trimethyl-cycl...)Show SMILES C\C(\C=C\c1ccoc1\C=C\C1=C(C)CCCC1(C)C)=C/C(O)=O |c:12| Show InChI InChI=1S/C21H26O3/c1-15(14-20(22)23)7-8-17-11-13-24-19(17)10-9-18-16(2)6-5-12-21(18,3)4/h7-11,13-14H,5-6,12H2,1-4H3,(H,22,23)/b8-7+,10-9+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-RA binding to retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 9: 589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2J38RQ5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha [200-419]
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | 7.9 | 4 |
CNRS
| Assay Description
Recombinant RAR protein expressed in E. coli was used in the direct binding assay. The apparent dissociation constants (Kd) were determined by the ch... |
J Mol Biol 302: 155-70 (2000)
Article DOI: 10.1006/jmbi.2000.4032
BindingDB Entry DOI: 10.7270/Q2BR8QHR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha [200-419]
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | 7.9 | 4 |
CNRS
| Assay Description
Recombinant RAR protein expressed in E. coli was used in the direct binding assay. The apparent dissociation constants (Kd) were determined by the ch... |
J Mol Biol 302: 155-70 (2000)
Article DOI: 10.1006/jmbi.2000.4032
BindingDB Entry DOI: 10.7270/Q2BR8QHR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha [200-419]
(Homo sapiens (Human)) | BDBM31887
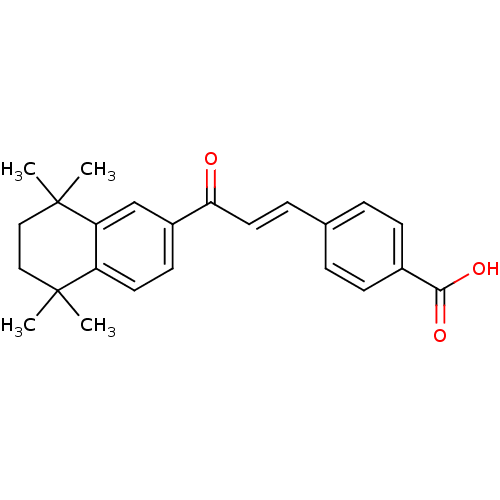
(BMS181156 | Ch 80)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)\C=C\c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H26O3/c1-23(2)13-14-24(3,4)20-15-18(10-11-19(20)23)21(25)12-7-16-5-8-17(9-6-16)22(26)27/h5-12,15H,13-14H2,1-4H3,(H,26,27)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | 7.9 | 4 |
CNRS
| Assay Description
Recombinant RAR protein expressed in E. coli was used in the direct binding assay. The apparent dissociation constants (Kd) were determined by the ch... |
J Mol Biol 302: 155-70 (2000)
Article DOI: 10.1006/jmbi.2000.4032
BindingDB Entry DOI: 10.7270/Q2BR8QHR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50471288

(CHEMBL312198)Show SMILES Cc1ccc(cc1)C1=CC(C)(C)Oc2c(Br)cc(cc12)C(=O)Nc1cc(F)c(C(O)=O)c(F)c1 |t:8| Show InChI InChI=1S/C26H20BrF2NO4/c1-13-4-6-14(7-5-13)18-12-26(2,3)34-23-17(18)8-15(9-19(23)27)24(31)30-16-10-20(28)22(25(32)33)21(29)11-16/h4-12H,1-3H3,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]ATRA binding to baculovirus expressed RAR alpha receptor |
J Med Chem 40: 2445-51 (1997)
Article DOI: 10.1021/jm9703911
BindingDB Entry DOI: 10.7270/Q2M61NZ2 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50120066

