

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50126720 ((S)-4-(2-(N-methylisoquinoline-5-sulfonamido)-3-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
Università di Ferrara Curated by ChEMBL | Assay Description Equilibrium constant for P2X purinoceptor 7 expressed in HEK 293 cells at 1 uM | Bioorg Med Chem Lett 14: 5709-12 (2004) Article DOI: 10.1016/j.bmcl.2004.07.095 BindingDB Entry DOI: 10.7270/Q2ZW1KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50126720 ((S)-4-(2-(N-methylisoquinoline-5-sulfonamido)-3-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
Università di Ferrara Curated by ChEMBL | Assay Description Maximum binding affinity towards P2X purinoceptor 7 expressed in HEK 293 cells | Bioorg Med Chem Lett 14: 5709-12 (2004) Article DOI: 10.1016/j.bmcl.2004.07.095 BindingDB Entry DOI: 10.7270/Q2ZW1KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50409686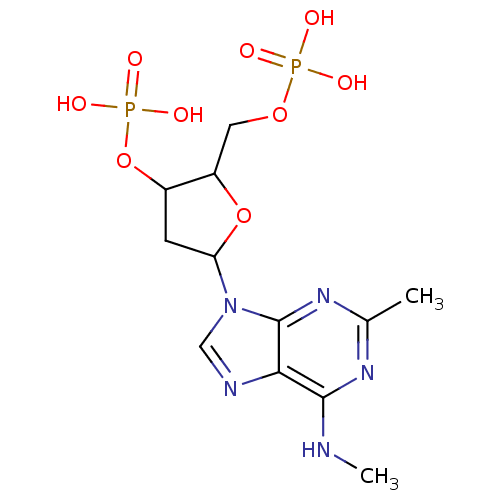 (CHEMBL173068) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 77.6 | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description pA2 value was evaluated against P2Y purinoceptor 1 | J Med Chem 45: 962-72 (2002) BindingDB Entry DOI: 10.7270/Q2ST7QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271557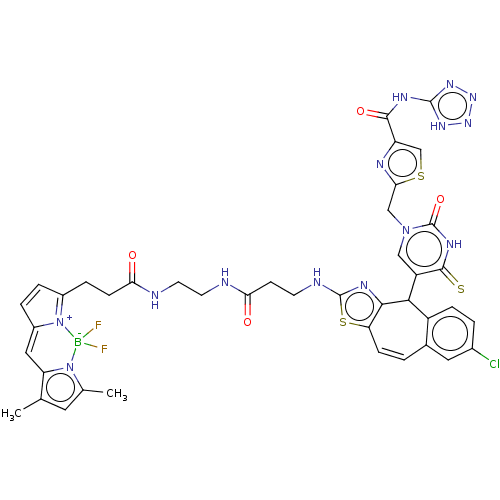 (CHEMBL4127889) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118229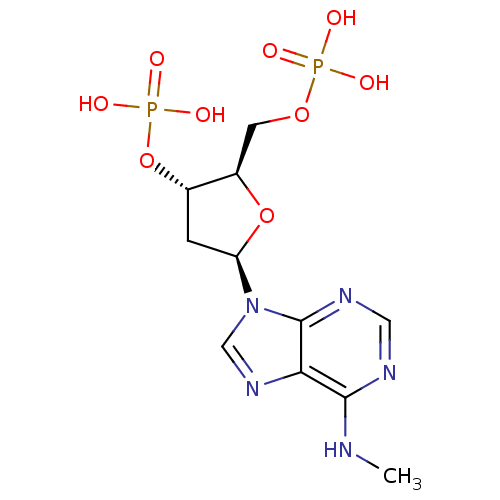 (CHEMBL129841 | MRS 2179) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | Bioorg Med Chem Lett 18: 3338-43 (2008) Article DOI: 10.1016/j.bmcl.2008.04.028 BindingDB Entry DOI: 10.7270/Q2X34Z95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271587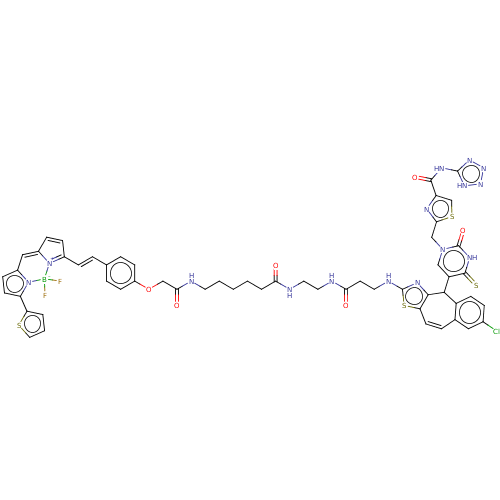 (CHEMBL4128532) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118229 (CHEMBL129841 | MRS 2179) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description pA2 value was evaluated against P2Y purinoceptor 1 | J Med Chem 45: 962-72 (2002) BindingDB Entry DOI: 10.7270/Q2ST7QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271589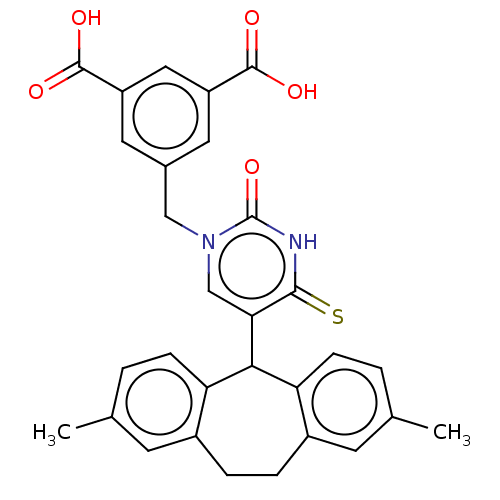 (CHEMBL4128024) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271588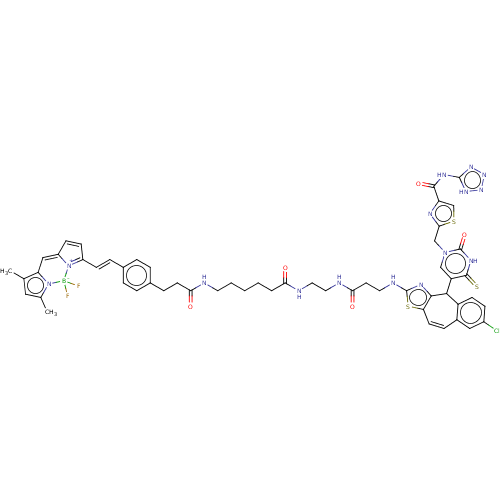 (CHEMBL4126655) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50586608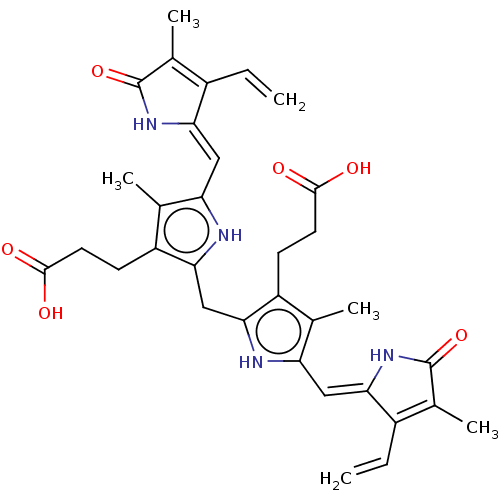 (CHEMBL5083184) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human wild type P2X7R assessed as dissociation constant by microscale thermophoresis analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128361 BindingDB Entry DOI: 10.7270/Q2D222JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271560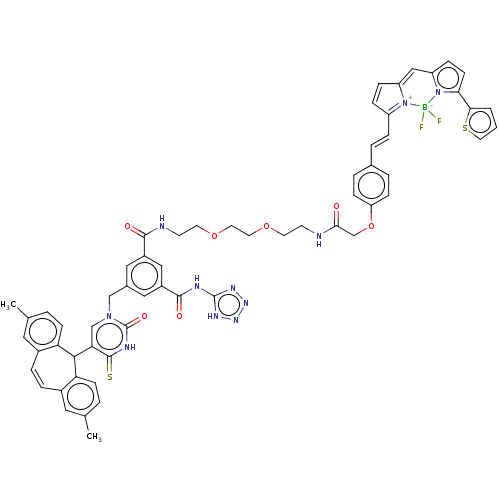 (CHEMBL4125853) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118237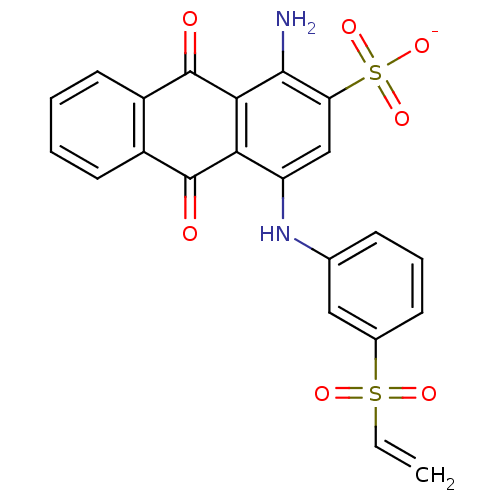 (CHEMBL130059 | Uniblue A) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Dissociation constant at P2X purinoceptor 1 from guinea pig taenia coli | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271561 (CHEMBL4125987) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271586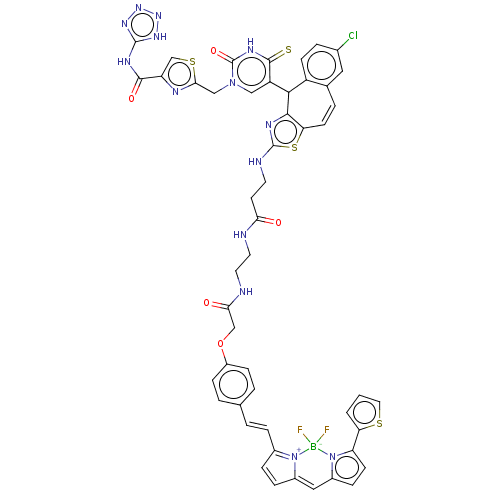 (CHEMBL4125833) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118211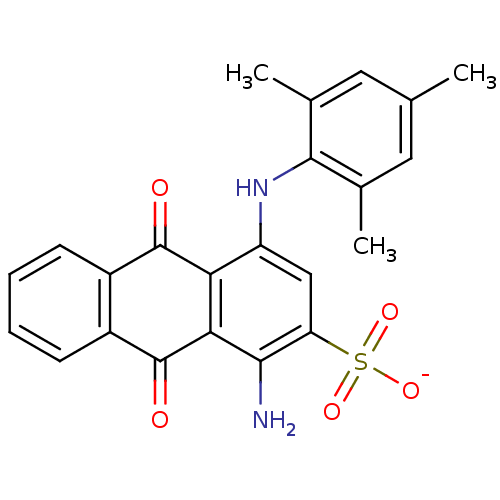 (Acid Blue 129 | CHEMBL133576 | cid_23675739 | sodi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Dissociative constant at P2Y purinoceptor 1 (P2Y1) from guinea pig taenia coli | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118231 (CHEMBL134193 | Cibachron Blue 3GA) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Dissociation constant of the copound at P2X purinoceptor 1 (P2X1) from rat vas deferens was reported | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271545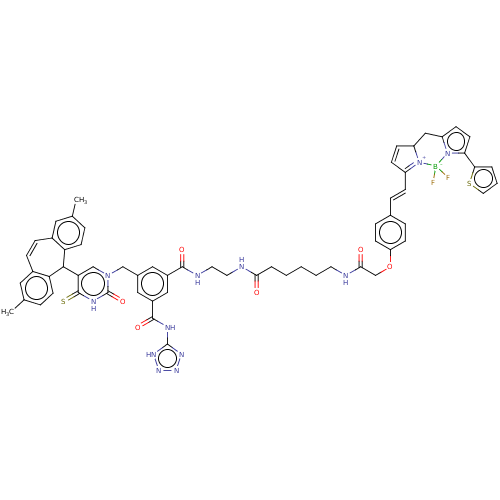 (CHEMBL4127230) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271569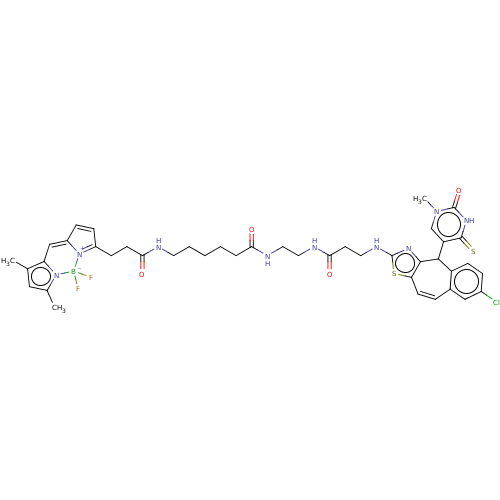 (CHEMBL4128318) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271559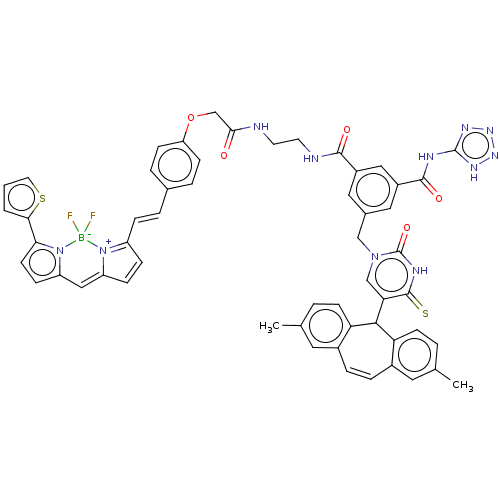 (CHEMBL4126826) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118231 (CHEMBL134193 | Cibachron Blue 3GA) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonistic activity at P2Y purinoceptor 1 (P2Y1) from guinea pig taenia coli | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118234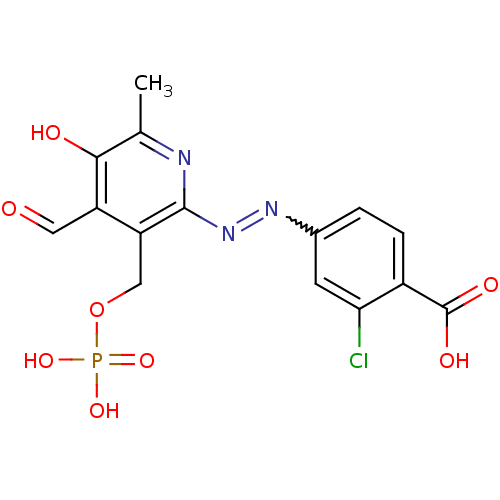 (CHEMBL129904 | MRS 2160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonistic activity at P2Y purinoceptor 1 (P2Y1) from guinea pig taenia coli | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271585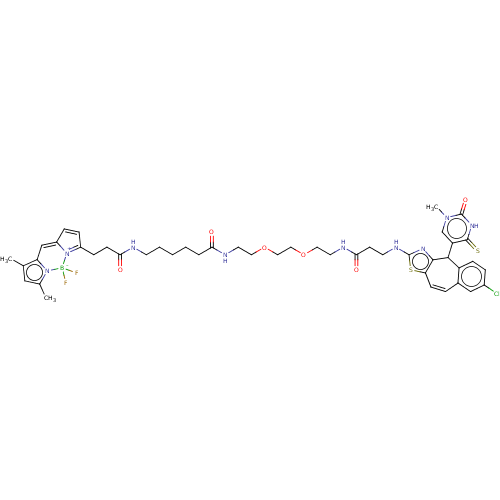 (CHEMBL4129129) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271568 (CHEMBL4129299) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50271546 (CHEMBL4129692) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal NLuc-tagged P2Y2R expressed in human 1321N1 cells incubated for 1 hr in presence of AR-C118925 by fu... | J Med Chem 61: 3089-3113 (2018) Article DOI: 10.1021/acs.jmedchem.8b00139 BindingDB Entry DOI: 10.7270/Q21C20CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118211 (Acid Blue 129 | CHEMBL133576 | cid_23675739 | sodi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against P2X purinoceptor 1 (P2X1) like receptor from rat vas deferens | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118237 (CHEMBL130059 | Uniblue A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Dissociation constant at P2Y purinoceptor 1 (P2Y1) from guinea pig taenia coli | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||