 Found 1053 hits of ki data for polymerid = 114
Found 1053 hits of ki data for polymerid = 114 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP-3). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-3 |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11875
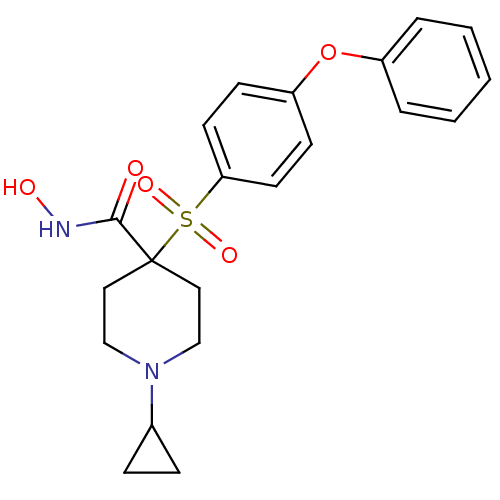
(1-Cyclopropyl-N-hydroxy-4-{[4-(phenoxyphenyl]-sulf...)Show SMILES ONC(=O)C1(CCN(CC1)C1CC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H24N2O5S/c24-20(22-25)21(12-14-23(15-13-21)16-6-7-16)29(26,27)19-10-8-18(9-11-19)28-17-4-2-1-3-5-17/h1-5,8-11,16,25H,6-7,12-15H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11874
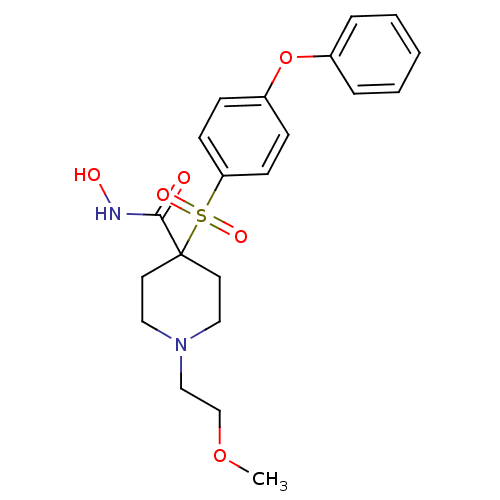
(N-Hydroxy-1-(2-methoxyethyl)-4-{[4-(phenoxyphenyl]...)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H26N2O6S/c1-28-16-15-23-13-11-21(12-14-23,20(24)22-25)30(26,27)19-9-7-18(8-10-19)29-17-5-3-2-4-6-17/h2-10,25H,11-16H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
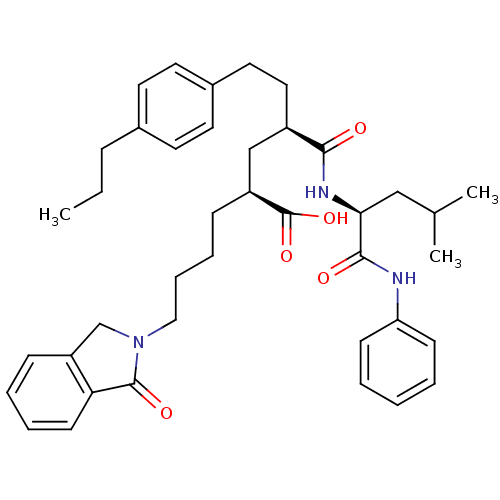
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50141575
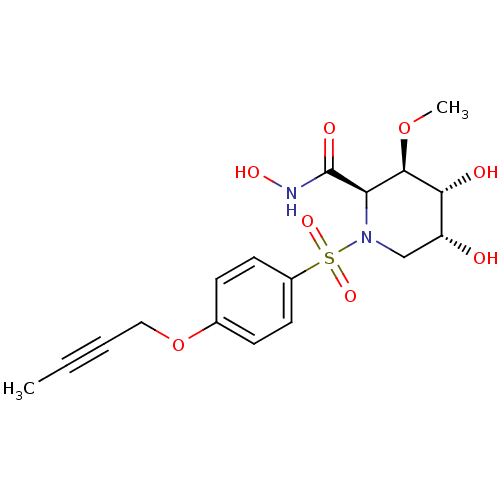
((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CO[C@H]1[C@H](O)[C@H](O)CN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCC#CC)cc1 Show InChI InChI=1S/C17H22N2O8S/c1-3-4-9-27-11-5-7-12(8-6-11)28(24,25)19-10-13(20)15(21)16(26-2)14(19)17(22)18-23/h5-8,13-16,20-21,23H,9-10H2,1-2H3,(H,18,22)/t13-,14-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 14: 1569-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.091