(2-Fluoro-4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydr...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H24FNO3/c1-21(2)9-10-22(3,4)17-11-13(5-8-16(17)21)19(25)24-14-6-7-15(20(26)27)18(23)12-14/h5-8,11-12H,9-10H2,1-4H3,(H,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity fo retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50029774
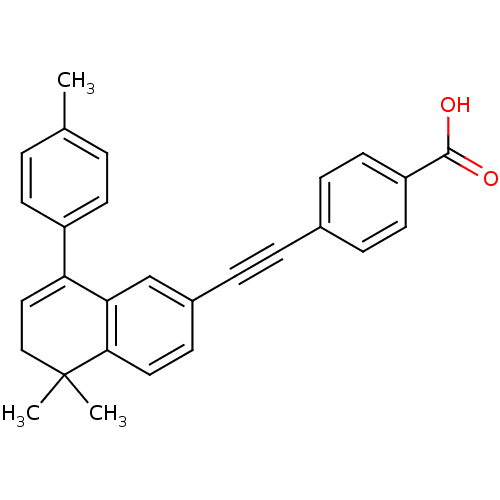
(4-(5,5-Dimethyl-8-p-tolyl-5,6-dihydro-naphthalen-2...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C28H24O2/c1-19-4-11-22(12-5-19)24-16-17-28(2,3)26-15-10-21(18-25(24)26)7-6-20-8-13-23(14-9-20)27(29)30/h4-5,8-16,18H,17H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding constant for baculovirus-expressed Retinoic acid receptor RAR alpha |
J Med Chem 38: 4764-7 (1996)
BindingDB Entry DOI: 10.7270/Q22N5194 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Apparent binding constant against Retinoic acid receptor alpha in HeLa cell GAl-4 transactivation assay |
J Med Chem 39: 2411-21 (1996)
Article DOI: 10.1021/jm9502293
BindingDB Entry DOI: 10.7270/Q2MS3RVK |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50471290

(CHEMBL81978)Show SMILES Cc1ccc(cc1)C1=CC(C)(C)Oc2c(Br)cc(cc12)C(=O)Nc1ccc(C(O)=O)c(F)c1 |t:8| Show InChI InChI=1S/C26H21BrFNO4/c1-14-4-6-15(7-5-14)20-13-26(2,3)33-23-19(20)10-16(11-21(23)27)24(30)29-17-8-9-18(25(31)32)22(28)12-17/h4-13H,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]ATRA binding to baculovirus expressed RAR alpha receptor |
J Med Chem 40: 2445-51 (1997)
Article DOI: 10.1021/jm9703911
BindingDB Entry DOI: 10.7270/Q2M61NZ2 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50120063

(2,6-Difluoro-4-[(3-hydroxy-5,5,8,8-tetramethyl-5,6...)Show SMILES CC1(C)CCC(C)(C)c2cc(C(=O)Nc3cc(F)c(C(O)=O)c(F)c3)c(O)cc12 Show InChI InChI=1S/C22H23F2NO4/c1-21(2)5-6-22(3,4)14-10-17(26)12(9-13(14)21)19(27)25-11-7-15(23)18(20(28)29)16(24)8-11/h7-10,26H,5-6H2,1-4H3,(H,25,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Effective concentration for retinoic acid receptor RAR alpha transcriptional activation |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50120065

