 Found 1287 hits of ki data for polymerid = 112
Found 1287 hits of ki data for polymerid = 112 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101499
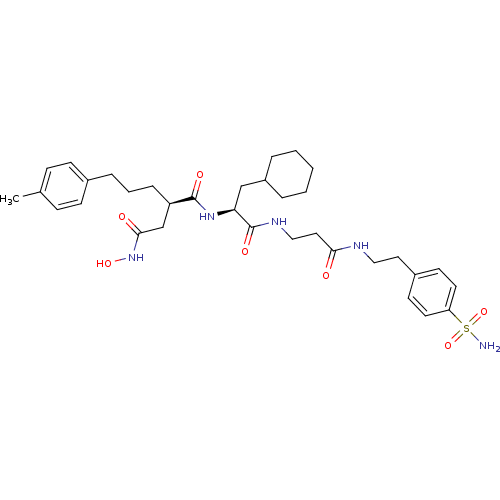
(CHEMBL74040 | N*1*-(2-Cyclohexyl-1-{2-[2-(4-sulfam...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C34H49N5O7S/c1-24-10-12-25(13-11-24)8-5-9-28(23-32(41)39-44)33(42)38-30(22-27-6-3-2-4-7-27)34(43)37-21-19-31(40)36-20-18-26-14-16-29(17-15-26)47(35,45)46/h10-17,27-28,30,44H,2-9,18-23H2,1H3,(H,36,40)(H,37,43)(H,38,42)(H,39,41)(H2,35,45,46)/t28-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101512
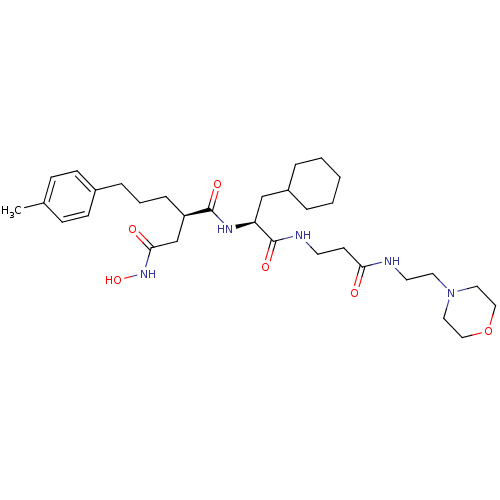
(CHEMBL306033 | N*1*-{2-Cyclohexyl-1-[2-(2-morpholi...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCN2CCOCC2)cc1 Show InChI InChI=1S/C32H51N5O6/c1-24-10-12-25(13-11-24)8-5-9-27(23-30(39)36-42)31(40)35-28(22-26-6-3-2-4-7-26)32(41)34-15-14-29(38)33-16-17-37-18-20-43-21-19-37/h10-13,26-28,42H,2-9,14-23H2,1H3,(H,33,38)(H,34,41)(H,35,40)(H,36,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101509
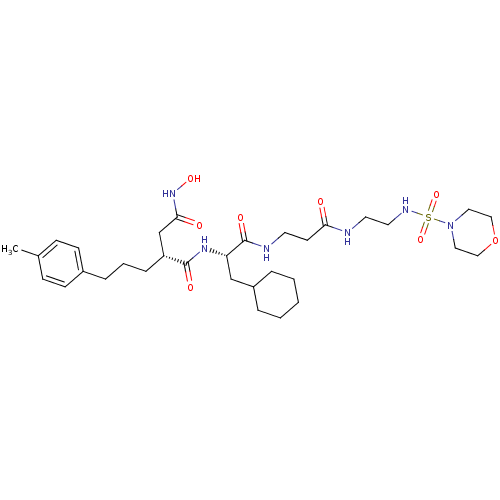
(CHEMBL306947 | N*1*-(2-Cyclohexyl-1-{2-[2-(morphol...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C32H52N6O8S/c1-24-10-12-25(13-11-24)8-5-9-27(23-30(40)37-43)31(41)36-28(22-26-6-3-2-4-7-26)32(42)34-15-14-29(39)33-16-17-35-47(44,45)38-18-20-46-21-19-38/h10-13,26-28,35,43H,2-9,14-23H2,1H3,(H,33,39)(H,34,42)(H,36,41)(H,37,40)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101508
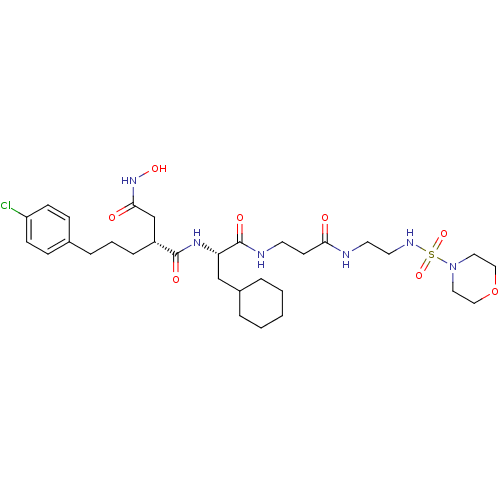
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-(2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C31H49ClN6O8S/c32-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-43)30(41)36-27(21-24-5-2-1-3-6-24)31(42)34-14-13-28(39)33-15-16-35-47(44,45)38-17-19-46-20-18-38/h9-12,24-25,27,35,43H,1-8,13-22H2,(H,33,39)(H,34,42)(H,36,41)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101505
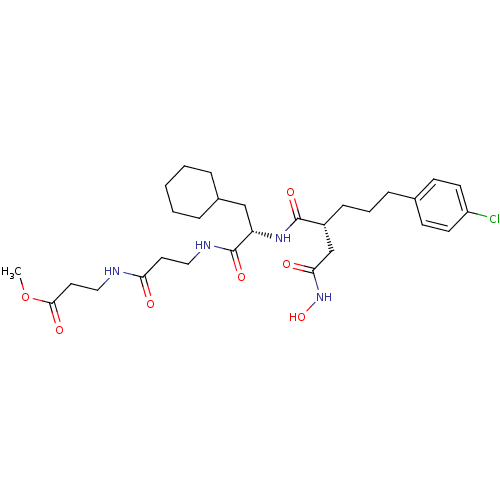
