 Found 140 hits of kd data for polymerid = 5049,5051,5051
Found 140 hits of kd data for polymerid = 5049,5051,5051 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489093
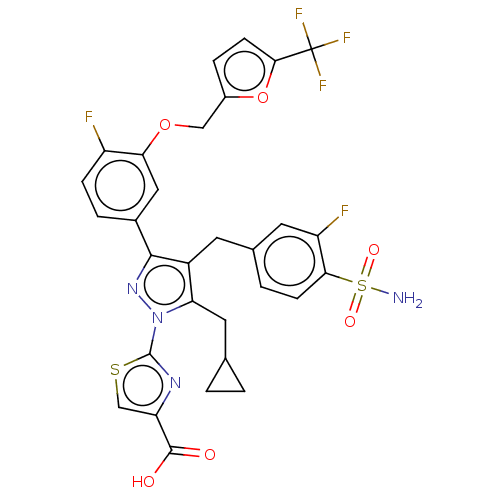
(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3-((5- (tri...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCc3ccc(o3)C(F)(F)F)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C30H23F5N4O6S2/c31-20-6-4-17(12-24(20)44-13-18-5-8-26(45-18)30(33,34)35)27-19(9-16-3-7-25(21(32)10-16)47(36,42)43)23(11-15-1-2-15)39(38-27)29-37-22(14-46-29)28(40)41/h3-8,10,12,14-15H,1-2,9,11,13H2,(H,40,41)(H2,36,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489092

(2-(3-(3- cydopropoxy-4- fluorophenyl)-5- (cyclopro...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OC3CC3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C27H24F2N4O5S2/c28-19-7-4-16(12-23(19)38-17-5-6-17)25-18(9-15-3-8-24(20(29)10-15)40(30,36)37)22(11-14-1-2-14)33(32-25)27-31-21(13-39-27)26(34)35/h3-4,7-8,10,12-14,17H,1-2,5-6,9,11H2,(H,34,35)(H2,30,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM537077

(US11247971, Cmpd ID 400)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CC3(F)F)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM537017
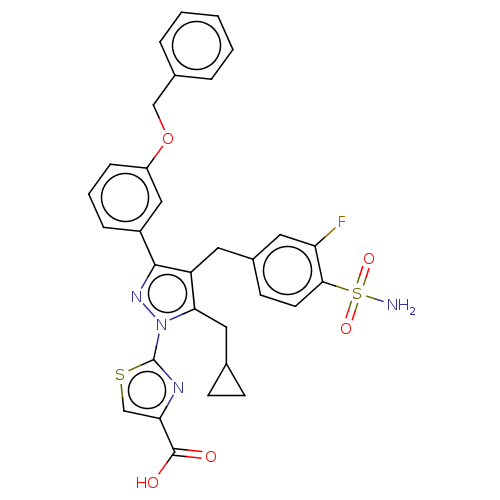
(US11247971, Cmpd ID 340)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(OCc3ccccc3)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546973
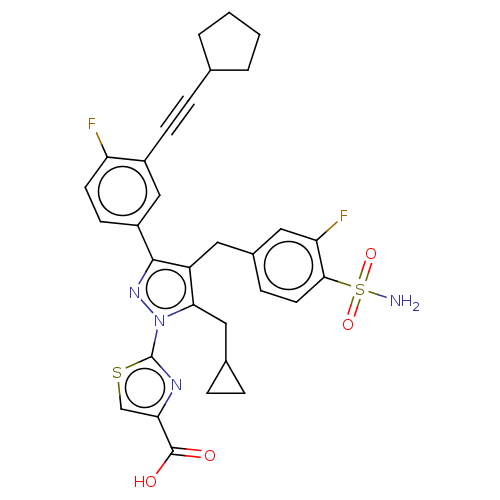
(CHEMBL4782843 | US11247971, Cmpd ID 258)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC2CCCC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.0193 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-tagged LDHA by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50547002
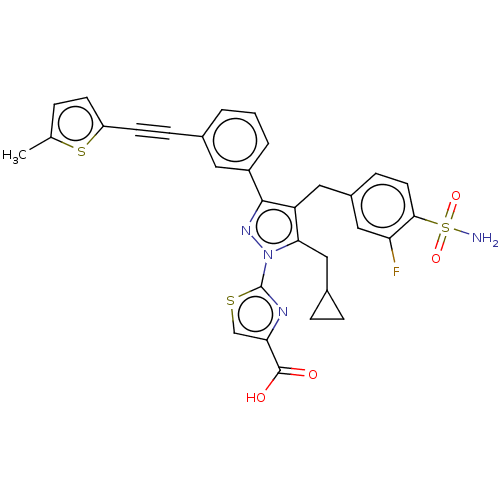
(CHEMBL4753379 | US11247971, Cmpd ID 449 | US117521...)Show SMILES Cc1ccc(s1)C#Cc1cccc(c1)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.0634 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-tagged LDHA by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546993
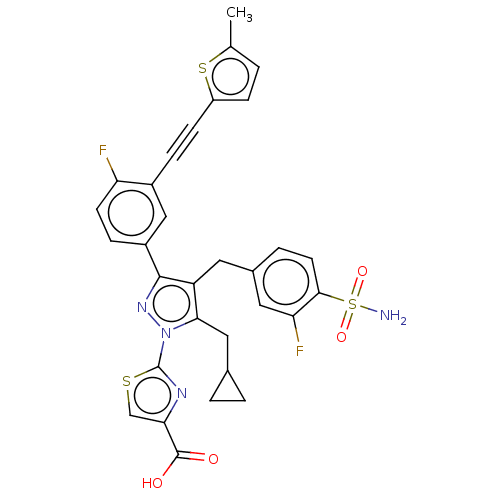
(CHEMBL4748782 | US11247971, Cmpd ID 408 | US117521...)Show SMILES Cc1ccc(s1)C#Cc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0667 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-tagged LDHA by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250656