(4-[(4-Chloro-3-hydroxy-5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES CC1(C)CCC(C)(C)c2c(Cl)c(O)c(cc12)C(=O)Nc1cc(F)c(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H22ClF2NO4/c1-21(2)5-6-22(3,4)16-12(21)9-11(18(27)17(16)23)19(28)26-10-7-13(24)15(20(29)30)14(25)8-10/h7-9,27H,5-6H2,1-4H3,(H,26,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity fo retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052412
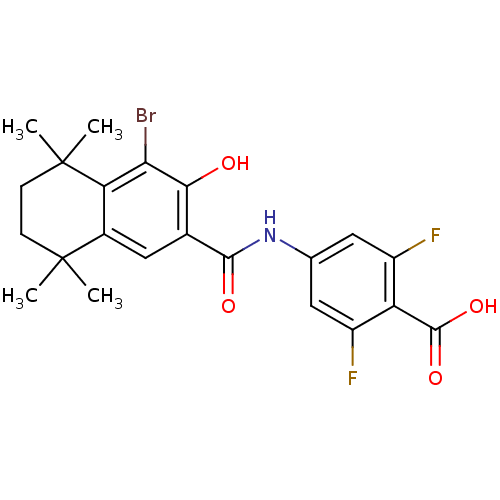
(4-[(4-Bromo-3-hydroxy-5,5,8,8-tetramethyl-5,6,7,8-...)Show SMILES CC1(C)CCC(C)(C)c2c(Br)c(O)c(cc12)C(=O)Nc1cc(F)c(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H22BrF2NO4/c1-21(2)5-6-22(3,4)16-12(21)9-11(18(27)17(16)23)19(28)26-10-7-13(24)15(20(29)30)14(25)8-10/h7-9,27H,5-6H2,1-4H3,(H,26,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity fo retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052413
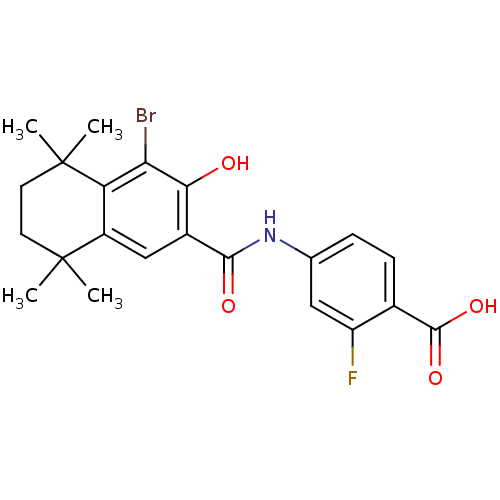
(4-[(4-Bromo-3-hydroxy-5,5,8,8-tetramethyl-5,6,7,8-...)Show SMILES CC1(C)CCC(C)(C)c2c(Br)c(O)c(cc12)C(=O)Nc1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H23BrFNO4/c1-21(2)7-8-22(3,4)16-14(21)10-13(18(26)17(16)23)19(27)25-11-5-6-12(20(28)29)15(24)9-11/h5-6,9-10,26H,7-8H2,1-4H3,(H,25,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity for baculovirus-expressed Retinoic acid receptor RAR alpha |
J Med Chem 39: 3035-8 (1996)
Article DOI: 10.1021/jm9603532
BindingDB Entry DOI: 10.7270/Q2W37VD3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075879
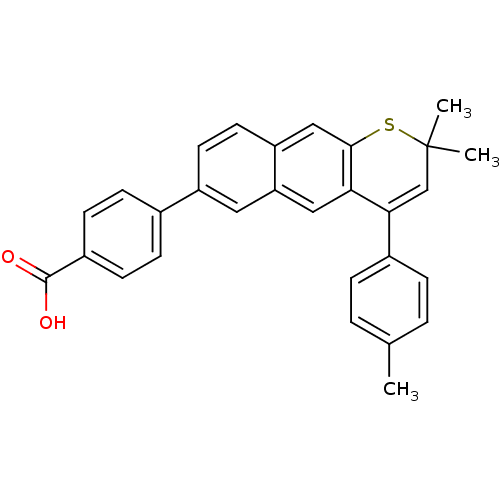
(4-(2,2-Dimethyl-4-p-tolyl-2H-1-thia-anthracen-6-yl...)Show SMILES Cc1ccc(cc1)C1=CC(C)(C)Sc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C29H24O2S/c1-18-4-6-20(7-5-18)26-17-29(2,3)32-27-16-23-13-12-22(14-24(23)15-25(26)27)19-8-10-21(11-9-19)28(30)31/h4-17H,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]- RA to baculovirus expressed human RAR alpha |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50471286

(CHEMBL82943)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C(=O)Nc1ccc(C(O)=O)c(F)c1 |t:8| Show InChI InChI=1S/C27H24FNO3/c1-16-4-6-17(7-5-16)20-12-13-27(2,3)23-11-8-18(14-22(20)23)25(30)29-19-9-10-21(26(31)32)24(28)15-19/h4-12,14-15H,13H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]ATRA binding to baculovirus expressed RAR alpha receptor |
J Med Chem 40: 2445-51 (1997)
Article DOI: 10.1021/jm9703911
BindingDB Entry DOI: 10.7270/Q2M61NZ2 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50120064
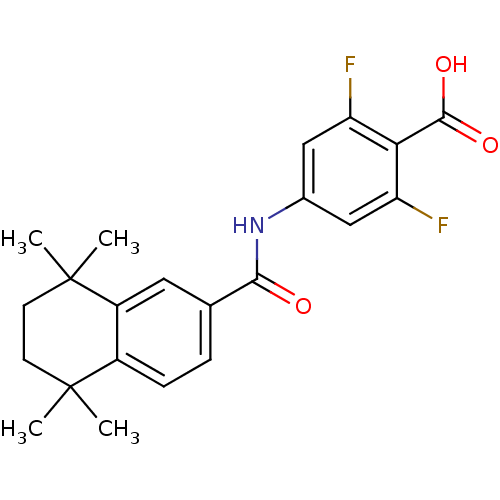
(2,6-Difluoro-4-[(5,5,8,8-tetramethyl-5,6,7,8-tetra...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1cc(F)c(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H23F2NO3/c1-21(2)7-8-22(3,4)15-9-12(5-6-14(15)21)19(26)25-13-10-16(23)18(20(27)28)17(24)11-13/h5-6,9-11H,7-8H2,1-4H3,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor RAR beta |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052412

