 Found 63 hits of kd data for polymerid = 49001077,49001080
Found 63 hits of kd data for polymerid = 49001077,49001080 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(RAT) | BDBM50038599
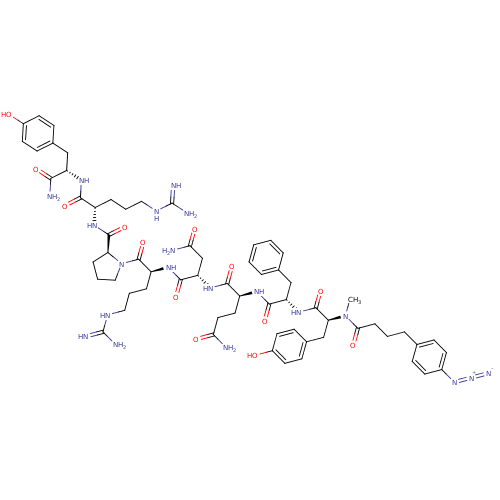
(4-N3-C6H4CH2CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C64H86N20O13/c1-83(54(89)15-5-11-37-16-22-41(23-17-37)81-82-72)51(35-40-20-26-43(86)27-21-40)61(96)80-48(34-38-9-3-2-4-10-38)58(93)75-45(28-29-52(65)87)57(92)79-49(36-53(66)88)59(94)77-46(13-7-31-74-64(70)71)62(97)84-32-8-14-50(84)60(95)76-44(12-6-30-73-63(68)69)56(91)78-47(55(67)90)33-39-18-24-42(85)25-19-39/h2-4,9-10,16-27,44-51,85-86H,5-8,11-15,28-36H2,1H3,(H2,65,87)(H2,66,88)(H2,67,90)(H,75,93)(H,76,95)(H,77,94)(H,78,91)(H,79,92)(H,80,96)(H4,68,69,73)(H4,70,71,74)/t44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038603
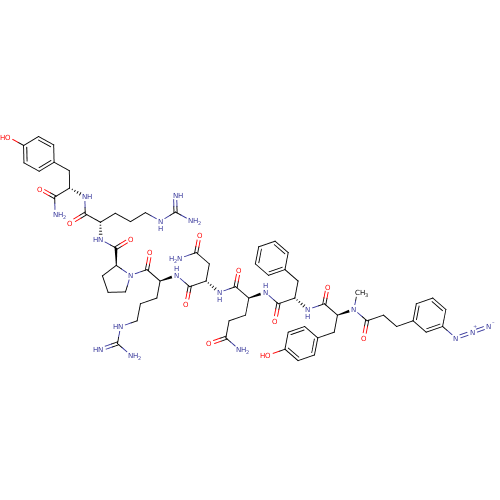
(3-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)27-20-37-11-5-12-40(31-37)80-81-71)50(34-39-18-23-42(85)24-19-39)60(95)79-47(33-36-9-3-2-4-10-36)57(92)74-44(25-26-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(14-7-29-73-63(69)70)61(96)83-30-8-15-49(83)59(94)75-43(13-6-28-72-62(67)68)55(90)77-46(54(66)89)32-38-16-21-41(84)22-17-38/h2-5,9-12,16-19,21-24,31,43-50,84-85H,6-8,13-15,20,25-30,32-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038602
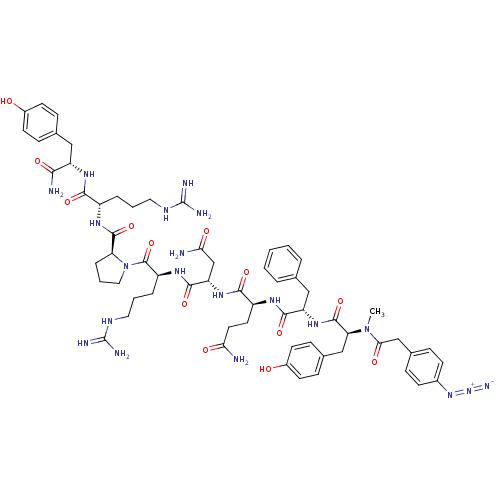
(4-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-13-19-39(20-14-38)79-80-70)49(32-37-17-23-41(84)24-18-37)59(94)78-46(31-35-8-3-2-4-9-35)56(91)73-43(25-26-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(11-6-28-72-62(68)69)60(95)82-29-7-12-48(82)58(93)74-42(10-5-27-71-61(66)67)54(89)76-45(53(65)88)30-36-15-21-40(83)22-16-36/h2-4,8-9,13-24,42-49,83-84H,5-7,10-12,25-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406623