(CHEMBL4081890 | US11247971, Cmpd ID 276)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(c2)-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C30H26N4O4S2/c31-40(37,38)24-13-11-19(12-14-24)15-25-27(16-20-9-10-20)34(30-32-26(18-39-30)29(35)36)33-28(25)23-8-4-7-22(17-23)21-5-2-1-3-6-21/h1-8,11-14,17-18,20H,9-10,15-16H2,(H,35,36)(H2,31,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-tagged LDHA by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250656

(CHEMBL4081890 | US11247971, Cmpd ID 276)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(c2)-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C30H26N4O4S2/c31-40(37,38)24-13-11-19(12-14-24)15-25-27(16-20-9-10-20)34(30-32-26(18-39-30)29(35)36)33-28(25)23-8-4-7-22(17-23)21-5-2-1-3-6-21/h1-8,11-14,17-18,20H,9-10,15-16H2,(H,35,36)(H2,31,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546988
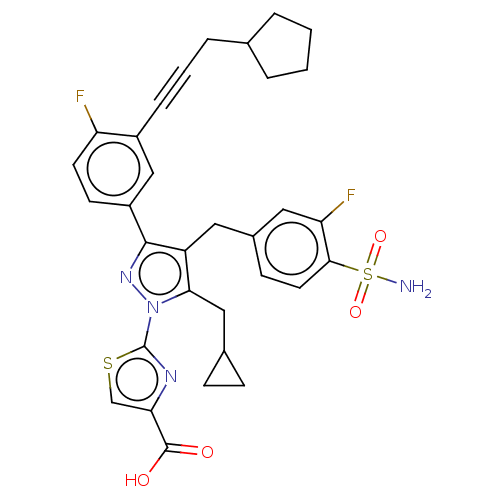
(CHEMBL4787852 | US11247971, Cmpd ID 411)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CCC2CCCC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-tagged LDHA by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250677
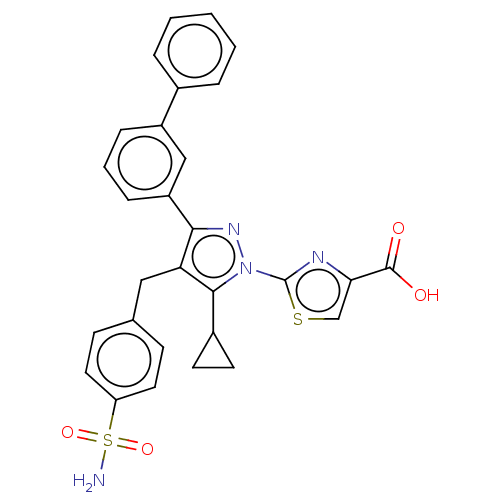
(CHEMBL4100845 | US10961200, Compound 52 | US112479...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(nn(-c3nc(cs3)C(O)=O)c2C2CC2)-c2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H24N4O4S2/c30-39(36,37)23-13-9-18(10-14-23)15-24-26(22-8-4-7-21(16-22)19-5-2-1-3-6-19)32-33(27(24)20-11-12-20)29-31-25(17-38-29)28(34)35/h1-10,13-14,16-17,20H,11-12,15H2,(H,34,35)(H2,30,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50569438
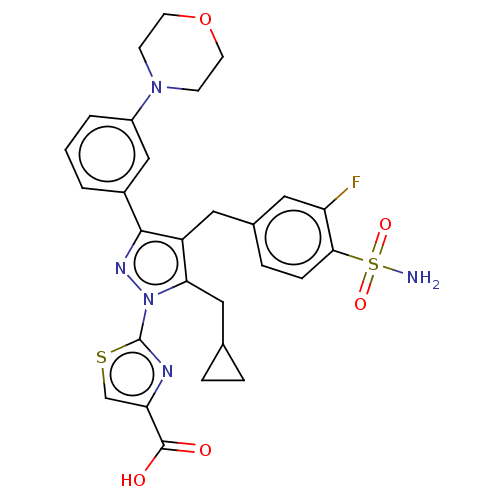
(CHEMBL4847591)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(c2)N2CCOCC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489147

(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3-((3- fluo...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCc3cccc(F)c3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C31H25F3N4O5S2/c32-21-3-1-2-19(10-21)15-43-27-14-20(7-8-23(27)33)29-22(11-18-6-9-28(24(34)12-18)45(35,41)42)26(13-17-4-5-17)38(37-29)31-36-25(16-44-31)30(39)40/h1-3,6-10,12,14,16-17H,4-5,11,13,15H2,(H,39,40)(H2,35,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489129

(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3-(p- tolyl...)Show SMILES Cc1ccc(Oc2cc(ccc2F)-c2nn(c(CC3CC3)c2Cc2ccc(c(F)c2)S(N)(=O)=O)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C31H26F2N4O5S2/c1-17-2-8-21(9-3-17)42-27-15-20(7-10-23(27)32)29-22(12-19-6-11-28(24(33)13-19)44(34,40)41)26(14-18-4-5-18)37(36-29)31-35-25(16-43-31)30(38)39/h2-3,6-11,13,15-16,18H,4-5,12,14H2,1H3,(H,38,39)(H2,34,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489104