(4-[(4-Bromo-3-hydroxy-5,5,8,8-tetramethyl-5,6,7,8-...)Show SMILES CC1(C)CCC(C)(C)c2c(Br)c(O)c(cc12)C(=O)Nc1cc(F)c(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H22BrF2NO4/c1-21(2)5-6-22(3,4)16-12(21)9-11(18(27)17(16)23)19(28)26-10-7-13(24)15(20(29)30)14(25)8-10/h7-9,27H,5-6H2,1-4H3,(H,26,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity for baculovirus-expressed Retinoic acid receptor RAR alpha |
J Med Chem 39: 3035-8 (1996)
Article DOI: 10.1021/jm9603532
BindingDB Entry DOI: 10.7270/Q2W37VD3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against retinoic acid receptor using 5 nM of [3H]-RA as a radioligand in baculovirus expressed receptor |
Bioorg Med Chem Lett 6: 213-218 (1996)
Article DOI: 10.1016/0960-894X(95)00588-K
BindingDB Entry DOI: 10.7270/Q2BC3ZHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075881

(4-[2,2-Dimethyl-4-(5-methyl-thiophen-2-yl)-2H-1-th...)Show SMILES Cc1ccc(s1)C1=CC(C)(C)Sc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:7| Show InChI InChI=1S/C27H22O2S2/c1-16-4-11-24(30-16)23-15-27(2,3)31-25-14-20-10-9-19(12-21(20)13-22(23)25)17-5-7-18(8-6-17)26(28)29/h4-15H,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]- RA to baculovirus expressed human RAR alpha |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075880