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES COC(=O)CCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C29H43ClN4O7/c1-41-27(37)15-17-31-25(35)14-16-32-29(39)24(18-21-6-3-2-4-7-21)33-28(38)22(19-26(36)34-40)9-5-8-20-10-12-23(30)13-11-20/h10-13,21-22,24,40H,2-9,14-19H2,1H3,(H,31,35)(H,32,39)(H,33,38)(H,34,36)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283704
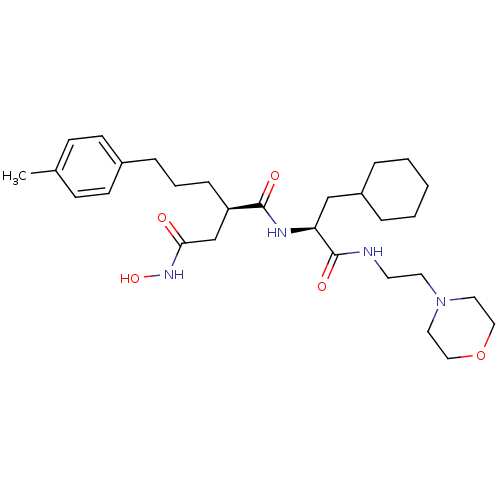
((R)-N*1*-[(S)-2-Cyclohexyl-1-(2-morpholin-4-yl-eth...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCN2CCOCC2)cc1 Show InChI InChI=1S/C29H46N4O5/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(34)32-37)28(35)31-26(20-24-6-3-2-4-7-24)29(36)30-14-15-33-16-18-38-19-17-33/h10-13,24-26,37H,2-9,14-21H2,1H3,(H,30,36)(H,31,35)(H,32,34)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283708
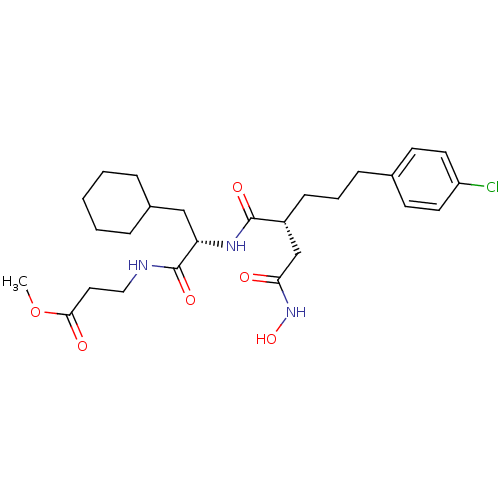
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283703
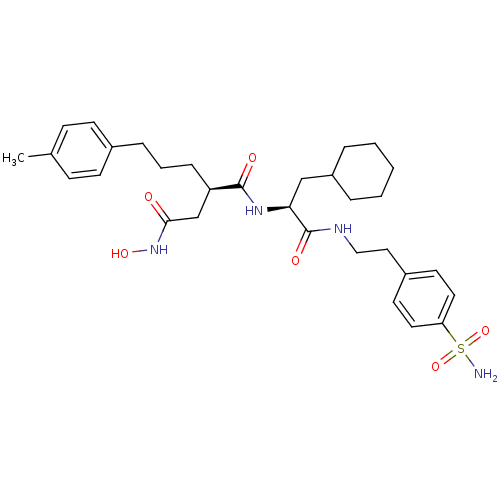
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(4-sulfamoyl-pheny...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C31H44N4O6S/c1-22-10-12-23(13-11-22)8-5-9-26(21-29(36)35-39)30(37)34-28(20-25-6-3-2-4-7-25)31(38)33-19-18-24-14-16-27(17-15-24)42(32,40)41/h10-17,25-26,28,39H,2-9,18-21H2,1H3,(H,33,38)(H,34,37)(H,35,36)(H2,32,40,41)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283701
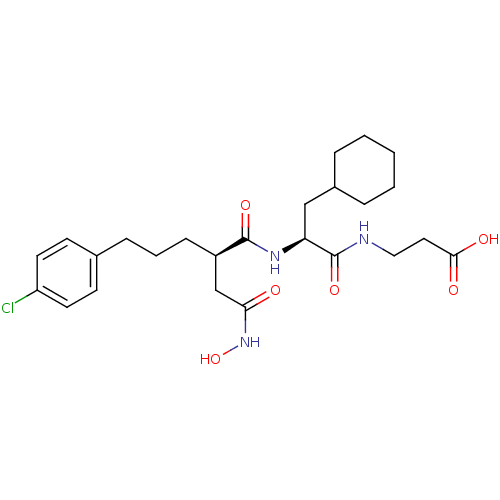
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283705
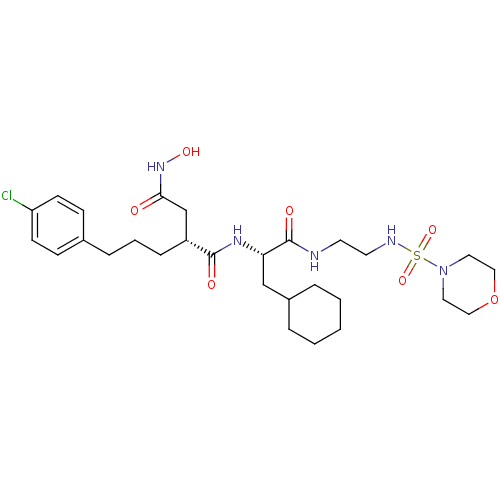
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C28H44ClN5O7S/c29-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-38)27(36)32-25(19-22-5-2-1-3-6-22)28(37)30-13-14-31-42(39,40)34-15-17-41-18-16-34/h9-12,22-23,25,31,38H,1-8,13-20H2,(H,30,37)(H,32,36)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283715
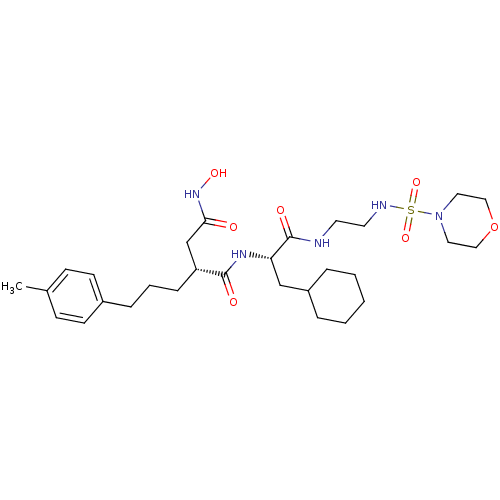
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(morpholine-4-sulf...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C29H47N5O7S/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(35)33-38)28(36)32-26(20-24-6-3-2-4-7-24)29(37)30-14-15-31-42(39,40)34-16-18-41-19-17-34/h10-13,24-26,31,38H,2-9,14-21H2,1H3,(H,30,37)(H,32,36)(H,33,35)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101516