(2-(5- (cyclopropylmethyl)- 3-(3- (phenylamino)phen...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(Nc3ccccc3)c2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C30H27N5O4S2/c31-41(38,39)24-13-11-19(12-14-24)15-25-27(16-20-9-10-20)35(30-33-26(18-40-30)29(36)37)34-28(25)21-5-4-8-23(17-21)32-22-6-2-1-3-7-22/h1-8,11-14,17-18,20,32H,9-10,15-16H2,(H,36,37)(H2,31,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LDHA (unknown origin) by SPR analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568
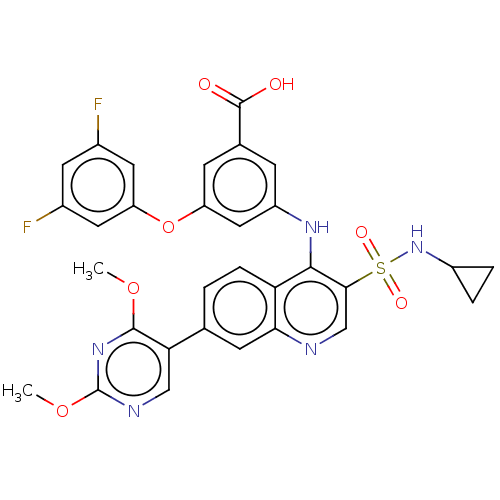
(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in absence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86138
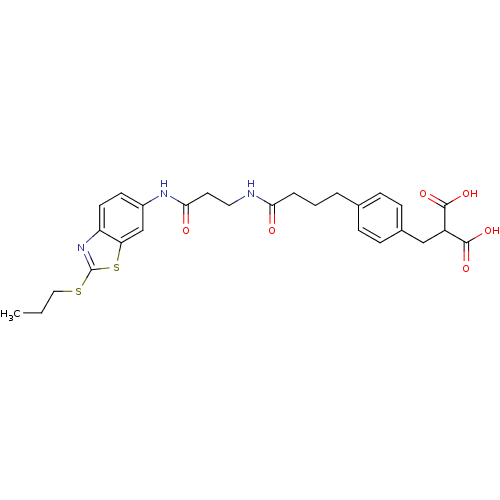
(LDHA Inhibitor, 34)Show SMILES CCCSc1nc2ccc(NC(=O)CCNC(=O)CCCc3ccc(CC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C27H31N3O6S2/c1-2-14-37-27-30-21-11-10-19(16-22(21)38-27)29-24(32)12-13-28-23(31)5-3-4-17-6-8-18(9-7-17)15-20(25(33)34)26(35)36/h6-11,16,20H,2-5,12-15H2,1H3,(H,28,31)(H,29,32)(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425797
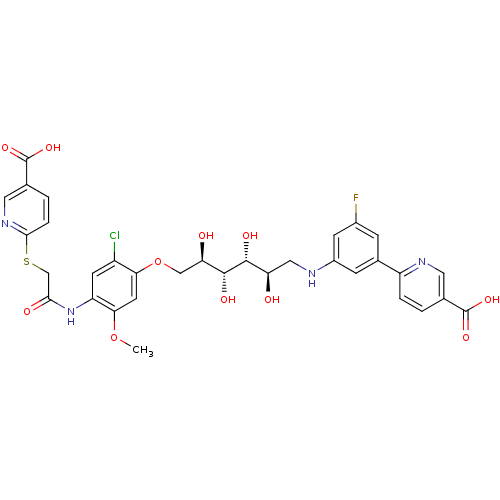
(CHEMBL2316885)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H32ClFN4O11S/c1-49-27-10-26(21(34)9-23(27)39-28(42)15-51-29-5-3-17(12-38-29)33(47)48)50-14-25(41)31(44)30(43)24(40)13-36-20-7-18(6-19(35)8-20)22-4-2-16(11-37-22)32(45)46/h2-12,24-25,30-31,36,40-41,43-44H,13-15H2,1H3,(H,39,42)(H,45,46)(H,47,48)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LDH-A by surface plasmon resonance analysis |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86131
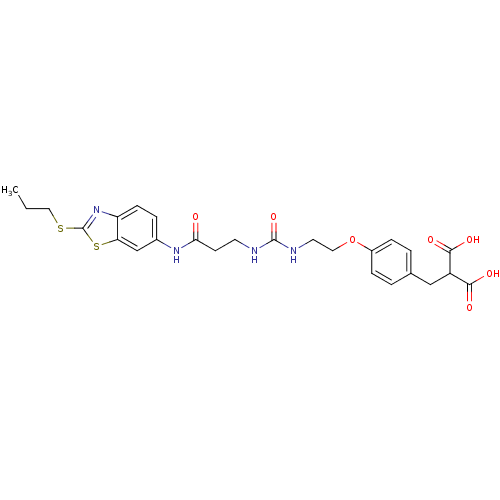
(LDHA Inhibitor, 27)Show SMILES CCCSc1nc2ccc(NC(=O)CCNC(=O)NCCOc3ccc(CC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C26H30N4O7S2/c1-2-13-38-26-30-20-8-5-17(15-21(20)39-26)29-22(31)9-10-27-25(36)28-11-12-37-18-6-3-16(4-7-18)14-19(23(32)33)24(34)35/h3-8,15,19H,2,9-14H2,1H3,(H,29,31)(H,32,33)(H,34,35)(H2,27,28,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 46 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86135
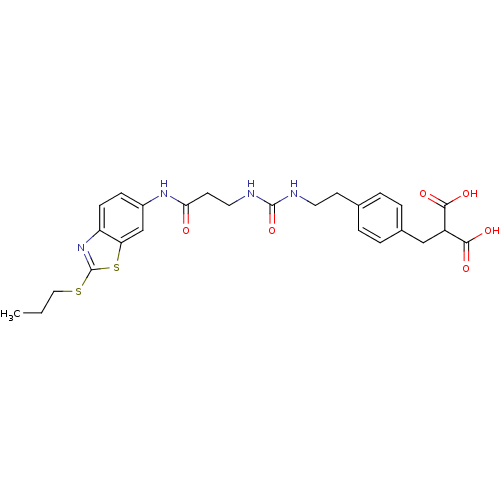
(LDHA Inhibitor, 31)Show SMILES CCCSc1nc2ccc(NC(=O)CCNC(=O)NCCc3ccc(CC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C26H30N4O6S2/c1-2-13-37-26-30-20-8-7-18(15-21(20)38-26)29-22(31)10-12-28-25(36)27-11-9-16-3-5-17(6-4-16)14-19(23(32)33)24(34)35/h3-8,15,19H,2,9-14H2,1H3,(H,29,31)(H,32,33)(H,34,35)(H2,27,28,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 60 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425798
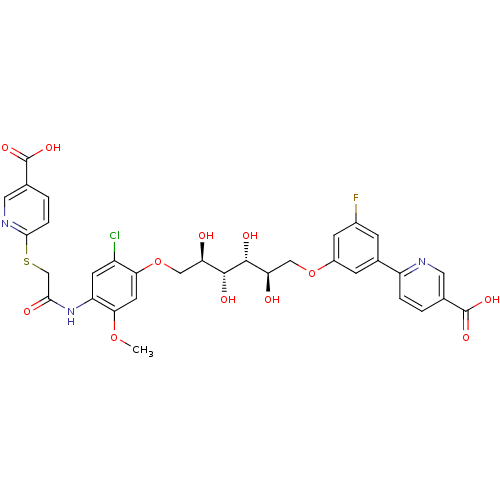