(4-(5,5-Dimethyl-8-p-tolyl-5,6-dihydro-anthracen-2-...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C30H26O2/c1-19-4-6-21(7-5-19)26-14-15-30(2,3)28-18-24-13-12-23(16-25(24)17-27(26)28)20-8-10-22(11-9-20)29(31)32/h4-14,16-18H,15H2,1-3H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonistic activity against RAR alpha in transcriptional activation assay with 32 nM TTNPB |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity fo retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit TTNPB-induced transactivation at retinoic acid receptor beta |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075878
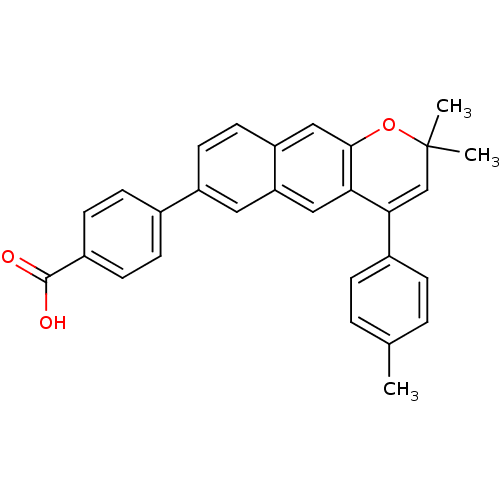
(4-(2,2-Dimethyl-4-p-tolyl-2H-benzo[g]chromen-7-yl)...)Show SMILES Cc1ccc(cc1)C1=CC(C)(C)Oc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C29H24O3/c1-18-4-6-20(7-5-18)26-17-29(2,3)32-27-16-23-13-12-22(14-24(23)15-25(26)27)19-8-10-21(11-9-19)28(30)31/h4-17H,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]- RA to baculovirus expressed human RAR alpha |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to human Retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 13: 261-4 (2002)
BindingDB Entry DOI: 10.7270/Q29P310V |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity for baculovirus-expressed Retinoic acid receptor RAR alpha |
J Med Chem 39: 3035-8 (1996)
Article DOI: 10.1021/jm9603532
BindingDB Entry DOI: 10.7270/Q2W37VD3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]ATRA binding to baculovirus expressed RAR alpha receptor |
J Med Chem 40: 2445-51 (1997)
Article DOI: 10.1021/jm9703911
BindingDB Entry DOI: 10.7270/Q2M61NZ2 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075877
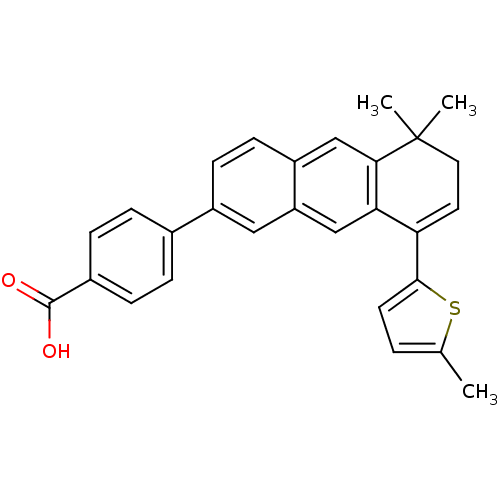
(4-[5,5-Dimethyl-8-(5-methyl-thiophen-2-yl)-5,6-dih...)Show SMILES Cc1ccc(s1)C1=CCC(C)(C)c2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:7| Show InChI InChI=1S/C28H24O2S/c1-17-4-11-26(31-17)23-12-13-28(2,3)25-16-21-10-9-20(14-22(21)15-24(23)25)18-5-7-19(8-6-18)27(29)30/h4-12,14-16H,13H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]- RA to baculovirus expressed human RAR alpha |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-RA binding to retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 9: 589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2J38RQ5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Ability to displace 3[H]-(all-E)-retinoic acid (5 nM) from alpha retinoic acid receptor (alpha RAR) using transactivation assay |
Bioorg Med Chem Lett 9: 573-6 (1999)
BindingDB Entry DOI: 10.7270/Q2SN084D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against retinoic acid receptor using 5 nM of [3H]-RA as a radioligand in baculovirus expressed receptor |
Bioorg Med Chem Lett 6: 213-218 (1996)
Article DOI: 10.1016/0960-894X(95)00588-K
BindingDB Entry DOI: 10.7270/Q2BC3ZHZ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to baculovirus expressed Retinoic acid receptor RAR alpha |
J Med Chem 39: 5027-30 (1997)
Article DOI: 10.1021/jm960687r
BindingDB Entry DOI: 10.7270/Q2TT4Q2F |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Retinoic acid receptor RAR alpha |
J Med Chem 42: 4961-9 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PX3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50029774

(4-(5,5-Dimethyl-8-p-tolyl-5,6-dihydro-naphthalen-2...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C28H24O2/c1-19-4-11-22(12-5-19)24-16-17-28(2,3)26-15-10-21(18-25(24)26)7-6-20-8-13-23(14-9-20)27(29)30/h4-5,8-16,18H,17H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Ability to displace 3[H]-(all-E)-retinoic acid (5 nM) from alpha retinoic acid receptor (alpha RAR) using transactivation assay |
Bioorg Med Chem Lett 9: 573-6 (1999)
BindingDB Entry DOI: 10.7270/Q2SN084D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075876
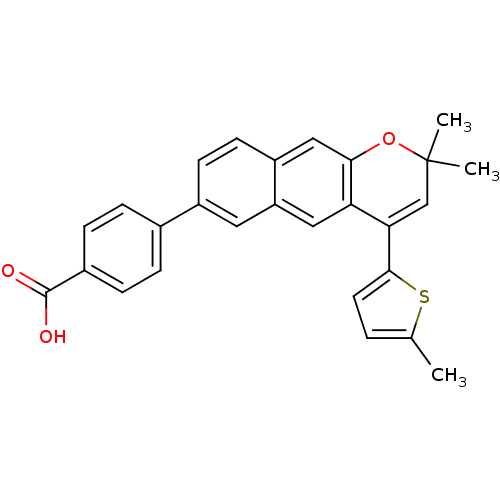
(4-[2,2-Dimethyl-4-(5-methyl-thiophen-2-yl)-2H-benz...)Show SMILES Cc1ccc(s1)C1=CC(C)(C)Oc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:7| Show InChI InChI=1S/C27H22O3S/c1-16-4-11-25(31-16)23-15-27(2,3)30-24-14-20-10-9-19(12-21(20)13-22(23)24)17-5-7-18(8-6-17)26(28)29/h4-15H,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]- RA to baculovirus expressed human RAR alpha |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50029774

