 Found 106 hits of kd data for polymerid = 49000266,50002396
Found 106 hits of kd data for polymerid = 49000266,50002396 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(RABBIT) | BDBM50003154
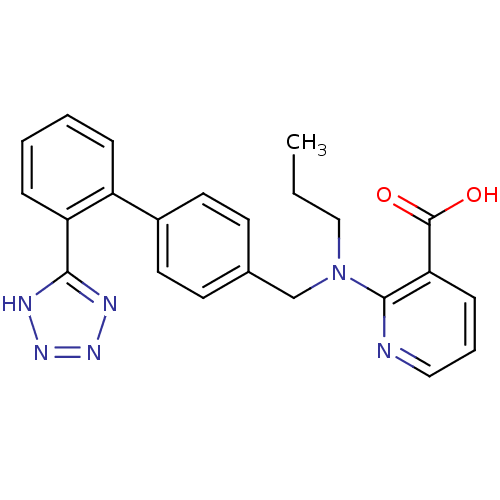
(2-{Propyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncccc1C(O)=O Show InChI InChI=1S/C23H22N6O2/c1-2-14-29(22-20(23(30)31)8-5-13-24-22)15-16-9-11-17(12-10-16)18-6-3-4-7-19(18)21-25-27-28-26-21/h3-13H,2,14-15H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50038189

(4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H28N4O3S/c1-4-27-32-28-20(2)18-21(3)31-29(28)34(27)19-22-14-16-23(17-15-22)25-12-8-9-13-26(25)38(36,37)33-30(35)24-10-6-5-7-11-24/h5-18H,4,19H2,1-3H3,(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50047126
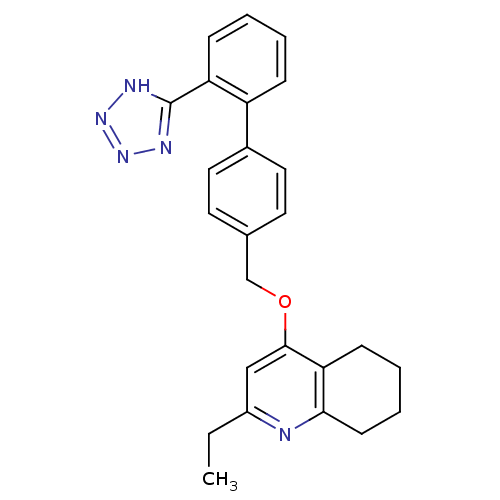
(2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2CCCCc2n1 Show InChI InChI=1S/C25H25N5O/c1-2-19-15-24(22-9-5-6-10-23(22)26-19)31-16-17-11-13-18(14-12-17)20-7-3-4-8-21(20)25-27-29-30-28-25/h3-4,7-8,11-15H,2,5-6,9-10,16H2,1H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50003154