BindingDB Entry DOI: 10.7270/Q2KS6QZB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109621
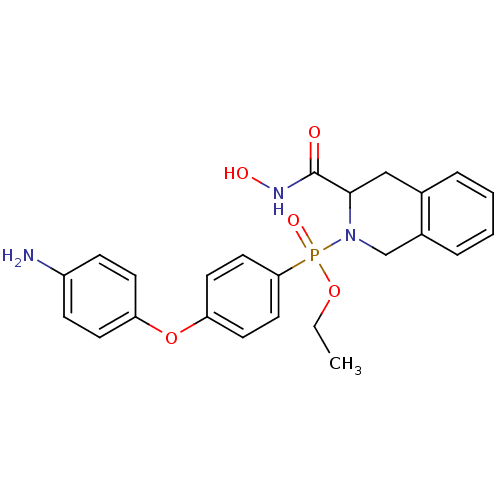
(CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccc(N)cc2)cc1 Show InChI InChI=1S/C24H26N3O5P/c1-2-31-33(30,22-13-11-21(12-14-22)32-20-9-7-19(25)8-10-20)27-16-18-6-4-3-5-17(18)15-23(27)24(28)26-29/h3-14,23,29H,2,15-16,25H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2737-40 (2003)
BindingDB Entry DOI: 10.7270/Q2PC31SG |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109635
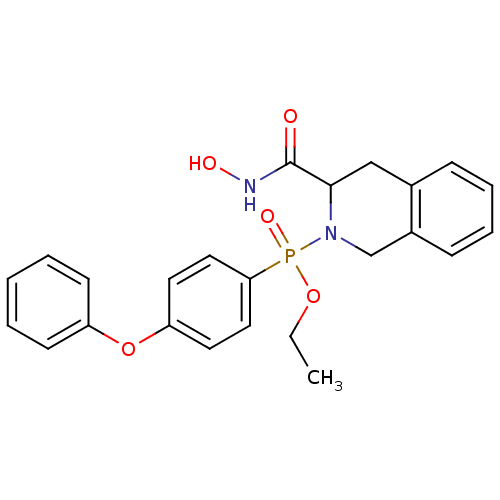
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283701
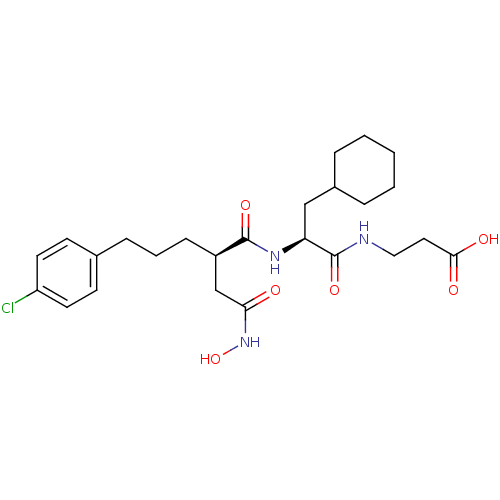
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101516
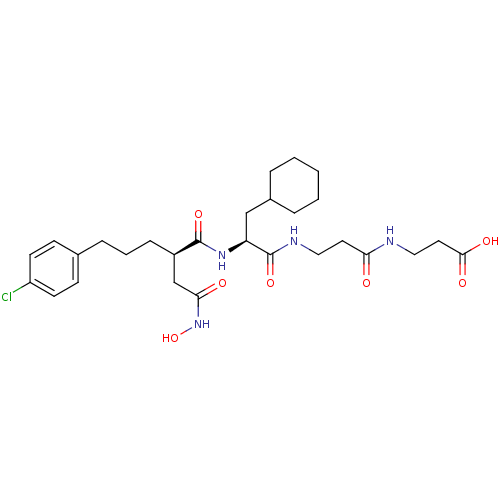
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(O)=O Show InChI InChI=1S/C28H41ClN4O7/c29-22-11-9-19(10-12-22)7-4-8-21(18-25(35)33-40)27(38)32-23(17-20-5-2-1-3-6-20)28(39)31-15-13-24(34)30-16-14-26(36)37/h9-12,20-21,23,40H,1-8,13-18H2,(H,30,34)(H,31,39)(H,32,38)(H,33,35)(H,36,37)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50183784
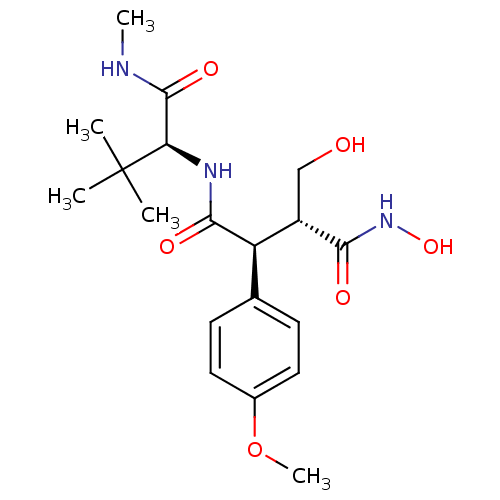
((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant MMP3 |
Bioorg Med Chem Lett 16: 2632-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.042
BindingDB Entry DOI: 10.7270/Q2JS9Q12 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50183784

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101505