(CHEMBL2115369)Show SMILES COc1ccc(C[C@@H]2NC(=O)C3(CCCC3)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(N)=O)cc1 Show InChI InChI=1S/C58H79N11O12S2/c1-34(2)48-55(78)65-44(31-47(60)71)52(75)66-45(56(79)69-27-11-15-46(69)54(77)62-40(14-7-10-26-59)50(73)63-41(49(61)72)28-36-16-20-38(70)21-17-36)32-82-83-33-58(24-8-9-25-58)57(80)67-43(30-37-18-22-39(81-3)23-19-37)51(74)64-42(53(76)68-48)29-35-12-5-4-6-13-35/h4-6,12-13,16-23,34,40-46,48,70H,7-11,14-15,24-33,59H2,1-3H3,(H2,60,71)(H2,61,72)(H,62,77)(H,63,73)(H,64,74)(H,65,78)(H,66,75)(H,67,80)(H,68,76)/t40-,41-,42-,43-,44-,45-,46-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406623

(CHEMBL2115369)Show SMILES COc1ccc(C[C@@H]2NC(=O)C3(CCCC3)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(N)=O)cc1 Show InChI InChI=1S/C58H79N11O12S2/c1-34(2)48-55(78)65-44(31-47(60)71)52(75)66-45(56(79)69-27-11-15-46(69)54(77)62-40(14-7-10-26-59)50(73)63-41(49(61)72)28-36-16-20-38(70)21-17-36)32-82-83-33-58(24-8-9-25-58)57(80)67-43(30-37-18-22-39(81-3)23-19-37)51(74)64-42(53(76)68-48)29-35-12-5-4-6-13-35/h4-6,12-13,16-23,34,40-46,48,70H,7-11,14-15,24-33,59H2,1-3H3,(H2,60,71)(H2,61,72)(H,62,77)(H,63,73)(H,64,74)(H,65,78)(H,66,75)(H,67,80)(H,68,76)/t40-,41-,42-,43-,44-,45-,46-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406623

(CHEMBL2115369)Show SMILES COc1ccc(C[C@@H]2NC(=O)C3(CCCC3)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(N)=O)cc1 Show InChI InChI=1S/C58H79N11O12S2/c1-34(2)48-55(78)65-44(31-47(60)71)52(75)66-45(56(79)69-27-11-15-46(69)54(77)62-40(14-7-10-26-59)50(73)63-41(49(61)72)28-36-16-20-38(70)21-17-36)32-82-83-33-58(24-8-9-25-58)57(80)67-43(30-37-18-22-39(81-3)23-19-37)51(74)64-42(53(76)68-48)29-35-12-5-4-6-13-35/h4-6,12-13,16-23,34,40-46,48,70H,7-11,14-15,24-33,59H2,1-3H3,(H2,60,71)(H2,61,72)(H,62,77)(H,63,73)(H,64,74)(H,65,78)(H,66,75)(H,67,80)(H,68,76)/t40-,41-,42-,43-,44-,45-,46-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.182 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406623

(CHEMBL2115369)Show SMILES COc1ccc(C[C@@H]2NC(=O)C3(CCCC3)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(N)=O)cc1 Show InChI InChI=1S/C58H79N11O12S2/c1-34(2)48-55(78)65-44(31-47(60)71)52(75)66-45(56(79)69-27-11-15-46(69)54(77)62-40(14-7-10-26-59)50(73)63-41(49(61)72)28-36-16-20-38(70)21-17-36)32-82-83-33-58(24-8-9-25-58)57(80)67-43(30-37-18-22-39(81-3)23-19-37)51(74)64-42(53(76)68-48)29-35-12-5-4-6-13-35/h4-6,12-13,16-23,34,40-46,48,70H,7-11,14-15,24-33,59H2,1-3H3,(H2,60,71)(H2,61,72)(H,62,77)(H,63,73)(H,64,74)(H,65,78)(H,66,75)(H,67,80)(H,68,76)/t40-,41-,42-,43-,44-,45-,46-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.182 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038601
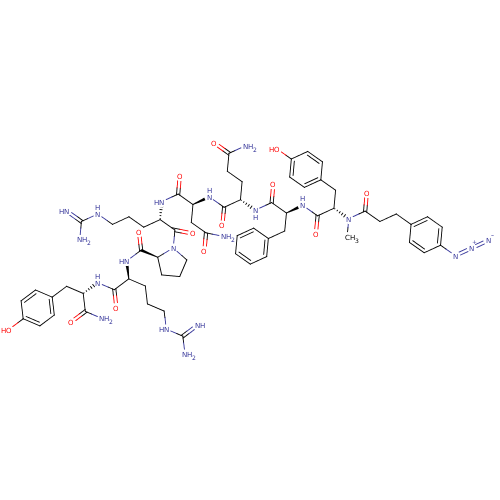
(4-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)28-19-36-13-20-40(21-14-36)80-81-71)50(34-39-17-24-42(85)25-18-39)60(95)79-47(33-37-8-3-2-4-9-37)57(92)74-44(26-27-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(11-6-30-73-63(69)70)61(96)83-31-7-12-49(83)59(94)75-43(10-5-29-72-62(67)68)55(90)77-46(54(66)89)32-38-15-22-41(84)23-16-38/h2-4,8-9,13-18,20-25,43-50,84-85H,5-7,10-12,19,26-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008097