(2-{Propyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncccc1C(O)=O Show InChI InChI=1S/C23H22N6O2/c1-2-14-29(22-20(23(30)31)8-5-13-24-22)15-16-9-11-17(12-10-16)18-6-3-4-7-19(18)21-25-27-28-26-21/h3-13H,2,14-15H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50006909
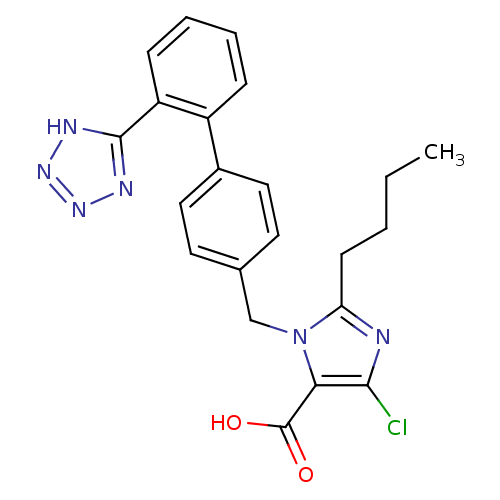
(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0813 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta assessed as reduction of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049188
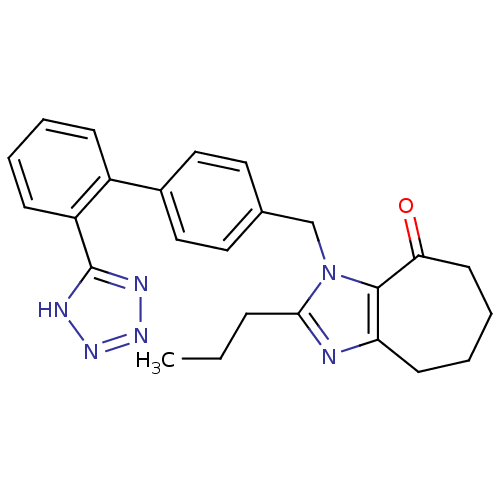
(2-Propyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCCc1nc2CCCCC(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H26N6O/c1-2-7-23-26-21-10-5-6-11-22(32)24(21)31(23)16-17-12-14-18(15-13-17)19-8-3-4-9-20(19)25-27-29-30-28-25/h3-4,8-9,12-15H,2,5-7,10-11,16H2,1H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0912 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403230
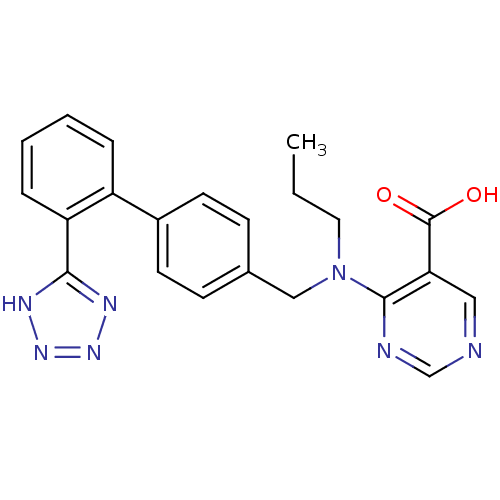
(A-81080 | CHEMBL49410)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncncc1C(O)=O Show InChI InChI=1S/C22H21N7O2/c1-2-11-29(21-19(22(30)31)12-23-14-24-21)13-15-7-9-16(10-8-15)17-5-3-4-6-18(17)20-25-27-28-26-20/h3-10,12,14H,2,11,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 0.117 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403230

(A-81080 | CHEMBL49410)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncncc1C(O)=O Show InChI InChI=1S/C22H21N7O2/c1-2-11-29(21-19(22(30)31)12-23-14-24-21)13-15-7-9-16(10-8-15)17-5-3-4-6-18(17)20-25-27-28-26-20/h3-10,12,14H,2,11,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.117 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049200
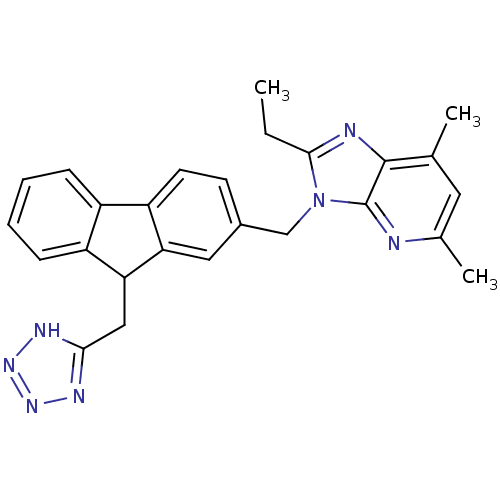
(2-Ethyl-5,7-dimethyl-3-[9-(1H-tetrazol-5-ylmethyl)...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc-2c(c1)C(Cc1nnn[nH]1)c1ccccc-21 Show InChI InChI=1S/C26H25N7/c1-4-24-28-25-15(2)11-16(3)27-26(25)33(24)14-17-9-10-20-18-7-5-6-8-19(18)22(21(20)12-17)13-23-29-31-32-30-23/h5-12,22H,4,13-14H2,1-3H3,(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aortic strip assessed as inhibition of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049205
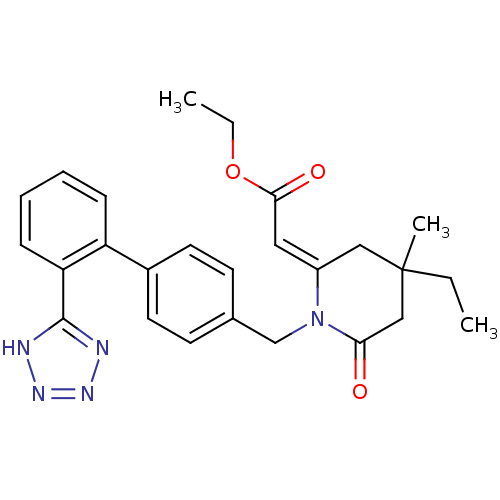
(CHEMBL48602 | RWJ-46458 | [4-Ethyl-4-methyl-6-oxo-...)Show SMILES CCOC(=O)\C=C1/CC(C)(CC)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H29N5O3/c1-4-26(3)15-20(14-24(33)34-5-2)31(23(32)16-26)17-18-10-12-19(13-11-18)21-8-6-7-9-22(21)25-27-29-30-28-25/h6-14H,4-5,15-17H2,1-3H3,(H,27,28,29,30)/b20-14+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403237
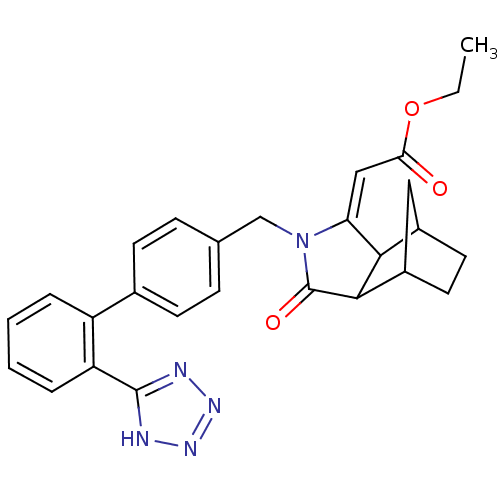
(CHEMBL122891)Show SMILES CCOC(=O)\C=C1/C2C3CCC(C3)C2C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H27N5O3/c1-2-35-23(33)14-22-24-18-11-12-19(13-18)25(24)27(34)32(22)15-16-7-9-17(10-8-16)20-5-3-4-6-21(20)26-28-30-31-29-26/h3-10,14,18-19,24-25H,2,11-13,15H2,1H3,(H,28,29,30,31)/b22-14+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta assessed as reduction of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50422230