(4-(5,5-Dimethyl-8-p-tolyl-5,6-dihydro-naphthalen-2...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C28H24O2/c1-19-4-11-22(12-5-19)24-16-17-28(2,3)26-15-10-21(18-25(24)26)7-6-20-8-13-23(14-9-20)27(29)30/h4-5,8-16,18H,17H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards retinoic acid receptor alpha was determined using [3H]-ATRA (5 nM) as radioligand |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50471287
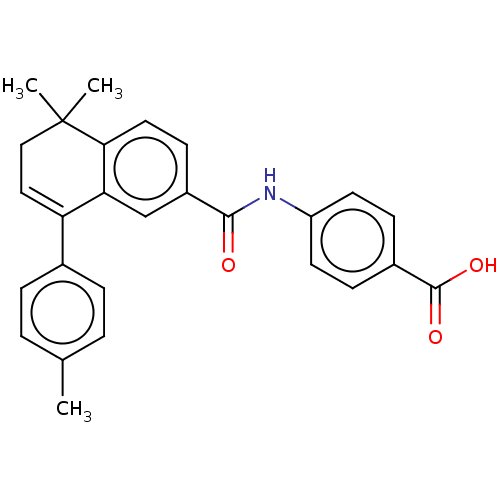
(CHEMBL81176)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C(=O)Nc1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C27H25NO3/c1-17-4-6-18(7-5-17)22-14-15-27(2,3)24-13-10-20(16-23(22)24)25(29)28-21-11-8-19(9-12-21)26(30)31/h4-14,16H,15H2,1-3H3,(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]ATRA binding to baculovirus expressed RAR alpha receptor |
J Med Chem 40: 2445-51 (1997)
Article DOI: 10.1021/jm9703911
BindingDB Entry DOI: 10.7270/Q2M61NZ2 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50101445
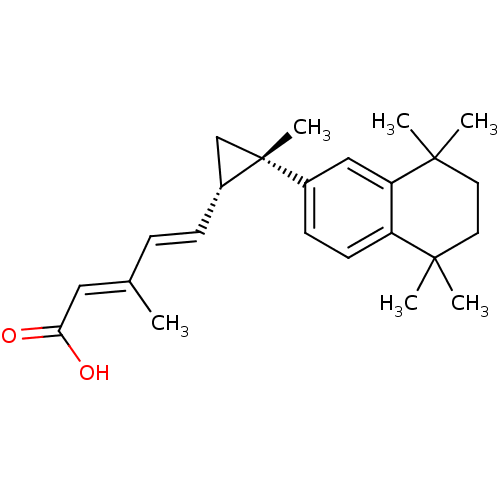
((2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@H]1C[C@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50471289