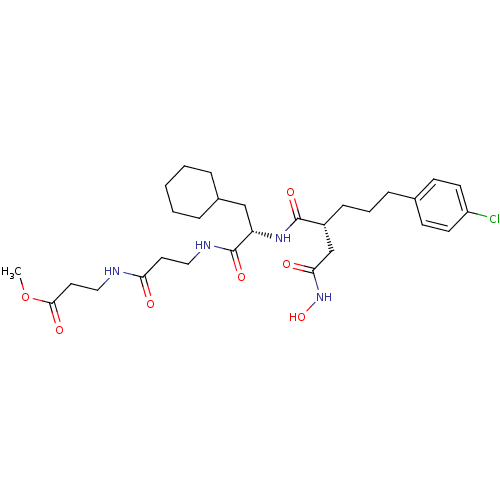
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES COC(=O)CCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C29H43ClN4O7/c1-41-27(37)15-17-31-25(35)14-16-32-29(39)24(18-21-6-3-2-4-7-21)33-28(38)22(19-26(36)34-40)9-5-8-20-10-12-23(30)13-11-20/h10-13,21-22,24,40H,2-9,14-19H2,1H3,(H,31,35)(H,32,39)(H,33,38)(H,34,36)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283708
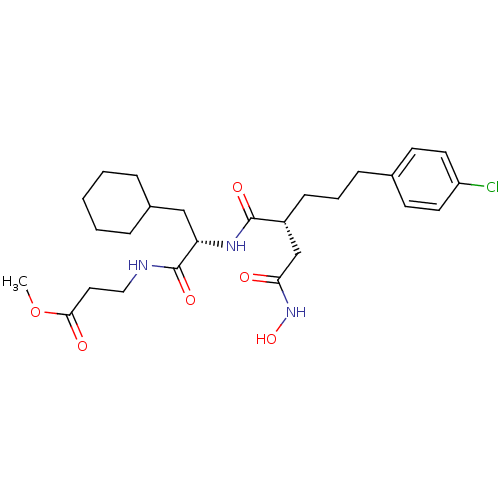
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101518
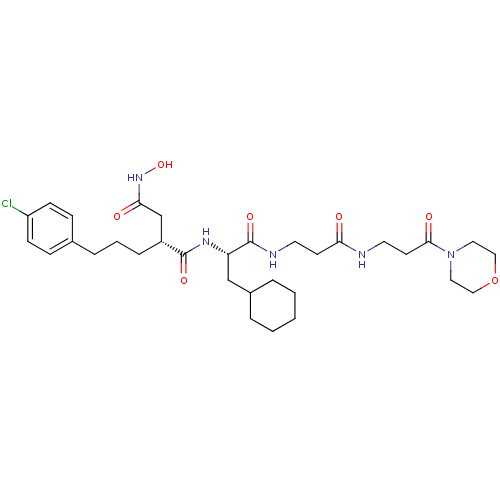
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C32H48ClN5O7/c33-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-44)31(42)36-27(21-24-5-2-1-3-6-24)32(43)35-15-13-28(39)34-16-14-30(41)38-17-19-45-20-18-38/h9-12,24-25,27,44H,1-8,13-22H2,(H,34,39)(H,35,43)(H,36,42)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288674
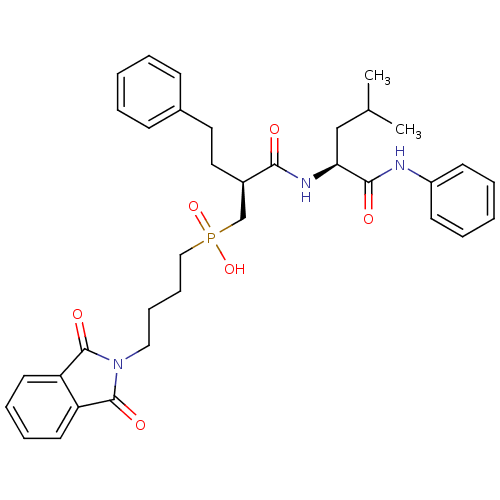
(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073839
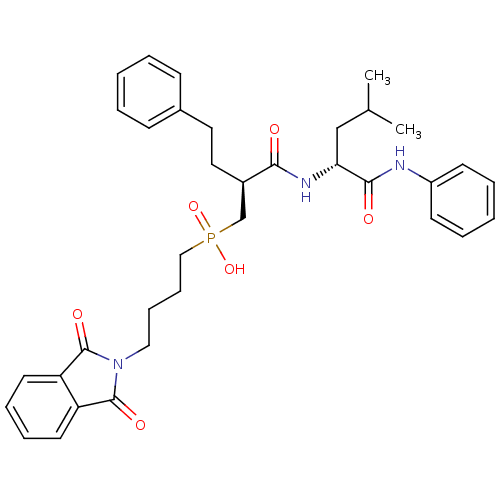
(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283711
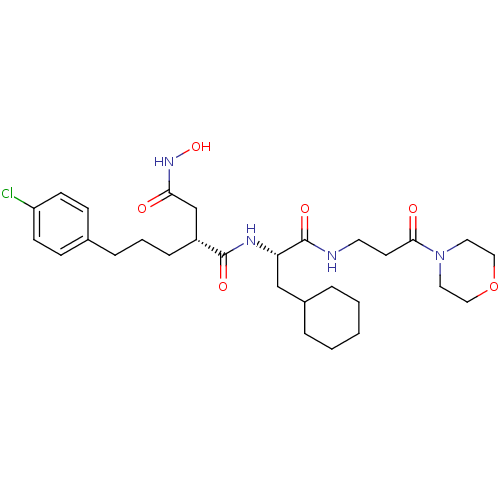
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C29H43ClN4O6/c30-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-39)28(37)32-25(19-22-5-2-1-3-6-22)29(38)31-14-13-27(36)34-15-17-40-18-16-34/h9-12,22-23,25,39H,1-8,13-20H2,(H,31,38)(H,32,37)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131386