(CHEMBL428475)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C48H69N13O10/c1-27(2)39(59-41(64)33(55-38(63)25-51-5)13-9-19-53-48(49)50)44(67)56-34(21-30-15-17-32(62)18-16-30)42(65)60-40(28(3)4)45(68)57-35(23-31-24-52-26-54-31)46(69)61-20-10-14-37(61)43(66)58-36(47(70)71)22-29-11-7-6-8-12-29/h6-8,11-12,15-18,24,26-28,33-37,39-40,51,62H,9-10,13-14,19-23,25H2,1-5H3,(H,52,54)(H,55,63)(H,56,67)(H,57,68)(H,58,66)(H,59,64)(H,60,65)(H,70,71)(H4,49,50,53)/t33-,34-,35-,36+,37-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Dissociation constant for [125 I] Ang binding to type 1 Angiotensin II receptorof bovine adrenocortical membranes |
J Med Chem 40: 3271-9 (1997)
Article DOI: 10.1021/jm9608669
BindingDB Entry DOI: 10.7270/Q2GQ6ZZM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049183
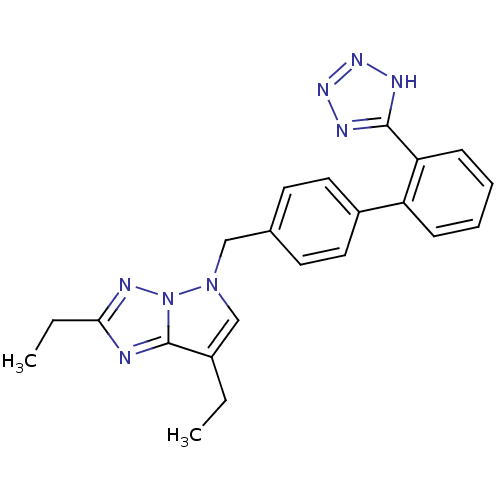
(2,7-Diethyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCc1nc2c(CC)cn(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)n2n1 Show InChI InChI=1S/C22H22N8/c1-3-16-14-29(30-22(16)23-20(4-2)26-30)13-15-9-11-17(12-10-15)18-7-5-6-8-19(18)21-24-27-28-25-21/h5-12,14H,3-4,13H2,1-2H3,(H,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50417812
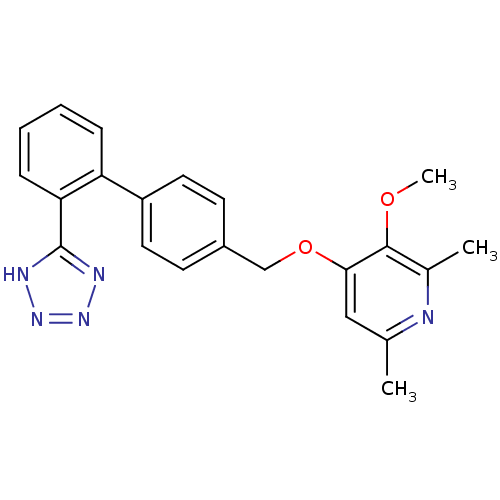
(CHEMBL352257 | ME-3221)Show SMILES COc1c(C)nc(C)cc1OCc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21N5O2/c1-14-12-20(21(28-3)15(2)23-14)29-13-16-8-10-17(11-9-16)18-6-4-5-7-19(18)22-24-26-27-25-22/h4-12H,13H2,1-3H3,(H,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta assessed as reduction of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50388737
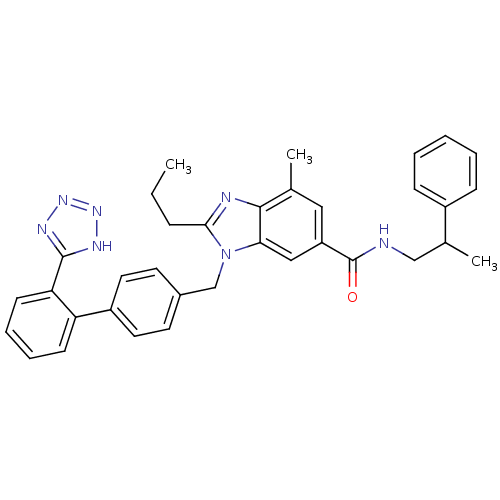
(CHEMBL2058860)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCC(C)c1ccccc1 Show InChI InChI=1S/C35H35N7O/c1-4-10-32-37-33-23(2)19-28(35(43)36-21-24(3)26-11-6-5-7-12-26)20-31(33)42(32)22-25-15-17-27(18-16-25)29-13-8-9-14-30(29)34-38-40-41-39-34/h5-9,11-20,24H,4,10,21-22H2,1-3H3,(H,36,43)(H,38,39,40,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Antagonist activity at AT1 receptor in Japanese white rabbit thoracic aorta assessed as inhibition of KCl-induced contraction incubated 30 mins post ... |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50388741