(CHEMBL310790)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C(=O)Nc1cc(F)c(C(O)=O)c(F)c1 |t:8| Show InChI InChI=1S/C27H23F2NO3/c1-15-4-6-16(7-5-15)19-10-11-27(2,3)21-9-8-17(12-20(19)21)25(31)30-18-13-22(28)24(26(32)33)23(29)14-18/h4-10,12-14H,11H2,1-3H3,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]ATRA binding to baculovirus expressed RAR alpha receptor |
J Med Chem 40: 2445-51 (1997)
Article DOI: 10.1021/jm9703911
BindingDB Entry DOI: 10.7270/Q2M61NZ2 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50097819
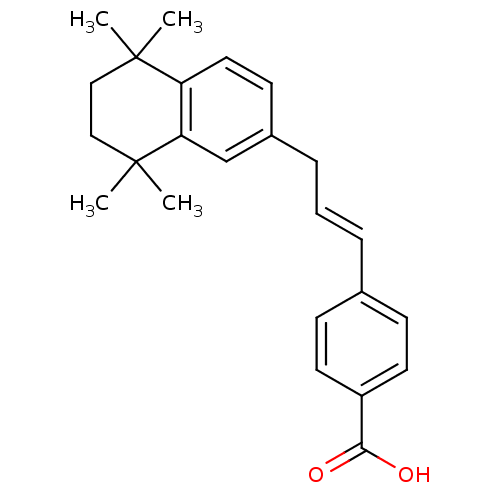
(4-[(E)-3-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(C\C=C\c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C24H28O2/c1-23(2)14-15-24(3,4)21-16-18(10-13-20(21)23)7-5-6-17-8-11-19(12-9-17)22(25)26/h5-6,8-13,16H,7,14-15H2,1-4H3,(H,25,26)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards retinoic acid receptor alpha was determined using [3H]-ATRA (5 nM) as radioligand |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219
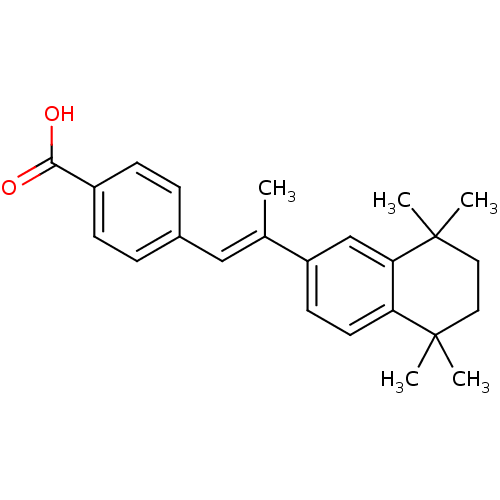
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052414

(4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-15(7-10-17(18)21)19(24)23-16-8-5-14(6-9-16)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity fo retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052414

(4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-15(7-10-17(18)21)19(24)23-16-8-5-14(6-9-16)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity for baculovirus-expressed Retinoic acid receptor RAR alpha |
J Med Chem 39: 3035-8 (1996)
Article DOI: 10.1021/jm9603532
BindingDB Entry DOI: 10.7270/Q2W37VD3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075353

(4-[8-(4-Cyano-phenyl)-5,5-dimethyl-5,6-dihydro-nap...)Show SMILES CC1(C)CC=C(c2ccc(cc2)C#N)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O |t:4| Show InChI InChI=1S/C28H21NO2/c1-28(2)16-15-24(22-10-7-21(18-29)8-11-22)25-17-20(9-14-26(25)28)4-3-19-5-12-23(13-6-19)27(30)31/h5-15,17H,16H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Ability to displace 3[H]-(all-E)-retinoic acid (5 nM) from alpha retinoic acid receptor (alpha RAR) using transactivation assay |
Bioorg Med Chem Lett 9: 573-6 (1999)
BindingDB Entry DOI: 10.7270/Q2SN084D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075349
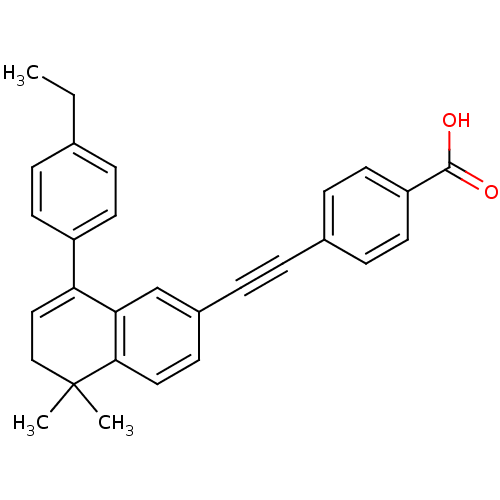
(4-[8-(4-Ethyl-phenyl)-5,5-dimethyl-5,6-dihydro-nap...)Show SMILES CCc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O |t:9| Show InChI InChI=1S/C29H26O2/c1-4-20-7-12-23(13-8-20)25-17-18-29(2,3)27-16-11-22(19-26(25)27)6-5-21-9-14-24(15-10-21)28(30)31/h7-17,19H,4,18H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Ability to displace 3[H]-(all-E)-retinoic acid (5 nM) from alpha retinoic acid receptor (alpha RAR) using transactivation assay |
Bioorg Med Chem Lett 9: 573-6 (1999)
BindingDB Entry DOI: 10.7270/Q2SN084D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075344