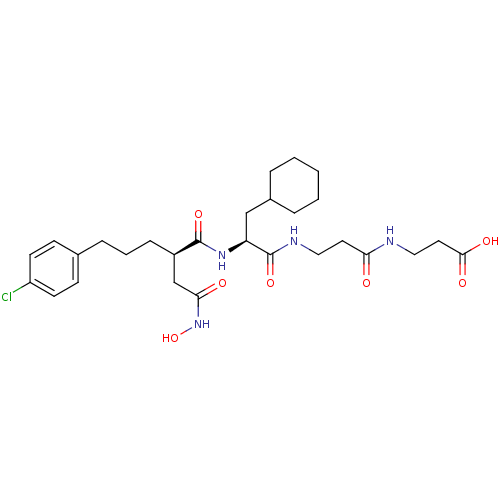
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(O)=O Show InChI InChI=1S/C28H41ClN4O7/c29-22-11-9-19(10-12-22)7-4-8-21(18-25(35)33-40)27(38)32-23(17-20-5-2-1-3-6-20)28(39)31-15-13-24(34)30-16-14-26(36)37/h9-12,20-21,23,40H,1-8,13-18H2,(H,30,34)(H,31,39)(H,32,38)(H,33,35)(H,36,37)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101520
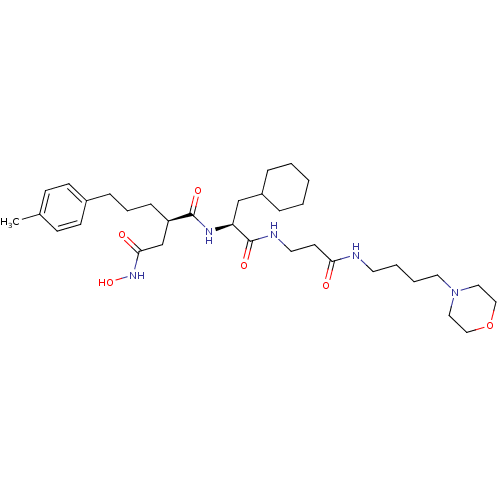
(CHEMBL77663 | N*1*-{2-Cyclohexyl-1-[2-(4-morpholin...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C34H55N5O6/c1-26-12-14-27(15-13-26)10-7-11-29(25-32(41)38-44)33(42)37-30(24-28-8-3-2-4-9-28)34(43)36-18-16-31(40)35-17-5-6-19-39-20-22-45-23-21-39/h12-15,28-30,44H,2-11,16-25H2,1H3,(H,35,40)(H,36,43)(H,37,42)(H,38,41)/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101504
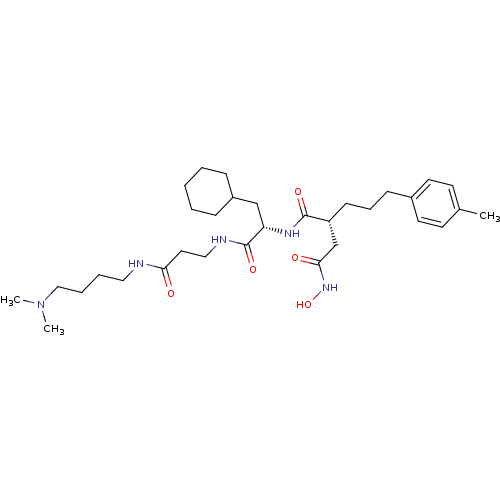
(CHEMBL77057 | N*1*-{2-Cyclohexyl-1-[2-(4-dimethyla...)Show SMILES CN(C)CCCCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(C)cc1)CC(=O)NO Show InChI InChI=1S/C32H53N5O5/c1-24-14-16-25(17-15-24)12-9-13-27(23-30(39)36-42)31(40)35-28(22-26-10-5-4-6-11-26)32(41)34-20-18-29(38)33-19-7-8-21-37(2)3/h14-17,26-28,42H,4-13,18-23H2,1-3H3,(H,33,38)(H,34,41)(H,35,40)(H,36,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283711
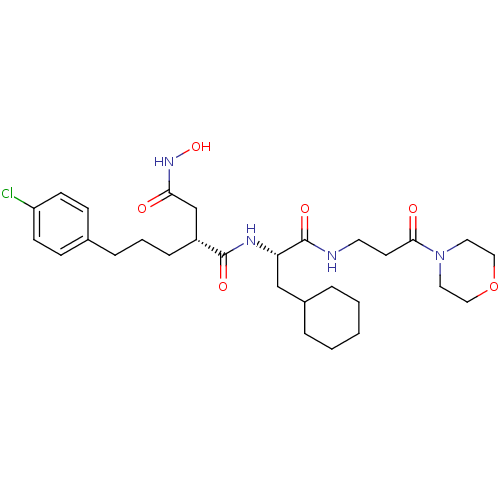
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C29H43ClN4O6/c30-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-39)28(37)32-25(19-22-5-2-1-3-6-22)29(38)31-14-13-27(36)34-15-17-40-18-16-34/h9-12,22-23,25,39H,1-8,13-20H2,(H,31,38)(H,32,37)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283710
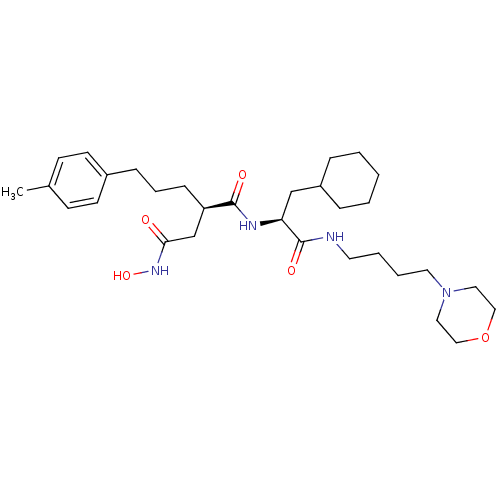
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-morpholin-4-yl-but...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C31H50N4O5/c1-24-12-14-25(15-13-24)10-7-11-27(23-29(36)34-39)30(37)33-28(22-26-8-3-2-4-9-26)31(38)32-16-5-6-17-35-18-20-40-21-19-35/h12-15,26-28,39H,2-11,16-23H2,1H3,(H,32,38)(H,33,37)(H,34,36)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283713