(CHEMBL2058864)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCCc1ccc(OC)c(OC)c1 Show InChI InChI=1S/C36H37N7O3/c1-5-8-33-38-34-23(2)19-27(36(44)37-18-17-24-13-16-31(45-3)32(20-24)46-4)21-30(34)43(33)22-25-11-14-26(15-12-25)28-9-6-7-10-29(28)35-39-41-42-40-35/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,44)(H,39,40,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Antagonist activity at AT1 receptor in Japanese white rabbit thoracic aorta assessed as inhibition of KCl-induced contraction incubated 30 mins post ... |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049212

(4'-(2-Cyclopropyl-7-methyl-imidazo[4,5-b]pyridin-3...)Show SMILES Cc1ccnc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(nc12)C1CC1 Show InChI InChI=1S/C24H21N3O2/c1-15-12-13-25-23-21(15)26-22(18-10-11-18)27(23)14-16-6-8-17(9-7-16)19-4-2-3-5-20(19)24(28)29/h2-9,12-13,18H,10-11,14H2,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049205

(CHEMBL48602 | RWJ-46458 | [4-Ethyl-4-methyl-6-oxo-...)Show SMILES CCOC(=O)\C=C1/CC(C)(CC)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H29N5O3/c1-4-26(3)15-20(14-24(33)34-5-2)31(23(32)16-26)17-18-10-12-19(13-11-18)21-8-6-7-9-22(21)25-27-29-30-28-25/h6-14H,4-5,15-17H2,1-3H3,(H,27,28,29,30)/b20-14+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049205