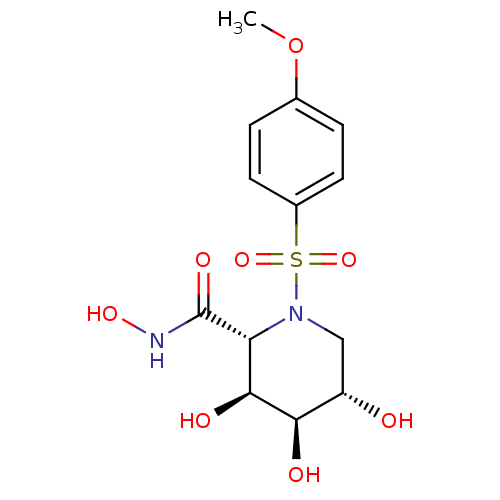
((2R,3S,4R,5S)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@H](O)[C@@H](O)[C@@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10+,11+,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2737-40 (2003)
BindingDB Entry DOI: 10.7270/Q2PC31SG |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283705
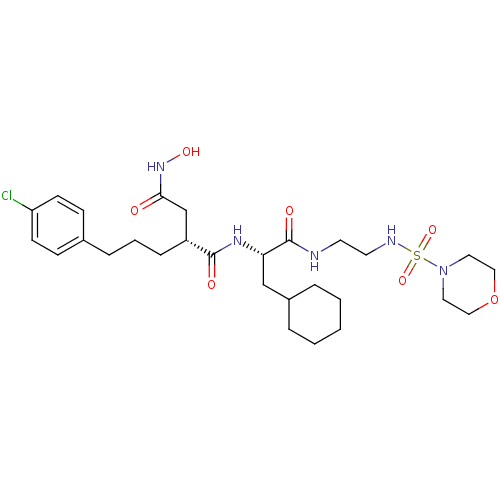
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C28H44ClN5O7S/c29-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-38)27(36)32-25(19-22-5-2-1-3-6-22)28(37)30-13-14-31-42(39,40)34-15-17-41-18-16-34/h9-12,22-23,25,31,38H,1-8,13-20H2,(H,30,37)(H,32,36)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101508
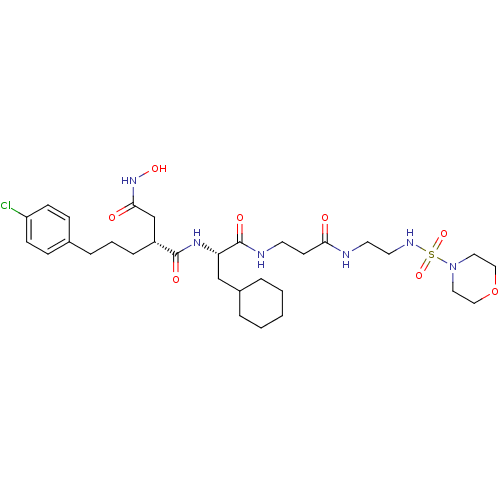
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-(2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C31H49ClN6O8S/c32-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-43)30(41)36-27(21-24-5-2-1-3-6-24)31(42)34-14-13-28(39)33-15-16-35-47(44,45)38-17-19-46-20-18-38/h9-12,24-25,27,35,43H,1-8,13-22H2,(H,33,39)(H,34,42)(H,36,41)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131382
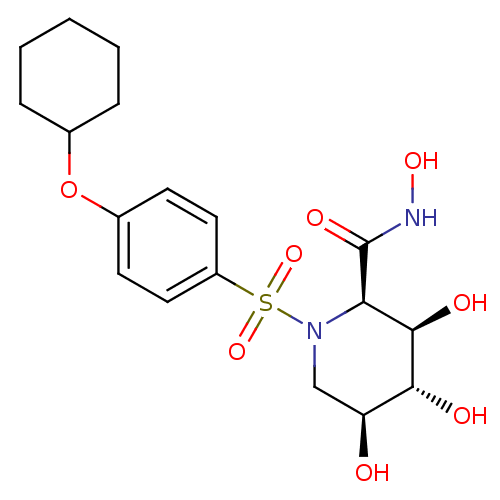
((2R,3R,4R,5S)-1-(4-Cyclohexyloxy-benzenesulfonyl)-...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(OC2CCCCC2)cc1 Show InChI InChI=1S/C18H26N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h6-9,11,14-17,21-23,25H,1-5,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2737-40 (2003)