(CHEMBL2316886)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H31ClFN3O12S/c1-48-27-10-26(21(34)9-23(27)38-28(41)15-51-29-5-3-17(12-37-29)33(46)47)50-14-25(40)31(43)30(42)24(39)13-49-20-7-18(6-19(35)8-20)22-4-2-16(11-36-22)32(44)45/h2-12,24-25,30-31,39-40,42-43H,13-15H2,1H3,(H,38,41)(H,44,45)(H,46,47)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LDH-A by surface plasmon resonance analysis |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86133
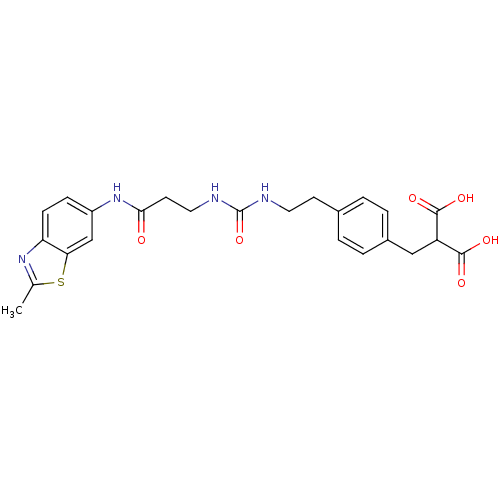
(LDHA Inhibitor, 29)Show SMILES Cc1nc2ccc(NC(=O)CCNC(=O)NCCc3ccc(CC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C24H26N4O6S/c1-14-27-19-7-6-17(13-20(19)35-14)28-21(29)9-11-26-24(34)25-10-8-15-2-4-16(5-3-15)12-18(22(30)31)23(32)33/h2-7,13,18H,8-12H2,1H3,(H,28,29)(H,30,31)(H,32,33)(H2,25,26,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 69 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86137
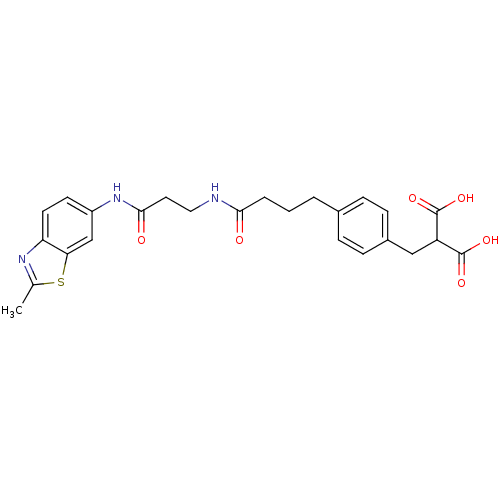
(LDHA Inhibitor, 33)Show SMILES Cc1nc2ccc(NC(=O)CCNC(=O)CCCc3ccc(CC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C25H27N3O6S/c1-15-27-20-10-9-18(14-21(20)35-15)28-23(30)11-12-26-22(29)4-2-3-16-5-7-17(8-6-16)13-19(24(31)32)25(33)34/h5-10,14,19H,2-4,11-13H2,1H3,(H,26,29)(H,28,30)(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 93 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86130
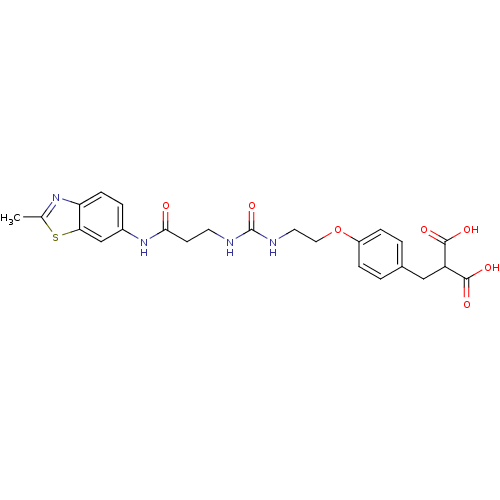
(LDHA Inhibitor, 26)Show SMILES Cc1nc2ccc(NC(=O)CCNC(=O)NCCOc3ccc(CC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C24H26N4O7S/c1-14-27-19-7-4-16(13-20(19)36-14)28-21(29)8-9-25-24(34)26-10-11-35-17-5-2-15(3-6-17)12-18(22(30)31)23(32)33/h2-7,13,18H,8-12H2,1H3,(H,28,29)(H,30,31)(H,32,33)(H2,25,26,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425796
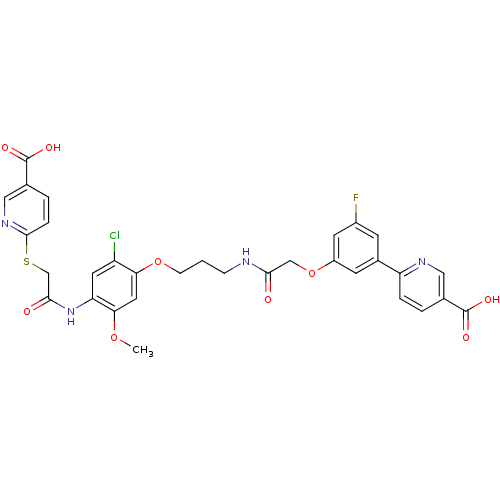
(CHEMBL2316884)Show SMILES COc1cc(OCCCNC(=O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O Show InChI InChI=1S/C32H28ClFN4O9S/c1-45-27-13-26(23(33)12-25(27)38-29(40)17-48-30-6-4-19(15-37-30)32(43)44)46-8-2-7-35-28(39)16-47-22-10-20(9-21(34)11-22)24-5-3-18(14-36-24)31(41)42/h3-6,9-15H,2,7-8,16-17H2,1H3,(H,35,39)(H,38,40)(H,41,42)(H,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LDH-A by surface plasmon resonance analysis |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86136
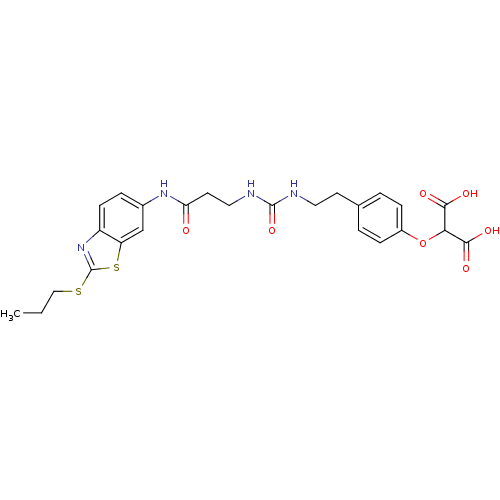
(LDHA Inhibitor, 32)Show SMILES CCCSc1nc2ccc(NC(=O)CCNC(=O)NCCc3ccc(OC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C25H28N4O7S2/c1-2-13-37-25-29-18-8-5-16(14-19(18)38-25)28-20(30)10-12-27-24(35)26-11-9-15-3-6-17(7-4-15)36-21(22(31)32)23(33)34/h3-8,14,21H,2,9-13H2,1H3,(H,28,30)(H,31,32)(H,33,34)(H2,26,27,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 180 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425794