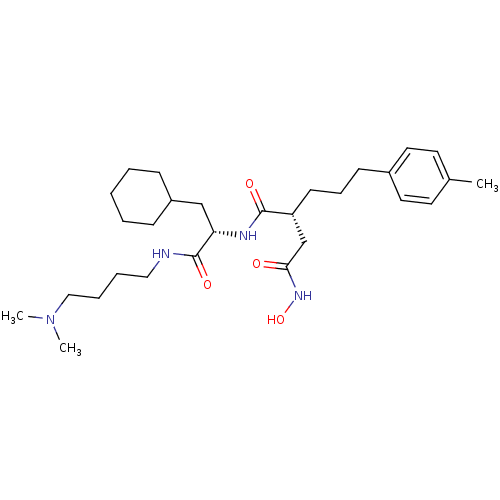
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-dimethylamino-buty...)Show SMILES CN(C)CCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(C)cc1)CC(=O)NO Show InChI InChI=1S/C29H48N4O4/c1-22-14-16-23(17-15-22)12-9-13-25(21-27(34)32-37)28(35)31-26(20-24-10-5-4-6-11-24)29(36)30-18-7-8-19-33(2)3/h14-17,24-26,37H,4-13,18-21H2,1-3H3,(H,30,36)(H,31,35)(H,32,34)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101518
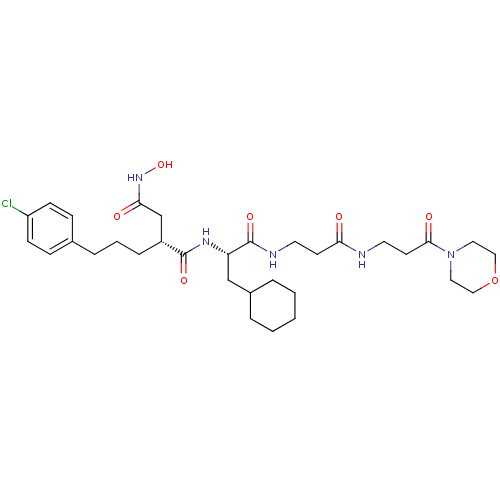
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C32H48ClN5O7/c33-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-44)31(42)36-27(21-24-5-2-1-3-6-24)32(43)35-15-13-28(39)34-16-14-30(41)38-17-19-45-20-18-38/h9-12,24-25,27,44H,1-8,13-22H2,(H,34,39)(H,35,43)(H,36,42)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101495
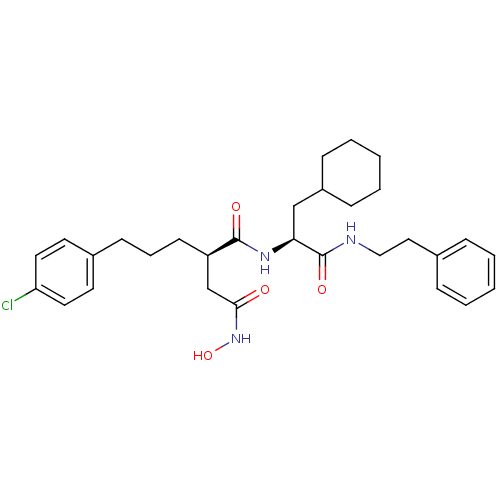
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-((S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40ClN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101503
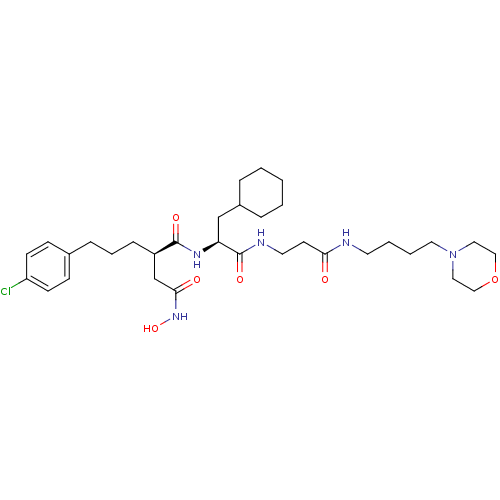
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C33H52ClN5O6/c34-28-13-11-25(12-14-28)9-6-10-27(24-31(41)38-44)32(42)37-29(23-26-7-2-1-3-8-26)33(43)36-17-15-30(40)35-16-4-5-18-39-19-21-45-22-20-39/h11-14,26-27,29,44H,1-10,15-24H2,(H,35,40)(H,36,43)(H,37,42)(H,38,41)/t27-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283702
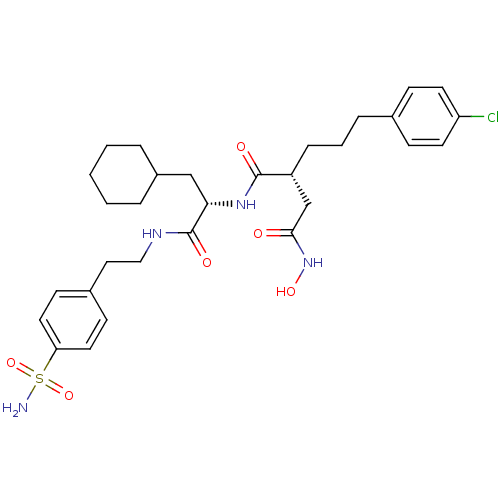
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](CCCc2ccc(Cl)cc2)CC(=O)NO)cc1 Show InChI InChI=1S/C30H41ClN4O6S/c31-25-13-9-21(10-14-25)7-4-8-24(20-28(36)35-39)29(37)34-27(19-23-5-2-1-3-6-23)30(38)33-18-17-22-11-15-26(16-12-22)42(32,40)41/h9-16,23-24,27,39H,1-8,17-20H2,(H,33,38)(H,34,37)(H,35,36)(H2,32,40,41)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101495