BindingDB Entry DOI: 10.7270/Q2PC31SG |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289128
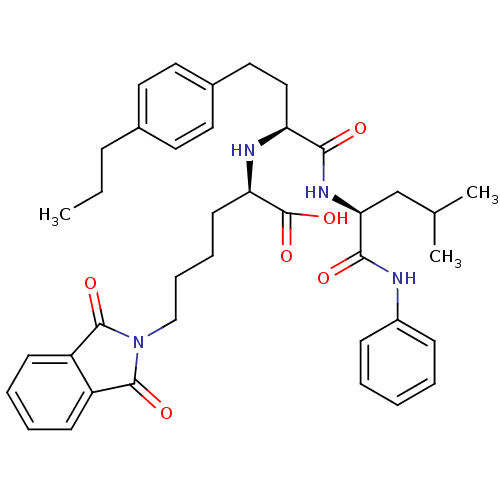
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50064340
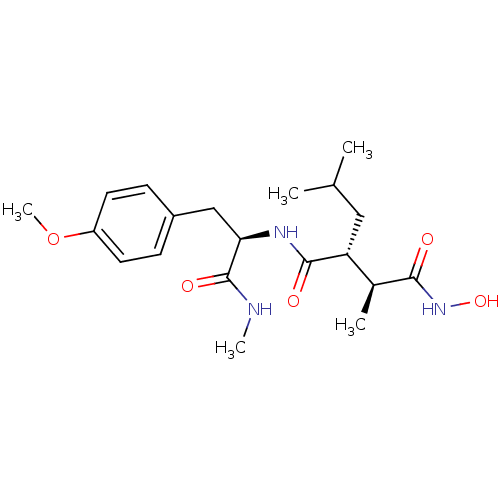
((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...)Show SMILES CNC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C20H31N3O5/c1-12(2)10-16(13(3)18(24)23-27)19(25)22-17(20(26)21-4)11-14-6-8-15(28-5)9-7-14/h6-9,12-13,16-17,27H,10-11H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-3. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50064340

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...)Show SMILES CNC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C20H31N3O5/c1-12(2)10-16(13(3)18(24)23-27)19(25)22-17(20(26)21-4)11-14-6-8-15(28-5)9-7-14/h6-9,12-13,16-17,27H,10-11H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3 (Matrix metalloproteinase-3) |
J Med Chem 41: 1745-8 (1998)
Article DOI: 10.1021/jm970849z
BindingDB Entry DOI: 10.7270/Q2GB235N |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057073
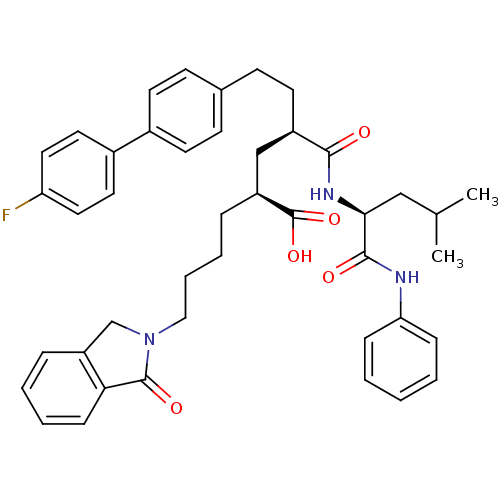
((2S,4R)-6-(4'-Fluoro-biphenyl-4-yl)-4-((S)-3-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccc(cc1)-c1ccc(F)cc1)C[C@H](CCCCN1Cc2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C43H48FN3O5/c1-29(2)26-39(41(49)45-37-12-4-3-5-13-37)46-40(48)33(20-17-30-15-18-31(19-16-30)32-21-23-36(44)24-22-32)27-34(43(51)52)10-8-9-25-47-28-35-11-6-7-14-38(35)42(47)50/h3-7,11-16,18-19,21-24,29,33-34,39H,8-10,17,20,25-28H2,1-2H3,(H,45,49)(H,46,48)(H,51,52)/t33-,34+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143731