(CHEMBL2028983 | [2-({1-[19-Amino-13-(4-azido-benzy...)Show SMILES [#6]-[#6@H](-[#7](-[#6])-[#6](=O)-[#6@H]-1-[#6]-[#16]-[#16]-[#6]C2([#6]-[#6]-[#6]-[#6]2)[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-c1ccc(cc1)-[#7]=[N+]=[#7-])-[#6](-[#7])=O |r| Show InChI InChI=1S/C60H83N19O12S2/c1-34(51(84)71-41(14-10-28-69-59(65)66)52(85)70-40(50(64)83)13-6-9-27-68-49(63)37-17-19-38(20-18-37)77-78-67)79(2)57(90)46-32-92-93-33-60(25-7-8-26-60)58(91)76-44(30-36-15-21-39(80)22-16-36)55(88)73-43(29-35-11-4-3-5-12-35)54(87)72-42(23-24-47(61)81)53(86)74-45(31-48(62)82)56(89)75-46/h3-5,11-12,15-22,34,40-46,80H,6-10,13-14,23-33H2,1-2H3,(H2,61,81)(H2,62,82)(H2,63,68)(H2,64,83)(H,70,85)(H,71,84)(H,72,87)(H,73,88)(H,74,86)(H,75,89)(H,76,91)(H4,65,66,69)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008097

(CHEMBL2028983 | [2-({1-[19-Amino-13-(4-azido-benzy...)Show SMILES [#6]-[#6@H](-[#7](-[#6])-[#6](=O)-[#6@H]-1-[#6]-[#16]-[#16]-[#6]C2([#6]-[#6]-[#6]-[#6]2)[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-c1ccc(cc1)-[#7]=[N+]=[#7-])-[#6](-[#7])=O |r| Show InChI InChI=1S/C60H83N19O12S2/c1-34(51(84)71-41(14-10-28-69-59(65)66)52(85)70-40(50(64)83)13-6-9-27-68-49(63)37-17-19-38(20-18-37)77-78-67)79(2)57(90)46-32-92-93-33-60(25-7-8-26-60)58(91)76-44(30-36-15-21-39(80)22-16-36)55(88)73-43(29-35-11-4-3-5-12-35)54(87)72-42(23-24-47(61)81)53(86)74-45(31-48(62)82)56(89)75-46/h3-5,11-12,15-22,34,40-46,80H,6-10,13-14,23-33H2,1-2H3,(H2,61,81)(H2,62,82)(H2,63,68)(H2,64,83)(H,70,85)(H,71,84)(H,72,87)(H,73,88)(H,74,86)(H,75,89)(H,76,91)(H4,65,66,69)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008097

(CHEMBL2028983 | [2-({1-[19-Amino-13-(4-azido-benzy...)Show SMILES [#6]-[#6@H](-[#7](-[#6])-[#6](=O)-[#6@H]-1-[#6]-[#16]-[#16]-[#6]C2([#6]-[#6]-[#6]-[#6]2)[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-c1ccc(cc1)-[#7]=[N+]=[#7-])-[#6](-[#7])=O |r| Show InChI InChI=1S/C60H83N19O12S2/c1-34(51(84)71-41(14-10-28-69-59(65)66)52(85)70-40(50(64)83)13-6-9-27-68-49(63)37-17-19-38(20-18-37)77-78-67)79(2)57(90)46-32-92-93-33-60(25-7-8-26-60)58(91)76-44(30-36-15-21-39(80)22-16-36)55(88)73-43(29-35-11-4-3-5-12-35)54(87)72-42(23-24-47(61)81)53(86)74-45(31-48(62)82)56(89)75-46/h3-5,11-12,15-22,34,40-46,80H,6-10,13-14,23-33H2,1-2H3,(H2,61,81)(H2,62,82)(H2,63,68)(H2,64,83)(H,70,85)(H,71,84)(H,72,87)(H,73,88)(H,74,86)(H,75,89)(H,76,91)(H4,65,66,69)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008097