((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-((S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40ClN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283707
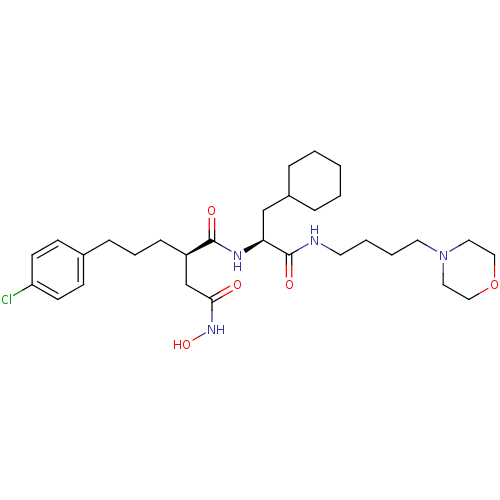
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C30H47ClN4O5/c31-26-13-11-23(12-14-26)9-6-10-25(22-28(36)34-39)29(37)33-27(21-24-7-2-1-3-8-24)30(38)32-15-4-5-16-35-17-19-40-20-18-35/h11-14,24-25,27,39H,1-10,15-22H2,(H,32,38)(H,33,37)(H,34,36)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101511
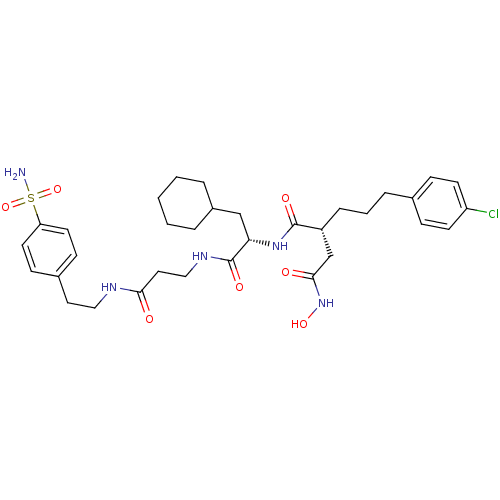
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-(2-cyclohexyl-...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](CCCc2ccc(Cl)cc2)CC(=O)NO)cc1 Show InChI InChI=1S/C33H46ClN5O7S/c34-27-13-9-23(10-14-27)7-4-8-26(22-31(41)39-44)32(42)38-29(21-25-5-2-1-3-6-25)33(43)37-20-18-30(40)36-19-17-24-11-15-28(16-12-24)47(35,45)46/h9-16,25-26,29,44H,1-8,17-22H2,(H,36,40)(H,37,43)(H,38,42)(H,39,41)(H2,35,45,46)/t26-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50563343
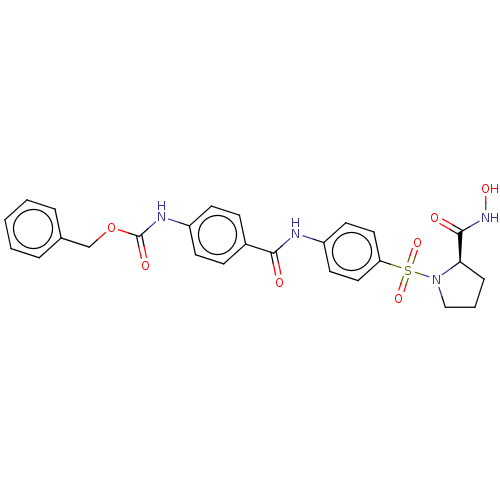
(CHEMBL4746390)Show SMILES ONC(=O)[C@H]1CCCN1S(=O)(=O)c1ccc(NC(=O)c2ccc(NC(=O)OCc3ccccc3)cc2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP2 (unknown origin) using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate incubated for 5 min followed by substrate addition and... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113260
BindingDB Entry DOI: 10.7270/Q2WW7NF0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101497
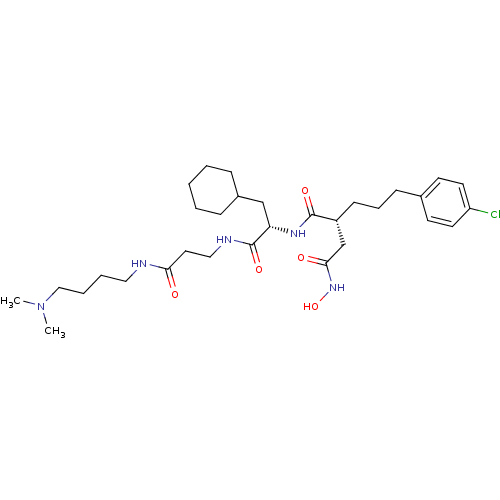
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES CN(C)CCCCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C31H50ClN5O5/c1-37(2)20-7-6-18-33-28(38)17-19-34-31(41)27(21-24-9-4-3-5-10-24)35-30(40)25(22-29(39)36-42)12-8-11-23-13-15-26(32)16-14-23/h13-16,24-25,27,42H,3-12,17-22H2,1-2H3,(H,33,38)(H,34,41)(H,35,40)(H,36,39)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283714
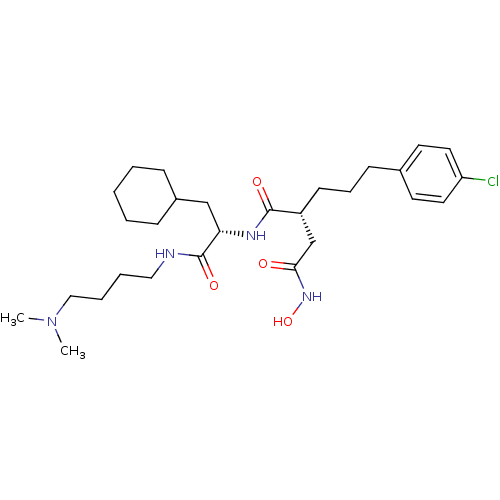