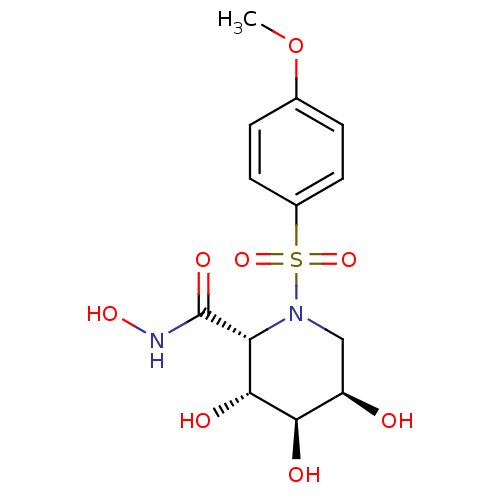
((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50031775
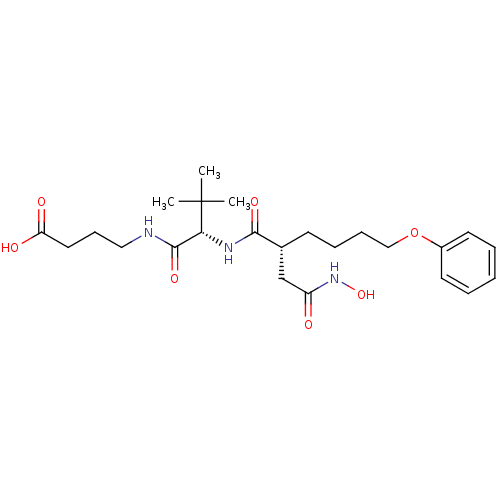
(4-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-6-phenoxy-h...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO)C(=O)NCCCC(O)=O Show InChI InChI=1S/C24H37N3O7/c1-24(2,3)21(23(32)25-14-9-13-20(29)30)26-22(31)17(16-19(28)27-33)10-7-8-15-34-18-11-5-4-6-12-18/h4-6,11-12,17,21,33H,7-10,13-16H2,1-3H3,(H,25,32)(H,26,31)(H,27,28)(H,29,30)/t17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibitory potency against human stromelysin, MMP-3 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057050
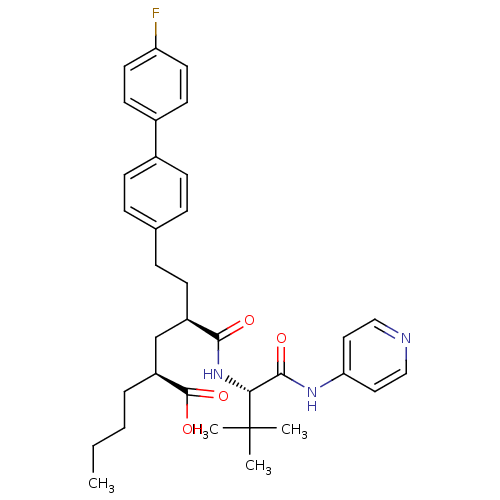
((2S,4R)-2-Butyl-4-[(S)-2,2-dimethyl-1-(pyridin-4-y...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(F)cc1)C(=O)N[C@H](C(=O)Nc1ccncc1)C(C)(C)C)C(O)=O Show InChI InChI=1S/C34H42FN3O4/c1-5-6-7-27(33(41)42)22-26(13-10-23-8-11-24(12-9-23)25-14-16-28(35)17-15-25)31(39)38-30(34(2,3)4)32(40)37-29-18-20-36-21-19-29/h8-9,11-12,14-21,26-27,30H,5-7,10,13,22H2,1-4H3,(H,38,39)(H,41,42)(H,36,37,40)/t26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109633
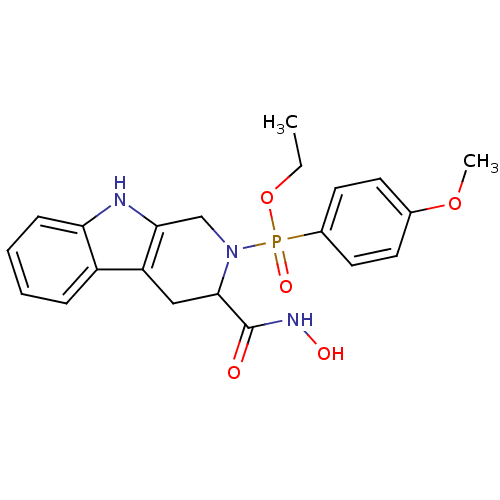