(CHEMBL2316883)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccccn1 |r| Show InChI InChI=1S/C32H32ClFN4O9S/c1-46-27-12-26(21(33)11-23(27)38-28(41)16-48-29-4-2-3-7-35-29)47-15-25(40)31(43)30(42)24(39)14-36-20-9-18(8-19(34)10-20)22-6-5-17(13-37-22)32(44)45/h2-13,24-25,30-31,36,39-40,42-43H,14-16H2,1H3,(H,38,41)(H,44,45)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LDH-A by surface plasmon resonance analysis |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052247

(CHEMBL3318482)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C19H13BrClNO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052331
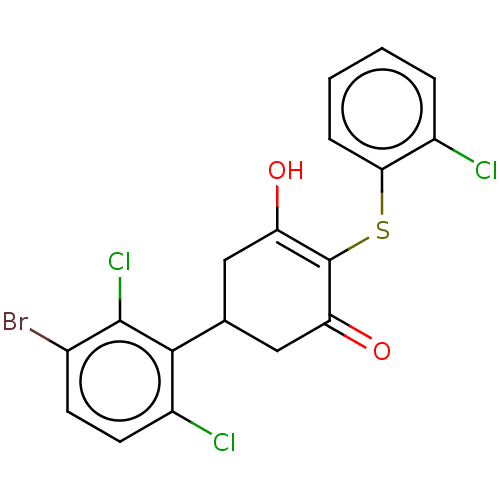
(CHEMBL3318476)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)ccc(Br)c1Cl |c:1| Show InChI InChI=1S/C18H12BrCl3O2S/c19-10-5-6-12(21)16(17(10)22)9-7-13(23)18(14(24)8-9)25-15-4-2-1-3-11(15)20/h1-6,9,23H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250674

(CHEMBL4076973 | US10961200, Compound 53 | US112479...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(O)n(nc2-c2ccc(F)c(F)c2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C20H14F2N4O5S2/c21-14-6-3-11(8-15(14)22)17-13(7-10-1-4-12(5-2-10)33(23,30)31)18(27)26(25-17)20-24-16(9-32-20)19(28)29/h1-6,8-9,27H,7H2,(H,28,29)(H2,23,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052246
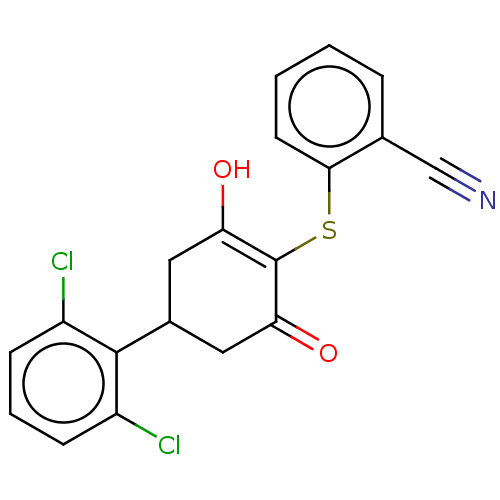
(CHEMBL3318481)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C19H13Cl2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052258

(CHEMBL3318471)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C18H13BrCl2O2S/c19-11-4-3-6-13(21)17(11)10-8-14(22)18(15(23)9-10)24-16-7-2-1-5-12(16)20/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052259
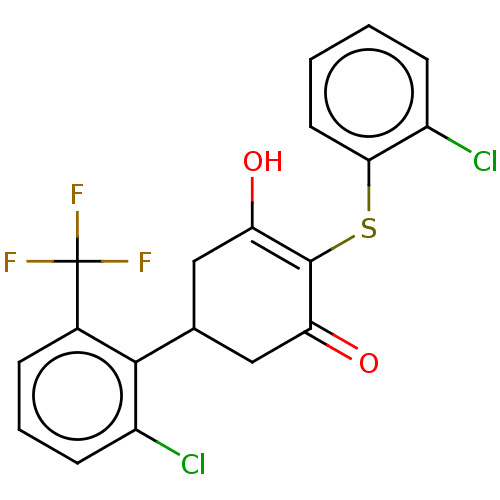
(CHEMBL3318472)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1C(F)(F)F |c:1| Show InChI InChI=1S/C19H13Cl2F3O2S/c20-12-5-1-2-7-16(12)27-18-14(25)8-10(9-15(18)26)17-11(19(22,23)24)4-3-6-13(17)21/h1-7,10,25H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
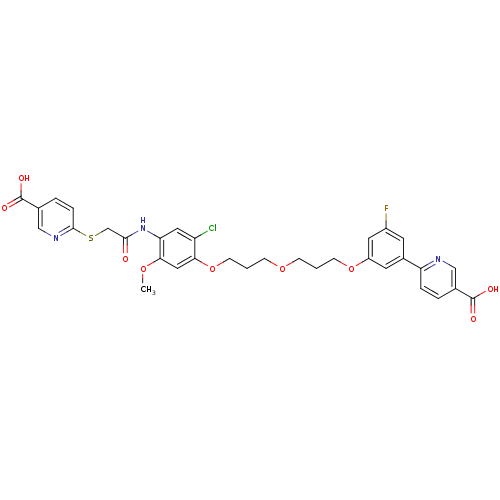
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425799
(CHEMBL2316888)Show SMILES COc1cc(OCCCOCCCOc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O Show InChI InChI=1S/C33H31ClFN3O9S/c1-44-29-16-28(25(34)15-27(29)38-30(39)19-48-31-7-5-21(18-37-31)33(42)43)47-11-3-9-45-8-2-10-46-24-13-22(12-23(35)14-24)26-6-4-20(17-36-26)32(40)41/h4-7,12-18H,2-3,8-11,19H2,1H3,(H,38,39)(H,40,41)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LDH-A by surface plasmon resonance analysis |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
