

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50078572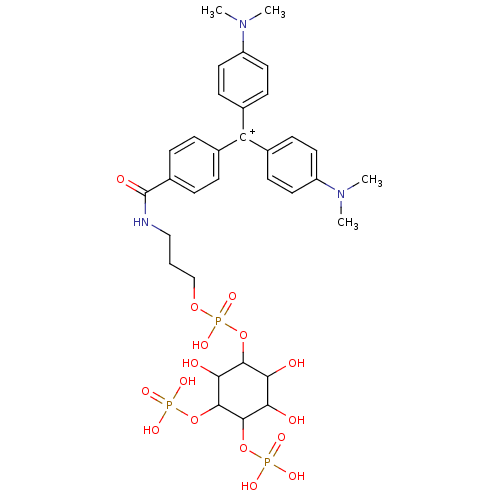 (CHEMBL172534 | CHEMBL297496 | {4-[(4-Dimethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111931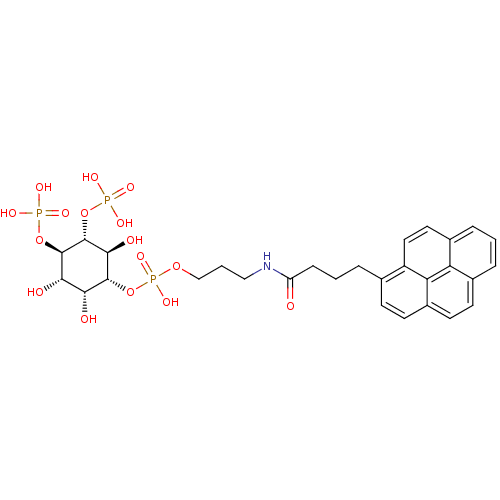 (CHEMBL434103 | Phosphoric acid 3-(4-pyren-1-yl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111931 (CHEMBL434103 | Phosphoric acid 3-(4-pyren-1-yl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111928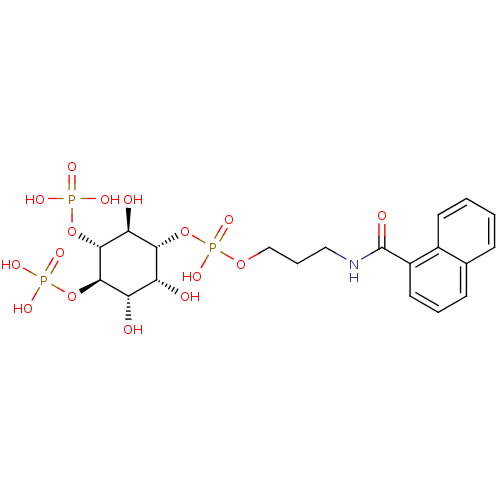 (CHEMBL169923 | Phosphoric acid 3-[(naphthalene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111928 (CHEMBL169923 | Phosphoric acid 3-[(naphthalene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50075183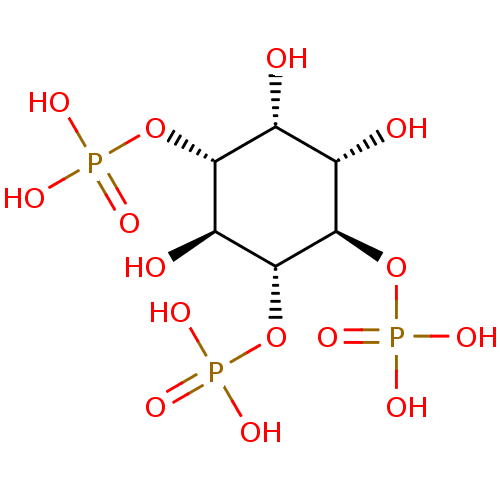 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111932 (CHEMBL369479 | inositol 1,4,5-Trisphosphate analog...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50078573 (CHEMBL297235 | Phosphoric acid 3-amino-propyl este...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111929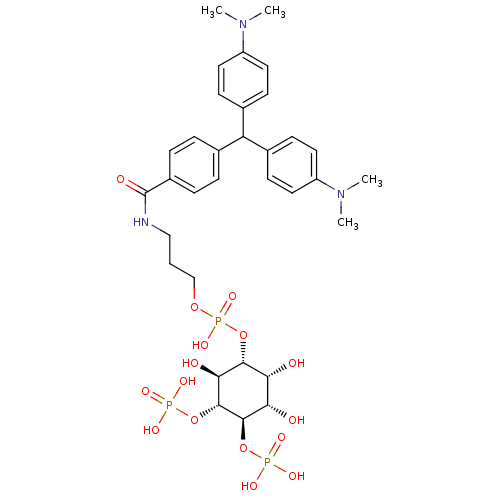 (CHEMBL352326 | Phosphoric acid 3-{4-[bis-(4-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Homo sapiens (Human)) | BDBM50111929 (CHEMBL352326 | Phosphoric acid 3-{4-[bis-(4-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Dissociation constant of the compound was determined using IP3-binding domain (IBD) of human Type 1 inositol 1,4,5-trisphosphate receptor | Bioorg Med Chem Lett 12: 911-3 (2002) BindingDB Entry DOI: 10.7270/Q2HX1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||