((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...)Show SMILES CCOP(=O)(N1Cc2[nH]c3ccccc3c2CC1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H24N3O5P/c1-3-29-30(27,15-10-8-14(28-2)9-11-15)24-13-19-17(12-20(24)21(25)23-26)16-6-4-5-7-18(16)22-19/h4-11,20,22,26H,3,12-13H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143732

((2R,3R,4R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288683
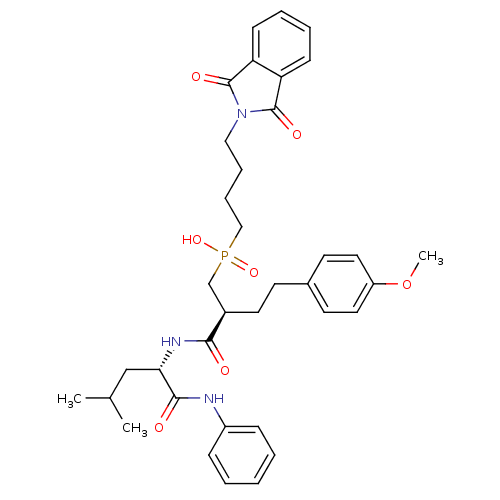
(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101503
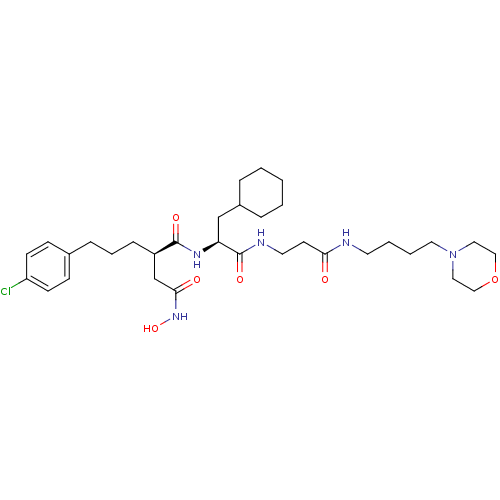
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C33H52ClN5O6/c34-28-13-11-25(12-14-28)9-6-10-27(24-31(41)38-44)32(42)37-29(23-26-7-2-1-3-8-26)33(43)36-17-15-30(40)35-16-4-5-18-39-19-21-45-22-20-39/h11-14,26-27,29,44H,1-10,15-24H2,(H,35,40)(H,36,43)(H,37,42)(H,38,41)/t27-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283707
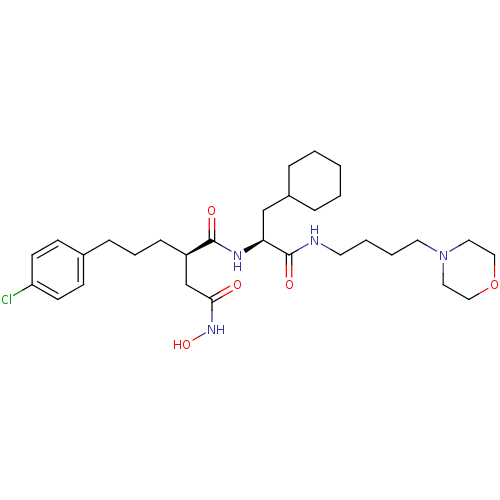
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C30H47ClN4O5/c31-26-13-11-23(12-14-26)9-6-10-25(22-28(36)34-39)29(37)33-27(21-24-7-2-1-3-8-24)30(38)32-15-4-5-16-35-17-19-40-20-18-35/h11-14,24-25,27,39H,1-10,15-22H2,(H,32,38)(H,33,37)(H,34,36)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-3. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109622

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES COc1ccc(cc1)P(=O)(OCC1CCCCC1)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C24H31N2O5P/c1-30-21-11-13-22(14-12-21)32(29,31-17-18-7-3-2-4-8-18)26-16-20-10-6-5-9-19(20)15-23(26)24(27)25-28/h5-6,9-14,18,23,28H,2-4,7-8,15-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