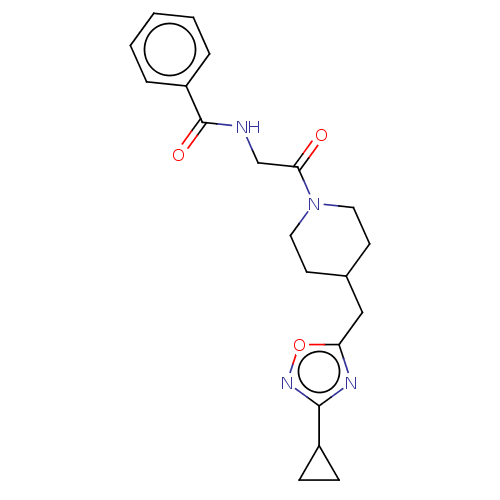
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50535762
(CHEMBL4563330)Show SMILES O=C(CNC(=O)c1ccccc1)N1CCC(Cc2nc(no2)C2CC2)CC1 Show InChI InChI=1S/C20H24N4O3/c25-18(13-21-20(26)16-4-2-1-3-5-16)24-10-8-14(9-11-24)12-17-22-19(23-27-17)15-6-7-15/h1-5,14-15H,6-13H2,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a |
The 5th Affiliated Hospital of Shenzhen University Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity to His-tagged human LDHA in presence of NAD+ by isothermal titration calorimetry |
Bioorg Med Chem Lett 29: 2459-2463 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.029
BindingDB Entry DOI: 10.7270/Q2QR51M3 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86134
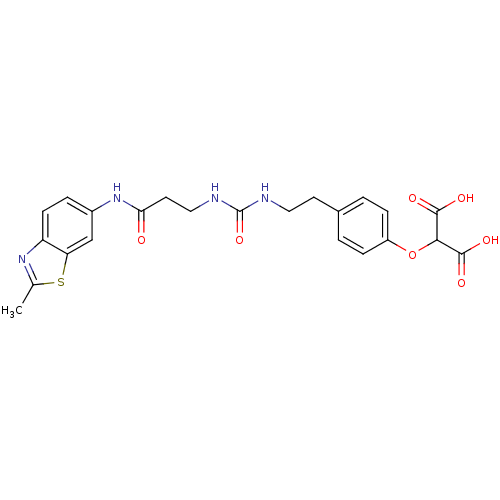
(LDHA Inhibitor, 30)Show SMILES Cc1nc2ccc(NC(=O)CCNC(=O)NCCc3ccc(OC(C(O)=O)C(O)=O)cc3)cc2s1 Show InChI InChI=1S/C23H24N4O7S/c1-13-26-17-7-4-15(12-18(17)35-13)27-19(28)9-11-25-23(33)24-10-8-14-2-5-16(6-3-14)34-20(21(29)30)22(31)32/h2-7,12,20H,8-11H2,1H3,(H,27,28)(H,29,30)(H,31,32)(H2,24,25,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052249
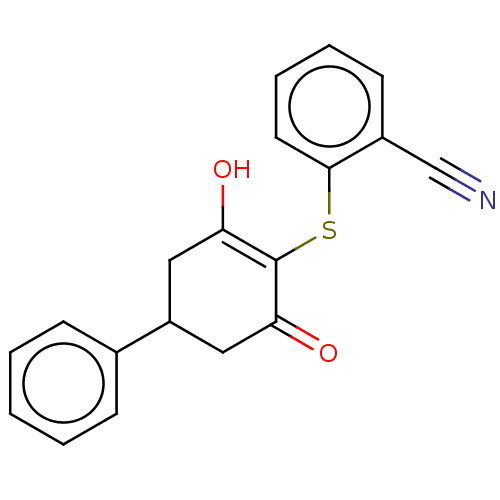
(CHEMBL3318423)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S/c20-12-14-8-4-5-9-18(14)23-19-16(21)10-15(11-17(19)22)13-6-2-1-3-7-13/h1-9,15,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052254
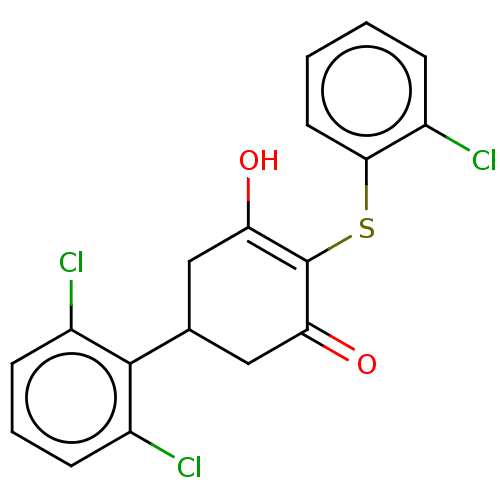
(2',6'-dichloro-4-((2-chlorophenyl)thio)-5-...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C18H13Cl3O2S/c19-11-4-1-2-7-16(11)24-18-14(22)8-10(9-15(18)23)17-12(20)5-3-6-13(17)21/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50433843
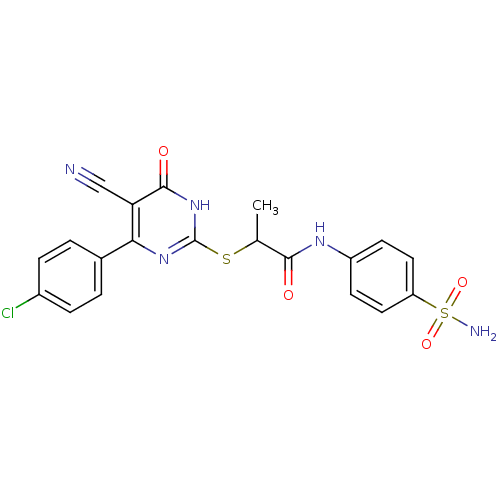
(CHEMBL2382401)Show SMILES CC(Sc1nc(-c2ccc(Cl)cc2)c(C#N)c(=O)[nH]1)C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H16ClN5O4S2/c1-11(18(27)24-14-6-8-15(9-7-14)32(23,29)30)31-20-25-17(16(10-22)19(28)26-20)12-2-4-13(21)5-3-12/h2-9,11H,1H3,(H,24,27)(H2,23,29,30)(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carboxy-terminal avi-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... |
Bioorg Med Chem Lett 23: 3186-94 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.001
BindingDB Entry DOI: 10.7270/Q29C6ZTT |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50539400
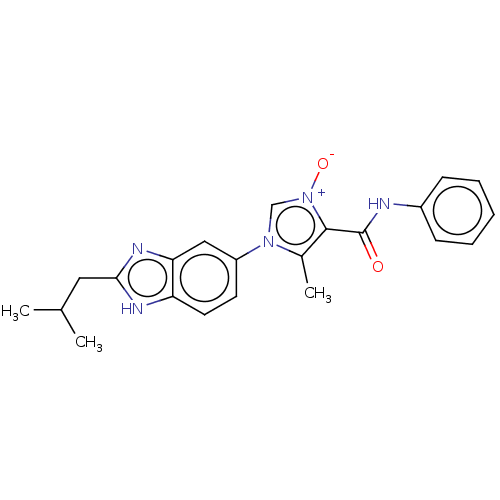
(CHEMBL4648371)Show SMILES CC(C)Cc1nc2cc(ccc2[nH]1)-n1c[n+]([O-])c(C(=O)Nc2ccccc2)c1C Show InChI InChI=1S/C22H23N5O2/c1-14(2)11-20-24-18-10-9-17(12-19(18)25-20)26-13-27(29)21(15(26)3)22(28)23-16-7-5-4-6-8-16/h4-10,12-14H,11H2,1-3H3,(H,23,28)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a |