(CHEMBL2028983 | [2-({1-[19-Amino-13-(4-azido-benzy...)Show SMILES [#6]-[#6@H](-[#7](-[#6])-[#6](=O)-[#6@H]-1-[#6]-[#16]-[#16]-[#6]C2([#6]-[#6]-[#6]-[#6]2)[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-c1ccc(cc1)-[#7]=[N+]=[#7-])-[#6](-[#7])=O |r| Show InChI InChI=1S/C60H83N19O12S2/c1-34(51(84)71-41(14-10-28-69-59(65)66)52(85)70-40(50(64)83)13-6-9-27-68-49(63)37-17-19-38(20-18-37)77-78-67)79(2)57(90)46-32-92-93-33-60(25-7-8-26-60)58(91)76-44(30-36-15-21-39(80)22-16-36)55(88)73-43(29-35-11-4-3-5-12-35)54(87)72-42(23-24-47(61)81)53(86)74-45(31-48(62)82)56(89)75-46/h3-5,11-12,15-22,34,40-46,80H,6-10,13-14,23-33H2,1-2H3,(H2,61,81)(H2,62,82)(H2,63,68)(H2,64,83)(H,70,85)(H,71,84)(H,72,87)(H,73,88)(H,74,86)(H,75,89)(H,76,91)(H4,65,66,69)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406622
(CHEMBL2115364)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N14O11S2/c1-31(2)45-52(78)63-41(28-44(57)71)49(75)64-42(53(79)69-24-10-14-43(69)51(77)60-37(13-8-9-23-56)47(73)61-38(46(58)72)25-34-17-21-36(70)22-18-34)29-81-82-30-55(3,4)54(80)65-40(27-33-15-19-35(20-16-33)67-68-59)48(74)62-39(50(76)66-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,70H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,71)(H2,58,72)(H,60,77)(H,61,73)(H,62,74)(H,63,78)(H,64,75)(H,65,80)(H,66,76)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406622

(CHEMBL2115364)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N14O11S2/c1-31(2)45-52(78)63-41(28-44(57)71)49(75)64-42(53(79)69-24-10-14-43(69)51(77)60-37(13-8-9-23-56)47(73)61-38(46(58)72)25-34-17-21-36(70)22-18-34)29-81-82-30-55(3,4)54(80)65-40(27-33-15-19-35(20-16-33)67-68-59)48(74)62-39(50(76)66-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,70H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,71)(H2,58,72)(H,60,77)(H,61,73)(H,62,74)(H,63,78)(H,64,75)(H,65,80)(H,66,76)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of the 3 nM VP stimulation of phospholipase-C in cultured WRK cells was used as a V1-mediated assay |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406622

(CHEMBL2115364)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N14O11S2/c1-31(2)45-52(78)63-41(28-44(57)71)49(75)64-42(53(79)69-24-10-14-43(69)51(77)60-37(13-8-9-23-56)47(73)61-38(46(58)72)25-34-17-21-36(70)22-18-34)29-81-82-30-55(3,4)54(80)65-40(27-33-15-19-35(20-16-33)67-68-59)48(74)62-39(50(76)66-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,70H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,71)(H2,58,72)(H,60,77)(H,61,73)(H,62,74)(H,63,78)(H,64,75)(H,65,80)(H,66,76)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406622

(CHEMBL2115364)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N14O11S2/c1-31(2)45-52(78)63-41(28-44(57)71)49(75)64-42(53(79)69-24-10-14-43(69)51(77)60-37(13-8-9-23-56)47(73)61-38(46(58)72)25-34-17-21-36(70)22-18-34)29-81-82-30-55(3,4)54(80)65-40(27-33-15-19-35(20-16-33)67-68-59)48(74)62-39(50(76)66-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,70H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,71)(H2,58,72)(H,60,77)(H,61,73)(H,62,74)(H,63,78)(H,64,75)(H,65,80)(H,66,76)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against V1 receptor in rat liver cells |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 0.646 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453149
(CHEMBL2115368)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N12O13S2/c1-31(2)45-52(76)62-41(28-44(57)69)49(73)63-42(53(77)66-24-10-14-43(66)51(75)59-37(13-8-9-23-56)47(71)60-38(46(58)70)25-34-17-21-36(68)22-18-34)29-81-82-30-55(3,4)54(78)64-40(27-33-15-19-35(20-16-33)67(79)80)48(72)61-39(50(74)65-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,68H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,69)(H2,58,70)(H,59,75)(H,60,71)(H,61,72)(H,62,76)(H,63,73)(H,64,78)(H,65,74)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453149