(CHEMBL48602 | RWJ-46458 | [4-Ethyl-4-methyl-6-oxo-...)Show SMILES CCOC(=O)\C=C1/CC(C)(CC)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H29N5O3/c1-4-26(3)15-20(14-24(33)34-5-2)31(23(32)16-26)17-18-10-12-19(13-11-18)21-8-6-7-9-22(21)25-27-29-30-28-25/h6-14H,4-5,15-17H2,1-3H3,(H,27,28,29,30)/b20-14+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403234
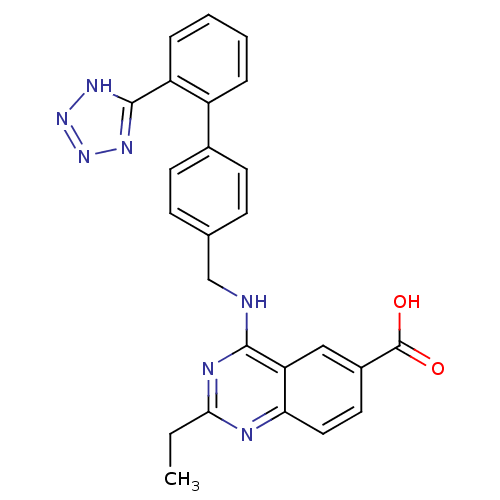
(CHEMBL49663)Show SMILES CCc1nc(NCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2cc(ccc2n1)C(O)=O Show InChI InChI=1S/C25H21N7O2/c1-2-22-27-21-12-11-17(25(33)34)13-20(21)23(28-22)26-14-15-7-9-16(10-8-15)18-5-3-4-6-19(18)24-29-31-32-30-24/h3-13H,2,14H2,1H3,(H,33,34)(H,26,27,28)(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403215
(CHEMBL299741)Show SMILES CCc1nc(NCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2cc(OC)ccc2n1 Show InChI InChI=1S/C25H23N7O/c1-3-23-27-22-13-12-18(33-2)14-21(22)24(28-23)26-15-16-8-10-17(11-9-16)19-6-4-5-7-20(19)25-29-31-32-30-25/h4-14H,3,15H2,1-2H3,(H,26,27,28)(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50069511
((2-Ethyl-quinazolin-4-yl)-[2'-(1H-tetrazol-5-yl)-b...)Show SMILES CCc1nc(NCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H21N7/c1-2-22-26-21-10-6-5-9-20(21)23(27-22)25-15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)24-28-30-31-29-24/h3-14H,2,15H2,1H3,(H,25,26,27)(H,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403238

(CHEMBL170196)Show SMILES CCOC(=O)\C=C1/CC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H27N5O3/c1-2-34-23(32)15-20-16-26(13-5-6-14-26)25(33)31(20)17-18-9-11-19(12-10-18)21-7-3-4-8-22(21)24-27-29-30-28-24/h3-4,7-12,15H,2,5-6,13-14,16-17H2,1H3,(H,27,28,29,30)/b20-15+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50363833

(CHEMBL1945010)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cc(OC)ccc1OC Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-26-20-28(43-3)15-16-32(26)44-4)21-31(34)39(33)22-24-11-13-25(14-12-24)29-9-6-7-10-30(29)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50049184

(2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nc2cccnc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-2-3-10-22-26-21-9-6-15-25-24(21)31(22)16-17-11-13-18(14-12-17)19-7-4-5-8-20(19)23-27-29-30-28-23/h4-9,11-15H,2-3,10,16H2,1H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50408260