Jilin University China-Japan Union Hospital
Curated by ChEMBL
| Assay Description
Binding affinity at human LDHA assessed as dissociation constant by isothermal titration calorimetry |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126909
BindingDB Entry DOI: 10.7270/Q2Z60SKH |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50433841
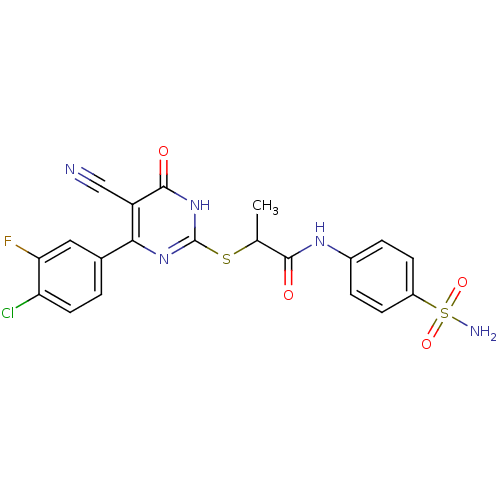
(CHEMBL2382403)Show SMILES CC(Sc1nc(-c2ccc(Cl)c(F)c2)c(C#N)c(=O)[nH]1)C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H15ClFN5O4S2/c1-10(18(28)25-12-3-5-13(6-4-12)33(24,30)31)32-20-26-17(14(9-23)19(29)27-20)11-2-7-15(21)16(22)8-11/h2-8,10H,1H3,(H,25,28)(H2,24,30,31)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carboxy-terminal avi-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... |
Bioorg Med Chem Lett 23: 3186-94 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.001
BindingDB Entry DOI: 10.7270/Q29C6ZTT |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425793
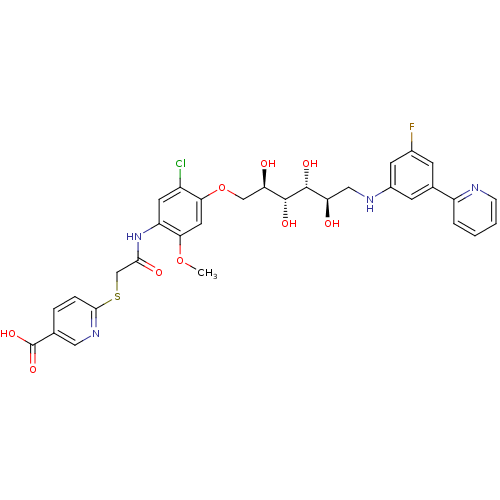
(CHEMBL2316881)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc2cc(F)cc(c2)-c2ccccn2)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C32H32ClFN4O9S/c1-46-27-12-26(21(33)11-23(27)38-28(41)16-48-29-6-5-17(13-37-29)32(44)45)47-15-25(40)31(43)30(42)24(39)14-36-20-9-18(8-19(34)10-20)22-4-2-3-7-35-22/h2-13,24-25,30-31,36,39-40,42-43H,14-16H2,1H3,(H,38,41)(H,44,45)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LDH-A by surface plasmon resonance analysis |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50433840
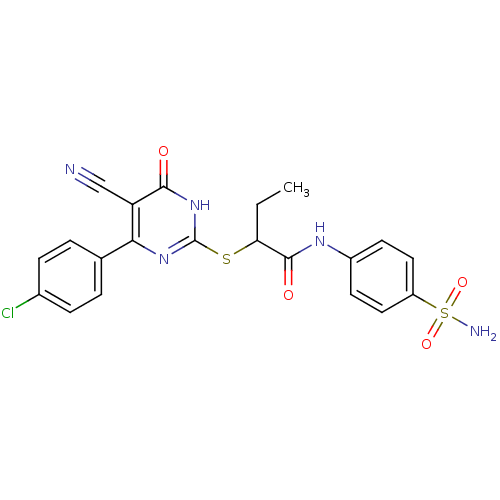
(CHEMBL2382404)Show SMILES CCC(Sc1nc(-c2ccc(Cl)cc2)c(C#N)c(=O)[nH]1)C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18ClN5O4S2/c1-2-17(20(29)25-14-7-9-15(10-8-14)33(24,30)31)32-21-26-18(16(11-23)19(28)27-21)12-3-5-13(22)6-4-12/h3-10,17H,2H2,1H3,(H,25,29)(H2,24,30,31)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carboxy-terminal avi-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... |
Bioorg Med Chem Lett 23: 3186-94 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.001
BindingDB Entry DOI: 10.7270/Q29C6ZTT |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50441120
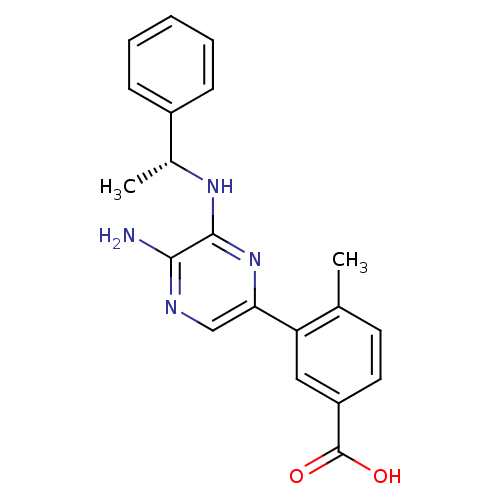
(CHEMBL2430732)Show SMILES C[C@@H](Nc1nc(cnc1N)-c1cc(ccc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O2/c1-12-8-9-15(20(25)26)10-16(12)17-11-22-18(21)19(24-17)23-13(2)14-6-4-3-5-7-14/h3-11,13H,1-2H3,(H2,21,22)(H,23,24)(H,25,26)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carboxy-terminal his-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... |