(CHEMBL2115368)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N12O13S2/c1-31(2)45-52(76)62-41(28-44(57)69)49(73)63-42(53(77)66-24-10-14-43(66)51(75)59-37(13-8-9-23-56)47(71)60-38(46(58)70)25-34-17-21-36(68)22-18-34)29-81-82-30-55(3,4)54(78)64-40(27-33-15-19-35(20-16-33)67(79)80)48(72)61-39(50(74)65-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,68H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,69)(H2,58,70)(H,59,75)(H,60,71)(H,61,72)(H,62,76)(H,63,73)(H,64,78)(H,65,74)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453149

(CHEMBL2115368)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N12O13S2/c1-31(2)45-52(76)62-41(28-44(57)69)49(73)63-42(53(77)66-24-10-14-43(66)51(75)59-37(13-8-9-23-56)47(71)60-38(46(58)70)25-34-17-21-36(68)22-18-34)29-81-82-30-55(3,4)54(78)64-40(27-33-15-19-35(20-16-33)67(79)80)48(72)61-39(50(74)65-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,68H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,69)(H2,58,70)(H,59,75)(H,60,71)(H,61,72)(H,62,76)(H,63,73)(H,64,78)(H,65,74)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against V1 receptor in rat liver cells |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453149

(CHEMBL2115368)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N12O13S2/c1-31(2)45-52(76)62-41(28-44(57)69)49(73)63-42(53(77)66-24-10-14-43(66)51(75)59-37(13-8-9-23-56)47(71)60-38(46(58)70)25-34-17-21-36(68)22-18-34)29-81-82-30-55(3,4)54(78)64-40(27-33-15-19-35(20-16-33)67(79)80)48(72)61-39(50(74)65-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,68H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,69)(H2,58,70)(H,59,75)(H,60,71)(H,61,72)(H,62,76)(H,63,73)(H,64,78)(H,65,74)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407342

(CHEMBL415160)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37-,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Compound was evaluated for the anti-vasopressor activity at V1a receptor. . |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008088

(CHEMBL2372416 | cyclo(Depa-Phe(N3)-Phe-Val-Asn-Cys...)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N14O11S2/c1-5-57(6-2)32-84-83-31-44(55(81)71-26-12-16-45(71)53(79)62-39(15-10-11-25-58)49(75)63-40(48(60)74)27-36-19-23-38(72)24-20-36)66-51(77)43(30-46(59)73)65-54(80)47(33(3)4)68-52(78)41(28-34-13-8-7-9-14-34)64-50(76)42(67-56(57)82)29-35-17-21-37(22-18-35)69-70-61/h7-9,13-14,17-24,33,39-45,47,72H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,73)(H2,60,74)(H,62,79)(H,63,75)(H,64,76)(H,65,80)(H,66,77)(H,67,82)(H,68,78)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453152

(CHEMBL2115365)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N12O13S2/c1-5-57(6-2)32-84-83-31-44(55(79)68-26-12-16-45(68)53(77)61-39(15-10-11-25-58)49(73)62-40(48(60)72)27-36-19-23-38(70)24-20-36)65-51(75)43(30-46(59)71)64-54(78)47(33(3)4)67-52(76)41(28-34-13-8-7-9-14-34)63-50(74)42(66-56(57)80)29-35-17-21-37(22-18-35)69(81)82/h7-9,13-14,17-24,33,39-45,47,70H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40-,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453152

(CHEMBL2115365)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N12O13S2/c1-5-57(6-2)32-84-83-31-44(55(79)68-26-12-16-45(68)53(77)61-39(15-10-11-25-58)49(73)62-40(48(60)72)27-36-19-23-38(70)24-20-36)65-51(75)43(30-46(59)71)64-54(78)47(33(3)4)67-52(76)41(28-34-13-8-7-9-14-34)63-50(74)42(66-56(57)80)29-35-17-21-37(22-18-35)69(81)82/h7-9,13-14,17-24,33,39-45,47,70H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40-,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008088

(CHEMBL2372416 | cyclo(Depa-Phe(N3)-Phe-Val-Asn-Cys...)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N14O11S2/c1-5-57(6-2)32-84-83-31-44(55(81)71-26-12-16-45(71)53(79)62-39(15-10-11-25-58)49(75)63-40(48(60)74)27-36-19-23-38(72)24-20-36)66-51(77)43(30-46(59)73)65-54(80)47(33(3)4)68-52(78)41(28-34-13-8-7-9-14-34)64-50(76)42(67-56(57)82)29-35-17-21-37(22-18-35)69-70-61/h7-9,13-14,17-24,33,39-45,47,72H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,73)(H2,60,74)(H,62,79)(H,63,75)(H,64,76)(H,65,80)(H,66,77)(H,67,82)(H,68,78)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453152