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES CN(C)CCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C28H45ClN4O4/c1-33(2)18-7-6-17-30-28(36)25(19-22-9-4-3-5-10-22)31-27(35)23(20-26(34)32-37)12-8-11-21-13-15-24(29)16-14-21/h13-16,22-23,25,37H,3-12,17-20H2,1-2H3,(H,30,36)(H,31,35)(H,32,34)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-2 |
J Med Chem 47: 325-36 (2004)
Article DOI: 10.1021/jm0308491
BindingDB Entry DOI: 10.7270/Q27080V1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101526
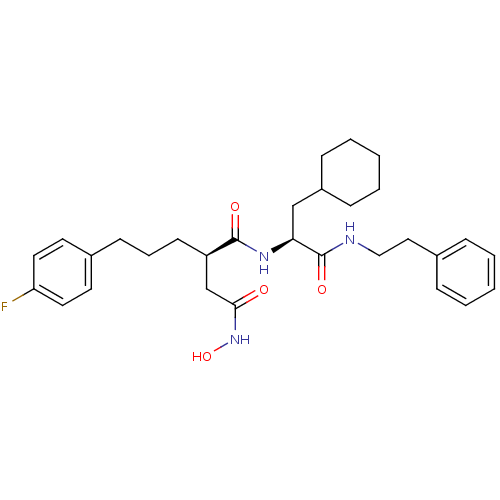
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(F)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40FN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101530
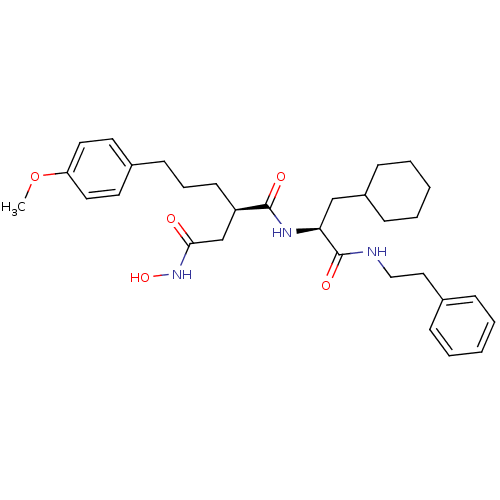
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES COc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O5/c1-39-27-17-15-24(16-18-27)13-8-14-26(22-29(35)34-38)30(36)33-28(21-25-11-6-3-7-12-25)31(37)32-20-19-23-9-4-2-5-10-23/h2,4-5,9-10,15-18,25-26,28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101526

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(F)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40FN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101530

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES COc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O5/c1-39-27-17-15-24(16-18-27)13-8-14-26(22-29(35)34-38)30(36)33-28(21-25-11-6-3-7-12-25)31(37)32-20-19-23-9-4-2-5-10-23/h2,4-5,9-10,15-18,25-26,28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492
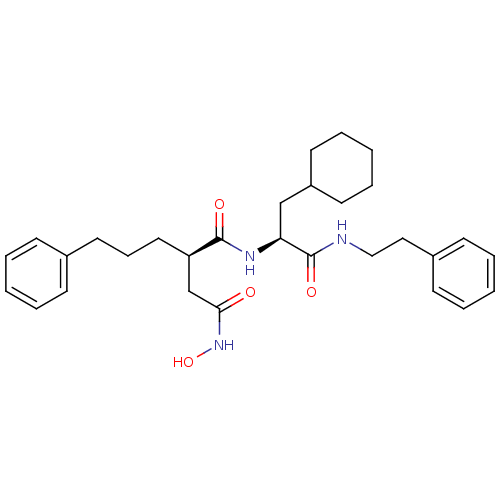
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101513

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O4/c1-23-15-17-25(18-16-23)13-8-14-27(22-29(35)34-38)30(36)33-28(21-26-11-6-3-7-12-26)31(37)32-20-19-24-9-4-2-5-10-24/h2,4-5,9-10,15-18,26-28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101513