(4-[5,5-Dimethyl-8-(4-trifluoromethyl-phenyl)-5,6-d...)Show SMILES CC1(C)CC=C(c2ccc(cc2)C(F)(F)F)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O |t:4| Show InChI InChI=1S/C28H21F3O2/c1-27(2)16-15-23(20-10-12-22(13-11-20)28(29,30)31)24-17-19(7-14-25(24)27)4-3-18-5-8-21(9-6-18)26(32)33/h5-15,17H,16H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Ability to displace 3[H]-(all-E)-retinoic acid (5 nM) from alpha retinoic acid receptor (alpha RAR) using transactivation assay |
Bioorg Med Chem Lett 9: 573-6 (1999)
BindingDB Entry DOI: 10.7270/Q2SN084D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290184
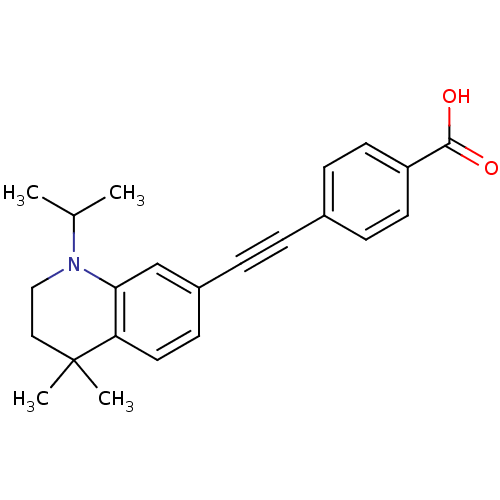
(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-12-9-18(15-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075352
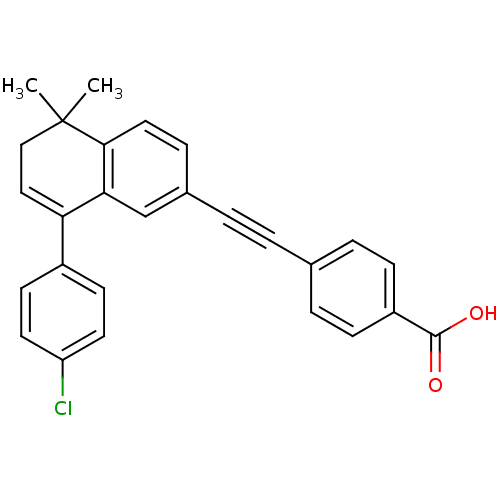
(4-[8-(4-Chloro-phenyl)-5,5-dimethyl-5,6-dihydro-na...)Show SMILES CC1(C)CC=C(c2ccc(Cl)cc2)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O |t:4| Show InChI InChI=1S/C27H21ClO2/c1-27(2)16-15-23(20-10-12-22(28)13-11-20)24-17-19(7-14-25(24)27)4-3-18-5-8-21(9-6-18)26(29)30/h5-15,17H,16H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Ability to displace 3[H]-(all-E)-retinoic acid (5 nM) from alpha retinoic acid receptor (alpha RAR) using transactivation assay |
Bioorg Med Chem Lett 9: 573-6 (1999)
BindingDB Entry DOI: 10.7270/Q2SN084D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290178
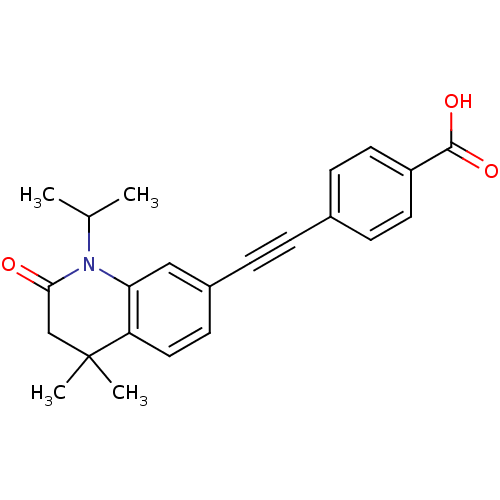
(4-(1-Isopropyl-4,4-dimethyl-2-oxo-1,2,3,4-tetrahyd...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H23NO3/c1-15(2)24-20-13-17(9-12-19(20)23(3,4)14-21(24)25)6-5-16-7-10-18(11-8-16)22(26)27/h7-13,15H,14H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data