(CHEMBL2115365)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N12O13S2/c1-5-57(6-2)32-84-83-31-44(55(79)68-26-12-16-45(68)53(77)61-39(15-10-11-25-58)49(73)62-40(48(60)72)27-36-19-23-38(70)24-20-36)65-51(75)43(30-46(59)71)64-54(78)47(33(3)4)67-52(76)41(28-34-13-8-7-9-14-34)63-50(74)42(66-56(57)80)29-35-17-21-37(22-18-35)69(81)82/h7-9,13-14,17-24,33,39-45,47,70H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40-,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453152

(CHEMBL2115365)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N12O13S2/c1-5-57(6-2)32-84-83-31-44(55(79)68-26-12-16-45(68)53(77)61-39(15-10-11-25-58)49(73)62-40(48(60)72)27-36-19-23-38(70)24-20-36)65-51(75)43(30-46(59)71)64-54(78)47(33(3)4)67-52(76)41(28-34-13-8-7-9-14-34)63-50(74)42(66-56(57)80)29-35-17-21-37(22-18-35)69(81)82/h7-9,13-14,17-24,33,39-45,47,70H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40-,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008092

(CHEMBL2372415 | cyclo(Ppa-Phe(NO2)-Phe-Val-Asn-Cys...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C2(CCCC2)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H76N12O13S2/c1-33(2)47-54(78)64-43(30-46(59)71)51(75)65-44(55(79)68-26-10-14-45(68)53(77)61-39(13-6-9-25-58)49(73)62-40(48(60)72)27-36-17-21-38(70)22-18-36)31-83-84-32-57(23-7-8-24-57)56(80)66-42(29-35-15-19-37(20-16-35)69(81)82)50(74)63-41(52(76)67-47)28-34-11-4-3-5-12-34/h3-5,11-12,15-22,33,39-45,47,70H,6-10,13-14,23-32,58H2,1-2H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008092

(CHEMBL2372415 | cyclo(Ppa-Phe(NO2)-Phe-Val-Asn-Cys...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C2(CCCC2)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H76N12O13S2/c1-33(2)47-54(78)64-43(30-46(59)71)51(75)65-44(55(79)68-26-10-14-45(68)53(77)61-39(13-6-9-25-58)49(73)62-40(48(60)72)27-36-17-21-38(70)22-18-36)31-83-84-32-57(23-7-8-24-57)56(80)66-42(29-35-15-19-37(20-16-35)69(81)82)50(74)63-41(52(76)67-47)28-34-11-4-3-5-12-34/h3-5,11-12,15-22,33,39-45,47,70H,6-10,13-14,23-32,58H2,1-2H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008091
(1-[16-(4-Azido-benzyl)-13-benzyl-7-carbamoylmethyl...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C53H70N14O11S2/c1-30(2)45-52(77)62-40(28-43(55)69)49(74)63-41(53(78)67-23-8-12-42(67)51(76)59-36(11-6-7-22-54)47(72)60-37(46(56)71)25-33-15-19-35(68)20-16-33)29-80-79-24-21-44(70)58-38(27-32-13-17-34(18-14-32)65-66-57)48(73)61-39(50(75)64-45)26-31-9-4-3-5-10-31/h3-5,9-10,13-20,30,36-42,45,68H,6-8,11-12,21-29,54H2,1-2H3,(H2,55,69)(H2,56,71)(H,58,70)(H,59,76)(H,60,72)(H,61,73)(H,62,77)(H,63,74)(H,64,75)/t36-,37+,38-,39-,40-,41-,42-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008091

(1-[16-(4-Azido-benzyl)-13-benzyl-7-carbamoylmethyl...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C53H70N14O11S2/c1-30(2)45-52(77)62-40(28-43(55)69)49(74)63-41(53(78)67-23-8-12-42(67)51(76)59-36(11-6-7-22-54)47(72)60-37(46(56)71)25-33-15-19-35(68)20-16-33)29-80-79-24-21-44(70)58-38(27-32-13-17-34(18-14-32)65-66-57)48(73)61-39(50(75)64-45)26-31-9-4-3-5-10-31/h3-5,9-10,13-20,30,36-42,45,68H,6-8,11-12,21-29,54H2,1-2H3,(H2,55,69)(H2,56,71)(H,58,70)(H,59,76)(H,60,72)(H,61,73)(H,62,77)(H,63,74)(H,64,75)/t36-,37+,38-,39-,40-,41-,42-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407333
(CHEMBL412942)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40+,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407332

(CHEMBL405005)Show SMILES CCN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40-,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407349