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O4/c1-23-15-17-25(18-16-23)13-8-14-27(22-29(35)34-38)30(36)33-28(21-26-11-6-3-7-12-26)31(37)32-20-19-24-9-4-2-5-10-24/h2,4-5,9-10,15-18,26-28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 (MMP-2). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (Matrix metalloprotease-2) |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50563342
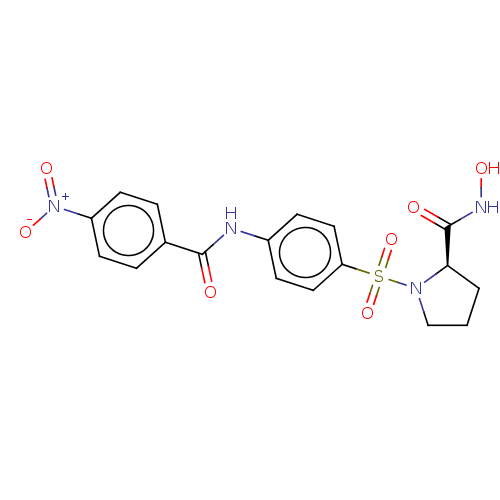
(CHEMBL4797032)Show SMILES ONC(=O)[C@H]1CCCN1S(=O)(=O)c1ccc(NC(=O)c2ccc(cc2)[N+]([O-])=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP2 (unknown origin) using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate incubated for 5 min followed by substrate addition and... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113260
BindingDB Entry DOI: 10.7270/Q2WW7NF0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101494
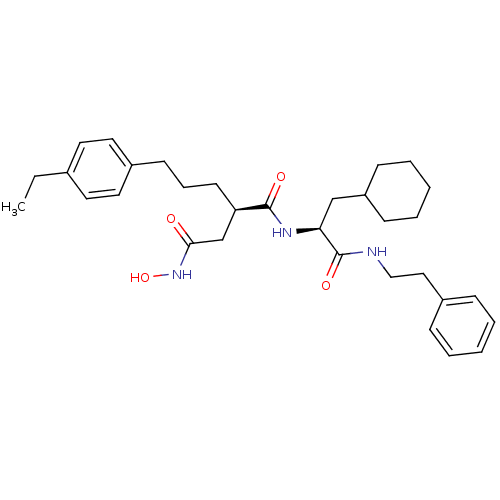
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES CCc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C32H45N3O4/c1-2-24-16-18-26(19-17-24)14-9-15-28(23-30(36)35-39)31(37)34-29(22-27-12-7-4-8-13-27)32(38)33-21-20-25-10-5-3-6-11-25/h3,5-6,10-11,16-19,27-29,39H,2,4,7-9,12-15,20-23H2,1H3,(H,33,38)(H,34,37)(H,35,36)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101494

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES CCc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C32H45N3O4/c1-2-24-16-18-26(19-17-24)14-9-15-28(23-30(36)35-39)31(37)34-29(22-27-12-7-4-8-13-27)32(38)33-21-20-25-10-5-3-6-11-25/h3,5-6,10-11,16-19,27-29,39H,2,4,7-9,12-15,20-23H2,1H3,(H,33,38)(H,34,37)(H,35,36)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11870

(4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H18ClNO6S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(23,24)18(17(21)20-22)9-11-25-12-10-18/h1-8,22H,9-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11873
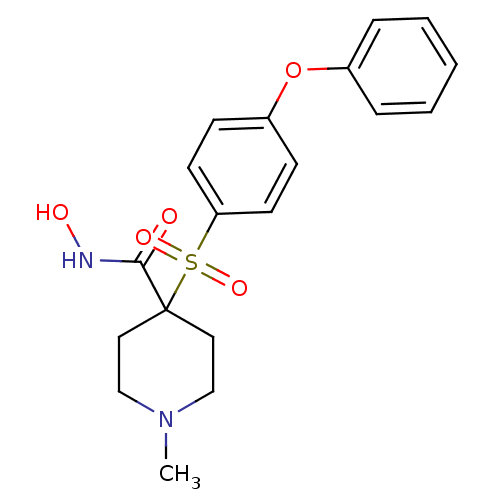
(N-Hydroxy-1-methyl-4-{[4-(phenoxyphenyl]sulfonyl}-...)Show SMILES CN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O5S/c1-21-13-11-19(12-14-21,18(22)20-23)27(24,25)17-9-7-16(8-10-17)26-15-5-3-2-4-6-15/h2-10,23H,11-14H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11889