(CHEMBL415157)Show SMILES CCCCCCCCCCCCCCCC(=O)Oc1ccc(C[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N2CCC[C@H]2C(=O)N[C@H](Cc2ccccc2)C(O)=O)cc1 Show InChI InChI=1S/C64H99N13O11/c1-7-8-9-10-11-12-13-14-15-16-17-18-22-29-54(79)88-47-32-30-45(31-33-47)36-49(72-60(83)55(42(2)3)75-57(80)48(71-53(78)40-67-6)27-23-34-69-64(65)66)58(81)76-56(43(4)5)61(84)73-50(38-46-39-68-41-70-46)62(85)77-35-24-28-52(77)59(82)74-51(63(86)87)37-44-25-20-19-21-26-44/h19-21,25-26,30-33,39,41-43,48-52,55-56,67H,7-18,22-24,27-29,34-38,40H2,1-6H3,(H,68,70)(H,71,78)(H,72,83)(H,73,84)(H,74,82)(H,75,80)(H,76,81)(H,86,87)(H4,65,66,69)/t48-,49-,50-,51+,52-,55-,56-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Dissociation constant for [125 I] Ang binding to type 1 Angiotensin II receptorof bovine adrenocortical membranes |
J Med Chem 40: 3271-9 (1997)
Article DOI: 10.1021/jm9608669
BindingDB Entry DOI: 10.7270/Q2GQ6ZZM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50209824
(CHEMBL245444 | methyl 3-(4-(2-(1H-tetrazol-5-yl)-1...)Show SMILES CCCCc1nc2cccc(C(=O)OC)c2n1Cc1ccc(cc1)-n1cccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H25N7O2/c1-3-4-10-22-26-20-8-5-7-19(25(33)34-2)23(20)32(22)16-17-11-13-18(14-12-17)31-15-6-9-21(31)24-27-29-30-28-24/h5-9,11-15H,3-4,10,16H2,1-2H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit thoracic aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50417813
(CHEMBL340315)Show SMILES CCCCCc1nc(SCc2ccc(cc2)-c2ccccc2C(O)=O)nn1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C35H33N3O4S/c1-2-3-4-13-32-36-35(43-23-25-16-20-27(21-17-25)29-10-6-8-12-31(29)34(41)42)37-38(32)22-24-14-18-26(19-15-24)28-9-5-7-11-30(28)33(39)40/h5-12,14-21H,2-4,13,22-23H2,1H3,(H,39,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta assessed as reduction of angiotensin 2-induced contractile response |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403241
(CHEMBL422912)Show SMILES CCOC(=O)\C=C1/CC2(CCCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H31N5O3/c1-2-36-25(34)17-22-18-28(15-7-3-4-8-16-28)27(35)33(22)19-20-11-13-21(14-12-20)23-9-5-6-10-24(23)26-29-31-32-30-26/h5-6,9-14,17H,2-4,7-8,15-16,18-19H2,1H3,(H,29,30,31,32)/b22-17+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50388736
(CHEMBL2058859)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C34H33N7O/c1-3-9-31-36-32-23(2)20-27(34(42)35-19-18-24-10-5-4-6-11-24)21-30(32)41(31)22-25-14-16-26(17-15-25)28-12-7-8-13-29(28)33-37-39-40-38-33/h4-8,10-17,20-21H,3,9,18-19,22H2,1-2H3,(H,35,42)(H,37,38,39,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Antagonist activity at AT1 receptor in Japanese white rabbit thoracic aorta assessed as inhibition of KCl-induced contraction incubated 30 mins post ... |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50363828
(CHEMBL1947131)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)cc1 Show InChI InChI=1S/C34H33N3O4/c1-4-7-31-36-32-22(2)18-26(33(38)35-20-23-12-16-27(41-3)17-13-23)19-30(32)37(31)21-24-10-14-25(15-11-24)28-8-5-6-9-29(28)34(39)40/h5-6,8-19H,4,7,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50280320
(CHEMBL51084 | [4-Oxo-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCOC(=O)\C=C1/CC2(CCCCC2)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H31N5O3/c1-2-36-26(35)16-22-17-28(14-6-3-7-15-28)18-25(34)33(22)19-20-10-12-21(13-11-20)23-8-4-5-9-24(23)27-29-31-32-30-27/h4-5,8-13,16H,2-3,6-7,14-15,17-19H2,1H3,(H,29,30,31,32)/b22-16+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50417814
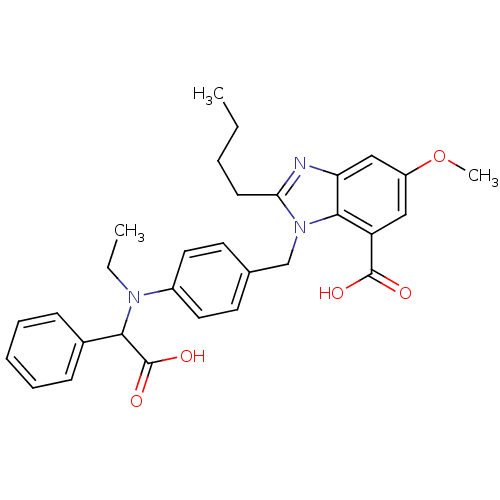
(CHEMBL1668002)Show SMILES CCCCc1nc2cc(OC)cc(C(O)=O)c2n1Cc1ccc(cc1)N(CC)C(C(O)=O)c1ccccc1 Show InChI InChI=1S/C30H33N3O5/c1-4-6-12-26-31-25-18-23(38-3)17-24(29(34)35)28(25)33(26)19-20-13-15-22(16-14-20)32(5-2)27(30(36)37)21-10-8-7-9-11-21/h7-11,13-18,27H,4-6,12,19H2,1-3H3,(H,34,35)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit thoracic aortic rings |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50003387
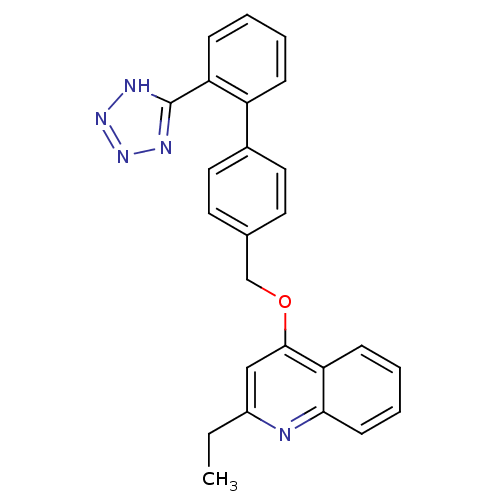
(2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H21N5O/c1-2-19-15-24(22-9-5-6-10-23(22)26-19)31-16-17-11-13-18(14-12-17)20-7-3-4-8-21(20)25-27-29-30-28-25/h3-15H,2,16H2,1H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin AT1 receptor in rabbit aorta |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403208