(CHEMBL265357)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38+,39-,40+,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407350

(CHEMBL263521)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C48H74N12O12S2/c1-4-27(2)40-46(71)55-31(16-17-36(50)62)42(67)56-32(23-37(51)63)43(68)57-33(47(72)60-21-9-11-34(60)44(69)54-30(10-8-20-49)41(66)53-25-38(52)64)26-73-74-48(18-6-5-7-19-48)24-39(65)59(3)35(45(70)58-40)22-28-12-14-29(61)15-13-28/h12-15,27,30-35,40,61H,4-11,16-26,49H2,1-3H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,66)(H,54,69)(H,55,71)(H,56,67)(H,57,68)(H,58,70)/t27-,30-,31-,32+,33-,34-,35-,40+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 11.0 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407336

(CHEMBL412616)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37+,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453148

(CHEMBL2372422)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7]=[N+]=[#7-])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H64N18O12S2/c47-27-21-77-78-22-33(45(76)64-16-2-4-34(64)44(75)57-28(3-1-15-54-46(51)52)39(70)55-20-37(50)68)61-43(74)32(19-36(49)67)60-40(71)29(13-14-35(48)66)56-41(72)31(17-23-5-9-25(10-6-23)62-63-53)59-42(73)30(58-38(27)69)18-24-7-11-26(65)12-8-24/h5-12,27-34,65H,1-4,13-22,47H2,(H2,48,66)(H2,49,67)(H2,50,68)(H,55,70)(H,56,72)(H,57,75)(H,58,69)(H,59,73)(H,60,71)(H,61,74)(H4,51,52,54)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453148

(CHEMBL2372422)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7]=[N+]=[#7-])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H64N18O12S2/c47-27-21-77-78-22-33(45(76)64-16-2-4-34(64)44(75)57-28(3-1-15-54-46(51)52)39(70)55-20-37(50)68)61-43(74)32(19-36(49)67)60-40(71)29(13-14-35(48)66)56-41(72)31(17-23-5-9-25(10-6-23)62-63-53)59-42(73)30(58-38(27)69)18-24-7-11-26(65)12-8-24/h5-12,27-34,65H,1-4,13-22,47H2,(H2,48,66)(H2,49,67)(H2,50,68)(H,55,70)(H,56,72)(H,57,75)(H,58,69)(H,59,73)(H,60,71)(H,61,74)(H4,51,52,54)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453150

(CHEMBL2372420)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C53H70N12O13S2/c1-30(2)45-52(75)61-40(28-43(55)67)49(72)62-41(53(76)64-23-8-12-42(64)51(74)58-36(11-6-7-22-54)47(70)59-37(46(56)69)25-33-15-19-35(66)20-16-33)29-80-79-24-21-44(68)57-38(27-32-13-17-34(18-14-32)65(77)78)48(71)60-39(50(73)63-45)26-31-9-4-3-5-10-31/h3-5,9-10,13-20,30,36-42,45,66H,6-8,11-12,21-29,54H2,1-2H3,(H2,55,67)(H2,56,69)(H,57,68)(H,58,74)(H,59,70)(H,60,71)(H,61,75)(H,62,72)(H,63,73)/t36-,37+,38-,39-,40-,41-,42-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453150

(CHEMBL2372420)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C53H70N12O13S2/c1-30(2)45-52(75)61-40(28-43(55)67)49(72)62-41(53(76)64-23-8-12-42(64)51(74)58-36(11-6-7-22-54)47(70)59-37(46(56)69)25-33-15-19-35(66)20-16-33)29-80-79-24-21-44(68)57-38(27-32-13-17-34(18-14-32)65(77)78)48(71)60-39(50(73)63-45)26-31-9-4-3-5-10-31/h3-5,9-10,13-20,30,36-42,45,66H,6-8,11-12,21-29,54H2,1-2H3,(H2,55,67)(H2,56,69)(H,57,68)(H,58,74)(H,59,70)(H,60,71)(H,61,75)(H,62,72)(H,63,73)/t36-,37+,38-,39-,40-,41-,42-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407338
(CHEMBL385062)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C51H73N13O10S2/c1-30(2)42-48(73)61-36(26-39(52)65)45(70)62-37(49(74)64-23-13-19-38(64)47(72)59-33(18-12-22-56-50(54)55)43(68)57-28-40(53)66)29-75-76-51(20-10-5-11-21-51)27-41(67)58-34(24-31-14-6-3-7-15-31)44(69)60-35(46(71)63-42)25-32-16-8-4-9-17-32/h3-4,6-9,14-17,30,33-38,42H,5,10-13,18-29H2,1-2H3,(H2,52,65)(H2,53,66)(H,57,68)(H,58,67)(H,59,72)(H,60,69)(H,61,73)(H,62,70)(H,63,71)(H4,54,55,56)/t33-,34+,35+,36+,37+,38-,42-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407335