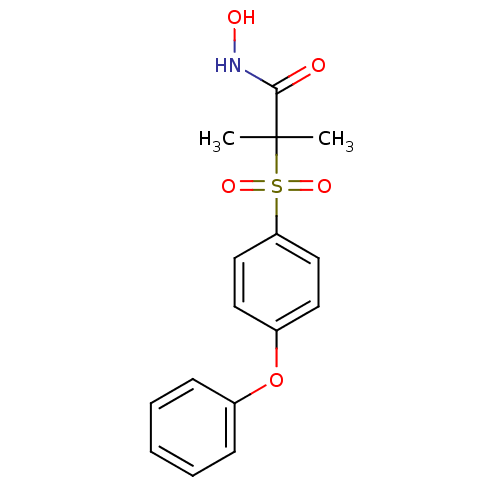
(N-Hydroxy-2-methyl-2-[(4-phenoxyphenyl)sulfonyl]-p...)Show InChI InChI=1S/C16H17NO5S/c1-16(2,15(18)17-19)23(20,21)14-10-8-13(9-11-14)22-12-6-4-3-5-7-12/h3-11,19H,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
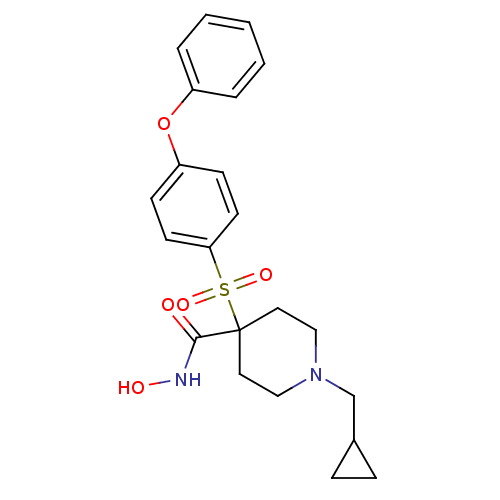
(Homo sapiens (Human)) | BDBM11876
(1-(Cyclopropylmethyl)-N-hydroxy-4-[(4-phenoxypheny...)Show SMILES ONC(=O)C1(CCN(CC2CC2)CC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C22H26N2O5S/c25-21(23-26)22(12-14-24(15-13-22)16-17-6-7-17)30(27,28)20-10-8-19(9-11-20)29-18-4-2-1-3-5-18/h1-5,8-11,17,26H,6-7,12-16H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
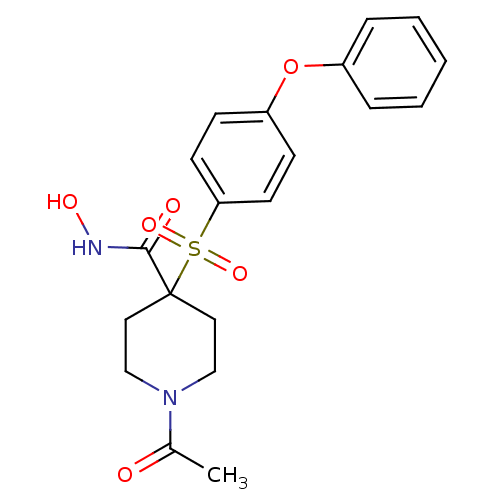
(Homo sapiens (Human)) | BDBM11878
(1-Acetyl-N-hydroxy-4-{[4-(phenoxyphenyl]sulfonyl}-...)Show SMILES CC(=O)N1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C20H22N2O6S/c1-15(23)22-13-11-20(12-14-22,19(24)21-25)29(26,27)18-9-7-17(8-10-18)28-16-5-3-2-4-6-16/h2-10,25H,11-14H2,1H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
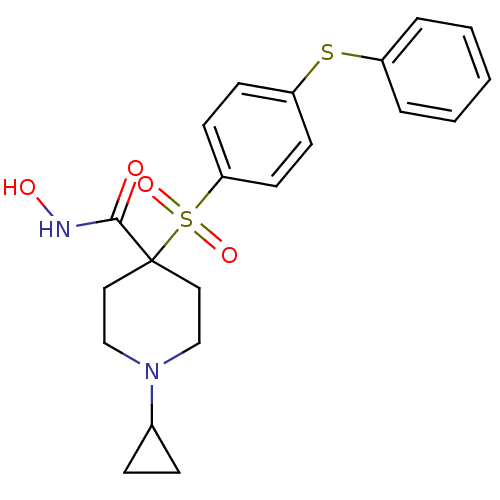
(Homo sapiens (Human)) | BDBM11883
(1-Cyclopropyl-N-hydroxy-4-{[4-(phenylthio)phenyl]-...)Show SMILES ONC(=O)C1(CCN(CC1)C1CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H24N2O4S2/c24-20(22-25)21(12-14-23(15-13-21)16-6-7-16)29(26,27)19-10-8-18(9-11-19)28-17-4-2-1-3-5-17/h1-5,8-11,16,25H,6-7,12-15H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11874
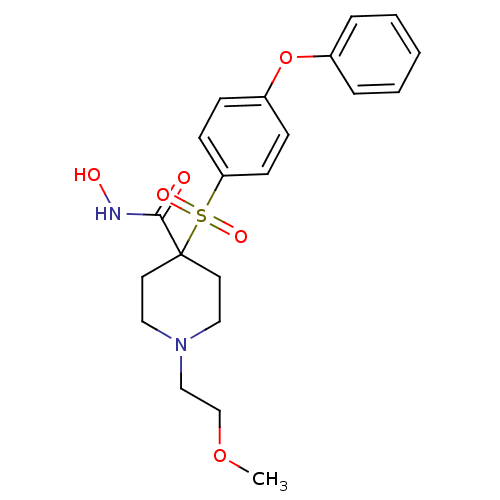
(N-Hydroxy-1-(2-methoxyethyl)-4-{[4-(phenoxyphenyl]...)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H26N2O6S/c1-28-16-15-23-13-11-21(12-14-23,20(24)22-25)30(26,27)19-9-7-18(8-10-19)29-17-5-3-2-4-6-17/h2-10,25H,11-16H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11877
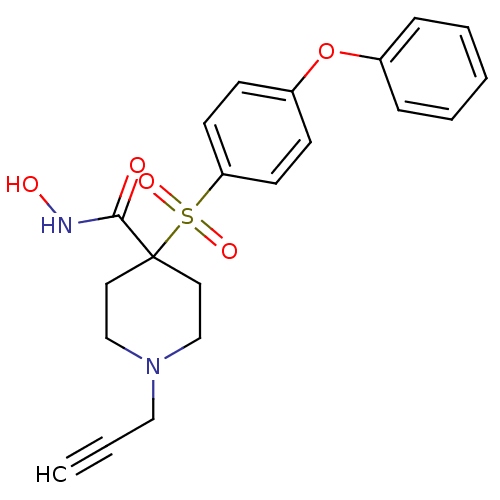
(N-Hydroxy-4-{[4-(phenoxyphenyl]sulfonyl}-1- (2-pro...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O5S/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data