(CHEMBL49528)Show SMILES CCc1nc(NCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ncn(C)c2n1 Show InChI InChI=1S/C22H21N9/c1-3-18-25-21(19-22(26-18)31(2)13-24-19)23-12-14-8-10-15(11-9-14)16-6-4-5-7-17(16)20-27-29-30-28-20/h4-11,13H,3,12H2,1-2H3,(H,23,25,26)(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Dissociation constant for [125 I] Ang binding to type 1 Angiotensin II receptorof bovine adrenocortical membranes |
J Med Chem 40: 3271-9 (1997)
Article DOI: 10.1021/jm9608669
BindingDB Entry DOI: 10.7270/Q2GQ6ZZM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403236

(CHEMBL123208)Show SMILES CCOC(=O)\C=C1/C2C(C3CCC2C=C3)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |c:14| Show InChI InChI=1S/C28H27N5O3/c1-2-36-24(34)15-23-25-19-11-13-20(14-12-19)26(25)28(35)33(23)16-17-7-9-18(10-8-17)21-5-3-4-6-22(21)27-29-31-32-30-27/h3-11,13,15,19-20,25-26H,2,12,14,16H2,1H3,(H,29,30,31,32)/b23-15+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403200
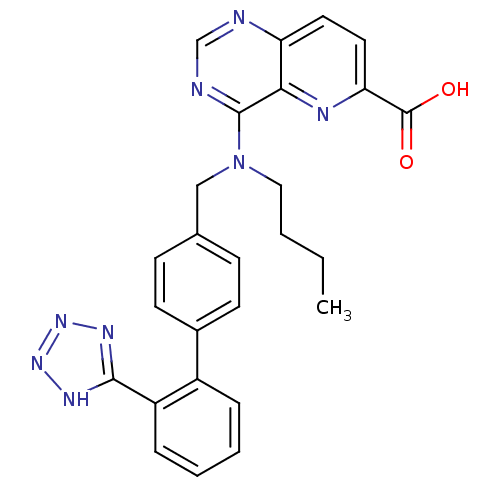
(CHEMBL48799)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncnc2ccc(nc12)C(O)=O Show InChI InChI=1S/C26H24N8O2/c1-2-3-14-34(25-23-21(27-16-28-25)12-13-22(29-23)26(35)36)15-17-8-10-18(11-9-17)19-6-4-5-7-20(19)24-30-32-33-31-24/h4-13,16H,2-3,14-15H2,1H3,(H,35,36)(H,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403211
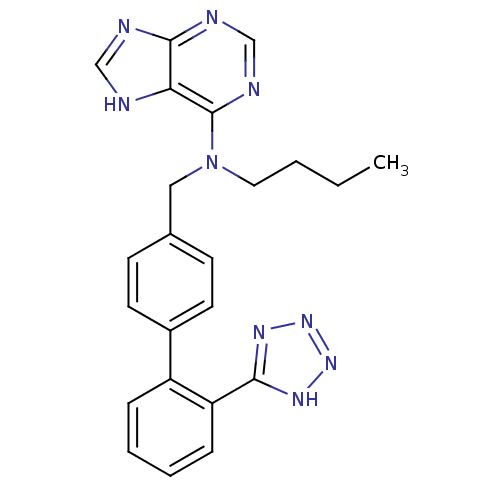
(CHEMBL46391)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncnc2nc[nH]c12 Show InChI InChI=1S/C23H23N9/c1-2-3-12-32(23-20-22(25-14-24-20)26-15-27-23)13-16-8-10-17(11-9-16)18-6-4-5-7-19(18)21-28-30-31-29-21/h4-11,14-15H,2-3,12-13H2,1H3,(H,24,25,26,27)(H,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50363826
(CHEMBL1947129)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1OC Show InChI InChI=1S/C34H33N3O4/c1-4-9-31-36-32-22(2)18-26(33(38)35-20-25-10-5-8-13-30(25)41-3)19-29(32)37(31)21-23-14-16-24(17-15-23)27-11-6-7-12-28(27)34(39)40/h5-8,10-19H,4,9,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403223
(CHEMBL46433)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncnc2n(CC(O)=O)cnc12 Show InChI InChI=1S/C24H23N9O2/c1-2-11-32(23-21-24(26-14-25-23)33(15-27-21)13-20(34)35)12-16-7-9-17(10-8-16)18-5-3-4-6-19(18)22-28-30-31-29-22/h3-10,14-15H,2,11-13H2,1H3,(H,34,35)(H,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50422226