Bioorg Med Chem Lett 23: 5533-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.060
BindingDB Entry DOI: 10.7270/Q2668FM1 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50441118
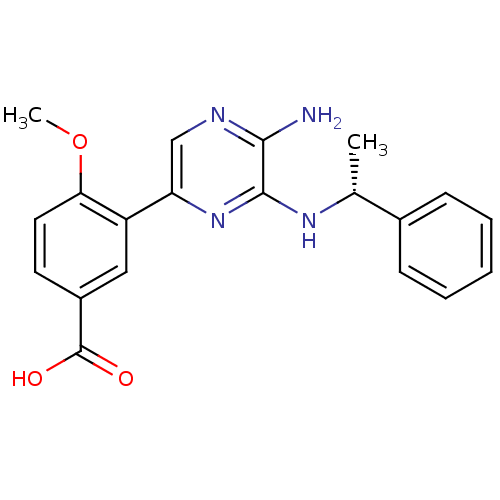
(CHEMBL2430734)Show SMILES COc1ccc(cc1-c1cnc(N)c(N[C@H](C)c2ccccc2)n1)C(O)=O |r| Show InChI InChI=1S/C20H20N4O3/c1-12(13-6-4-3-5-7-13)23-19-18(21)22-11-16(24-19)15-10-14(20(25)26)8-9-17(15)27-2/h3-12H,1-2H3,(H2,21,22)(H,23,24)(H,25,26)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carboxy-terminal his-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... |
Bioorg Med Chem Lett 23: 5533-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.060
BindingDB Entry DOI: 10.7270/Q2668FM1 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Rattus norvegicus (Rat)) | BDBM86132
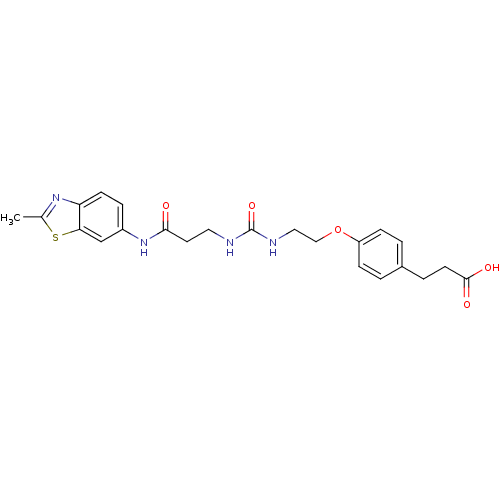
(LDHA Inhibitor, 28)Show SMILES Cc1nc2ccc(NC(=O)CCNC(=O)NCCOc3ccc(CCC(O)=O)cc3)cc2s1 Show InChI InChI=1S/C23H26N4O5S/c1-15-26-19-8-5-17(14-20(19)33-15)27-21(28)10-11-24-23(31)25-12-13-32-18-6-2-16(3-7-18)4-9-22(29)30/h2-3,5-8,14H,4,9-13H2,1H3,(H,27,28)(H,29,30)(H2,24,25,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | 7.4 | n/a |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052248

(5-hydroxy-4-((2-nitrophenyl)thio)-1,6-dihydro-[1,1...)Show SMILES OC1=C(Sc2ccccc2[N+]([O-])=O)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15NO4S/c20-15-10-13(12-6-2-1-3-7-12)11-16(21)18(15)24-17-9-5-4-8-14(17)19(22)23/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay in presence of +NADH |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50040030
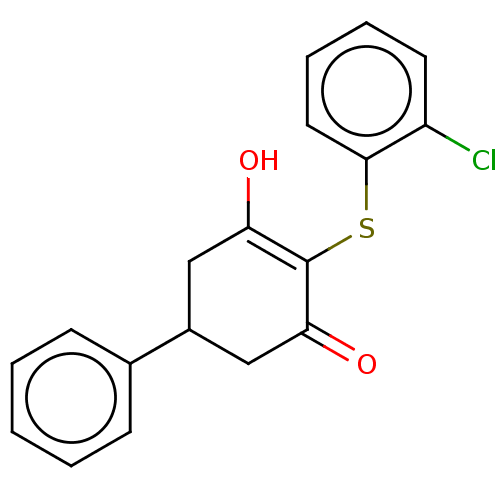
(4-((2-chlorophenyl)thio)-5-hydroxy-1,6-dihydro-[1,...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15ClO2S/c19-14-8-4-5-9-17(14)22-18-15(20)10-13(11-16(18)21)12-6-2-1-3-7-12/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by SPR assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50441115

(CHEMBL2430713)Show SMILES C[C@@H](Nc1nc(cnc1N)-c1cccc(c1)C(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H17ClN4O2/c1-11(12-5-7-15(20)8-6-12)23-18-17(21)22-10-16(24-18)13-3-2-4-14(9-13)19(25)26/h2-11H,1H3,(H2,21,22)(H,23,24)(H,25,26)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carboxy-terminal his-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... |
Bioorg Med Chem Lett 23: 5533-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.060
BindingDB Entry DOI: 10.7270/Q2668FM1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data