

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM552953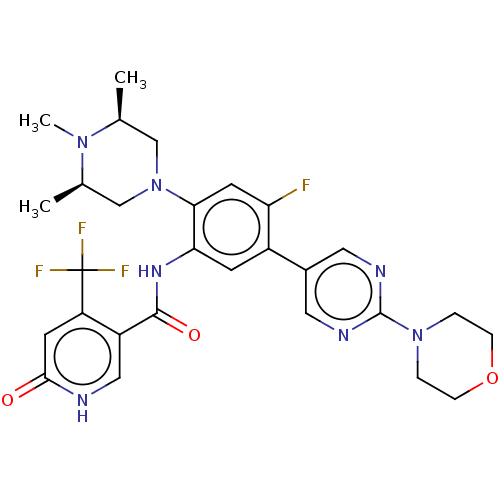 (N-[4-fluoro-5-(2- morpholin-4-ylpyrimidin- 5-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplary compounds of the application were dissolved in 100% DMSO at 10 mM, assayed fresh, and then stored at −20° C. for repeat studies and o... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM553008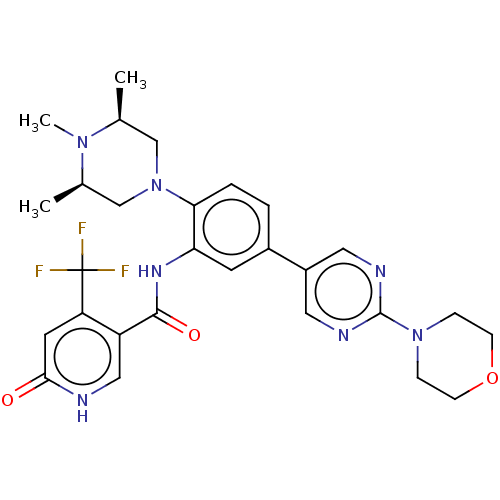 (N-(5-(2- morpholinopyrimidin-5- yl)-2-((3R,5S)-3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplary compounds of the application were dissolved in 100% DMSO at 10 mM, assayed fresh, and then stored at −20° C. for repeat studies and o... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM553032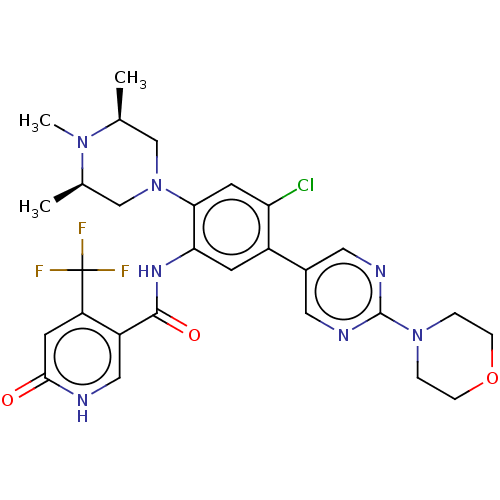 (N-[4-chloro-5-(2- morpholin-4-ylpyrimidin- 5-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplary compounds of the application were dissolved in 100% DMSO at 10 mM, assayed fresh, and then stored at −20° C. for repeat studies and o... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM553011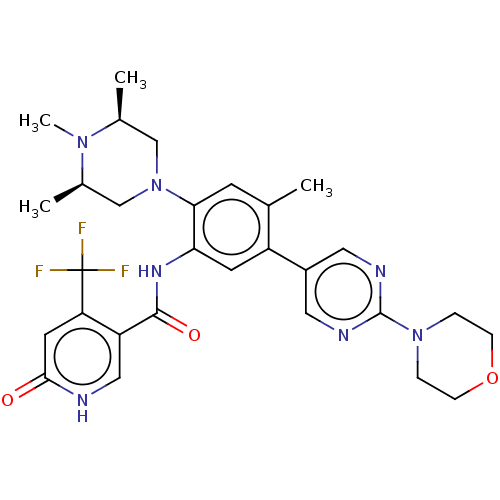 (N-[4-methyl-5-(2- morpholin-4-ylpyrimidin- 5-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplary compounds of the application were dissolved in 100% DMSO at 10 mM, assayed fresh, and then stored at −20° C. for repeat studies and o... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM553005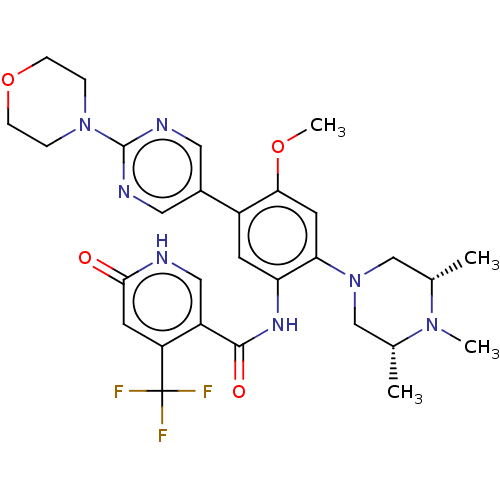 (N-[4-methoxy-5-(2- morpholin-4- ylpyrimidin-5-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >200 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplary compounds of the application were dissolved in 100% DMSO at 10 mM, assayed fresh, and then stored at −20° C. for repeat studies and o... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM50092310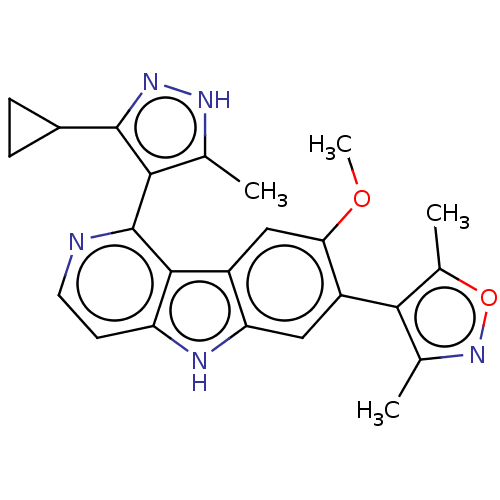 (CHEMBL3581661 | US9675697, Cpd. No. 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to biotinylated MLL1 (1566 to 1784 amino acid residues) (unknown origin) expressed in Rosetta2 cells by bio-layer interferometry met... | J Med Chem 58: 4927-39 (2015) Article DOI: 10.1021/acs.jmedchem.5b00613 BindingDB Entry DOI: 10.7270/Q2XD13FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM553009 (N-[5-(2-morpholin-4- ylpyrimidin-5-yl)-2-[rac- (3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplary compounds of the application were dissolved in 100% DMSO at 10 mM, assayed fresh, and then stored at −20° C. for repeat studies and o... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM50282115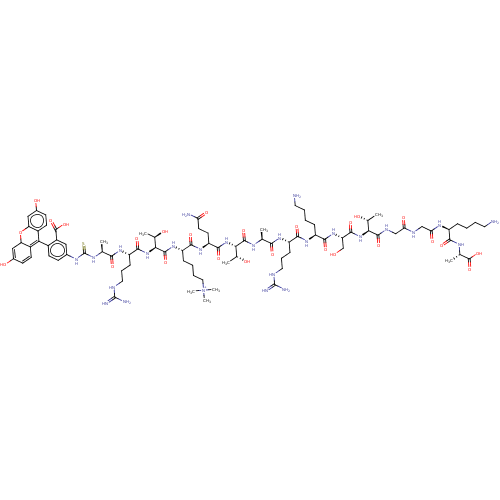 (CHEMBL4165111) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Binding affinity to 15N-labeled GST-tagged MLL1 PHD3 finger domain (1565 to 1627 residues) (unknown origin) expressed in Escherichia coli Rosetta2 BL... | Eur J Med Chem 136: 14-35 (2017) Article DOI: 10.1016/j.ejmech.2017.04.047 BindingDB Entry DOI: 10.7270/Q2F76G3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||