(CHEMBL412717)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C48H69N13O11/c1-26(2)38(59-41(65)33(12-8-18-53-48(50)51)55-40(64)32(49)24-62)44(68)56-34(20-29-14-16-31(63)17-15-29)42(66)60-39(27(3)4)45(69)57-35(22-30-23-52-25-54-30)46(70)61-19-9-13-37(61)43(67)58-36(47(71)72)21-28-10-6-5-7-11-28/h5-7,10-11,14-17,23,25-27,32-39,62-63H,8-9,12-13,18-22,24,49H2,1-4H3,(H,52,54)(H,55,64)(H,56,68)(H,57,69)(H,58,67)(H,59,65)(H,60,66)(H,71,72)(H4,50,51,53)/t32-,33-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Dissociation constant for [125 I] Ang binding to type 1 Angiotensin II receptorof bovine adrenocortical membranes |
J Med Chem 40: 3271-9 (1997)
Article DOI: 10.1021/jm9608669
BindingDB Entry DOI: 10.7270/Q2GQ6ZZM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403220

(CHEMBL52036)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncnc2nc(C)[nH]c12 Show InChI InChI=1S/C24H25N9/c1-3-4-13-33(24-21-23(25-15-26-24)28-16(2)27-21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)22-29-31-32-30-22/h5-12,15H,3-4,13-14H2,1-2H3,(H,25,26,27,28)(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 7.76 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50280333
(CHEMBL49207 | [4,4-Diethyl-6-oxo-1-[2'-(1H-tetrazo...)Show SMILES CCOC(=O)\C=C1/CC(CC)(CC)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H31N5O3/c1-4-27(5-2)16-21(15-25(34)35-6-3)32(24(33)17-27)18-19-11-13-20(14-12-19)22-9-7-8-10-23(22)26-28-30-31-29-26/h7-15H,4-6,16-18H2,1-3H3,(H,28,29,30,31)/b21-15+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50280333

(CHEMBL49207 | [4,4-Diethyl-6-oxo-1-[2'-(1H-tetrazo...)Show SMILES CCOC(=O)\C=C1/CC(CC)(CC)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H31N5O3/c1-4-27(5-2)16-21(15-25(34)35-6-3)32(24(33)17-27)18-19-11-13-20(14-12-19)22-9-7-8-10-23(22)26-28-30-31-29-26/h7-15H,4-6,16-18H2,1-3H3,(H,28,29,30,31)/b21-15+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings |
Bioorg Med Chem Lett 4: 87-92 (1994)
Article DOI: 10.1016/S0960-894X(01)81127-6
BindingDB Entry DOI: 10.7270/Q2BC40QF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403227

(CHEMBL300194)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncnc2n(C)cnc12 Show InChI InChI=1S/C24H25N9/c1-3-4-13-33(24-21-23(25-15-26-24)32(2)16-27-21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)22-28-30-31-29-22/h5-12,15-16H,3-4,13-14H2,1-2H3,(H,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403231
(CHEMBL50174)Show SMILES CCc1nc(NCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2c(OC)cccc2n1 Show InChI InChI=1S/C25H23N7O/c1-3-22-27-20-9-6-10-21(33-2)23(20)25(28-22)26-15-16-11-13-17(14-12-16)18-7-4-5-8-19(18)24-29-31-32-30-24/h4-14H,3,15H2,1-2H3,(H,26,27,28)(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
pA2 value determined in vitro against angiotensin II receptor, type 1 using rabbit aortic rings |
Bioorg Med Chem Lett 4: 173-176 (1994)
Article DOI: 10.1016/S0960-894X(01)81142-2
BindingDB Entry DOI: 10.7270/Q2G44RFS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data