(CHEMBL428187)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35-,36-,41+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407343
(CHEMBL412806)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35+,36-,41+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453151
(CHEMBL2372421)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7]=[N+]=[#7-])-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N18O12S2/c47-27-21-77-78-22-33(45(76)64-16-2-4-34(64)44(75)57-28(3-1-15-54-46(51)52)39(70)55-20-37(50)68)61-43(74)32(19-36(49)67)60-40(71)29(13-14-35(48)66)56-41(72)31(17-23-5-9-25(10-6-23)62-63-53)59-42(73)30(58-38(27)69)18-24-7-11-26(65)12-8-24/h5-12,27-34,65H,1-4,13-22,47H2,(H2,48,66)(H2,49,67)(H2,50,68)(H,55,70)(H,56,72)(H,57,75)(H,58,69)(H,59,73)(H,60,71)(H,61,74)(H4,51,52,54)/t27-,28-,29-,30+,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453151

(CHEMBL2372421)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7]=[N+]=[#7-])-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N18O12S2/c47-27-21-77-78-22-33(45(76)64-16-2-4-34(64)44(75)57-28(3-1-15-54-46(51)52)39(70)55-20-37(50)68)61-43(74)32(19-36(49)67)60-40(71)29(13-14-35(48)66)56-41(72)31(17-23-5-9-25(10-6-23)62-63-53)59-42(73)30(58-38(27)69)18-24-7-11-26(65)12-8-24/h5-12,27-34,65H,1-4,13-22,47H2,(H2,48,66)(H2,49,67)(H2,50,68)(H,55,70)(H,56,72)(H,57,75)(H,58,69)(H,59,73)(H,60,71)(H,61,74)(H4,51,52,54)/t27-,28-,29-,30+,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008096
(CHEMBL2372414 | cyclo(Ppa-Phe(N3)-Phe-Val-Asn-Cys)...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C2(CCCC2)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H76N14O11S2/c1-33(2)47-54(80)65-43(30-46(59)73)51(77)66-44(55(81)71-26-10-14-45(71)53(79)62-39(13-6-9-25-58)49(75)63-40(48(60)74)27-36-17-21-38(72)22-18-36)31-83-84-32-57(23-7-8-24-57)56(82)67-42(29-35-15-19-37(20-16-35)69-70-61)50(76)64-41(52(78)68-47)28-34-11-4-3-5-12-34/h3-5,11-12,15-22,33,39-45,47,72H,6-10,13-14,23-32,58H2,1-2H3,(H2,59,73)(H2,60,74)(H,62,79)(H,63,75)(H,64,76)(H,65,80)(H,66,77)(H,67,82)(H,68,78)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008096

(CHEMBL2372414 | cyclo(Ppa-Phe(N3)-Phe-Val-Asn-Cys)...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C2(CCCC2)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H76N14O11S2/c1-33(2)47-54(80)65-43(30-46(59)73)51(77)66-44(55(81)71-26-10-14-45(71)53(79)62-39(13-6-9-25-58)49(75)63-40(48(60)74)27-36-17-21-38(72)22-18-36)31-83-84-32-57(23-7-8-24-57)56(82)67-42(29-35-15-19-37(20-16-35)69-70-61)50(76)64-41(52(78)68-47)28-34-11-4-3-5-12-34/h3-5,11-12,15-22,33,39-45,47,72H,6-10,13-14,23-32,58H2,1-2H3,(H2,59,73)(H2,60,74)(H,62,79)(H,63,75)(H,64,76)(H,65,80)(H,66,77)(H,67,82)(H,68,78)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008084
(1-[13-Benzyl-7-carbamoylmethyl-10-isopropyl-16-(4-...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7+](-[#8-])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H64N16O14S2/c47-27-21-77-78-22-33(45(74)61-16-2-4-34(61)44(73)56-28(3-1-15-53-46(51)52)39(68)54-20-37(50)66)60-43(72)32(19-36(49)65)59-40(69)29(13-14-35(48)64)55-41(70)31(17-23-5-9-25(10-6-23)62(75)76)58-42(71)30(57-38(27)67)18-24-7-11-26(63)12-8-24/h5-12,27-34,63H,1-4,13-22,47H2,(H2,48,64)(H2,49,65)(H2,50,66)(H,54,68)(H,55,70)(H,56,73)(H,57,67)(H,58,71)(H,59,69)(H,60,72)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data