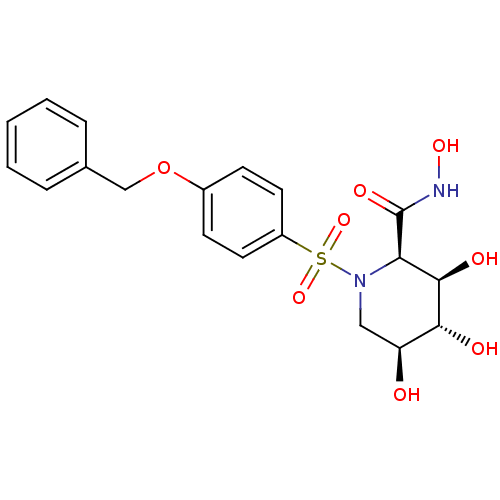
(Homo sapiens (Human)) | BDBM50131381
((2R,3R,4R,5S)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16+,17+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2737-40 (2003)
BindingDB Entry DOI: 10.7270/Q2PC31SG |
More data for this
Ligand-Target Pair | |
Stromelysin-1
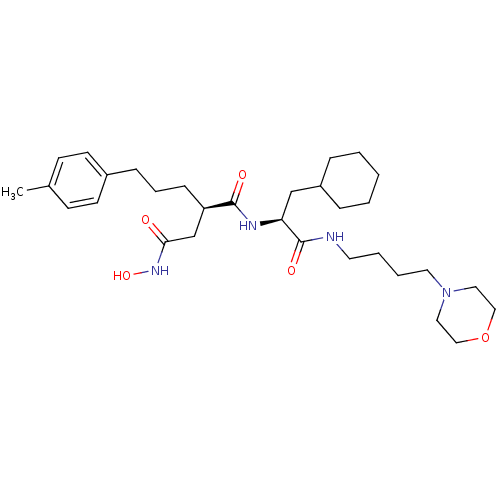
(Homo sapiens (Human)) | BDBM50283710
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-morpholin-4-yl-but...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C31H50N4O5/c1-24-12-14-25(15-13-24)10-7-11-27(23-29(36)34-39)30(37)33-28(22-26-8-3-2-4-9-26)31(38)32-16-5-6-17-35-18-20-40-21-19-35/h12-15,26-28,39H,2-11,16-23H2,1H3,(H,32,38)(H,33,37)(H,34,36)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
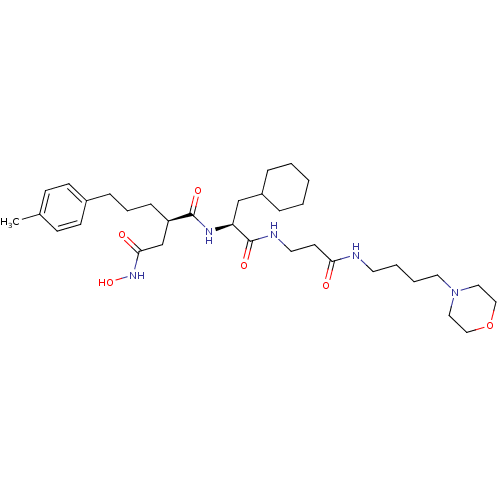
(Homo sapiens (Human)) | BDBM50101520
(CHEMBL77663 | N*1*-{2-Cyclohexyl-1-[2-(4-morpholin...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C34H55N5O6/c1-26-12-14-27(15-13-26)10-7-11-29(25-32(41)38-44)33(42)37-30(24-28-8-3-2-4-9-28)34(43)36-18-16-31(40)35-17-5-6-19-39-20-22-45-23-21-39/h12-15,28-30,44H,2-11,16-25H2,1H3,(H,35,40)(H,36,43)(H,37,42)(H,38,41)/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101509
(CHEMBL306947 | N*1*-(2-Cyclohexyl-1-{2-[2-(morphol...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C32H52N6O8S/c1-24-10-12-25(13-11-24)8-5-9-27(23-30(40)37-43)31(41)36-28(22-26-6-3-2-4-7-26)32(42)34-15-14-29(39)33-16-17-35-47(44,45)38-18-20-46-21-19-38/h10-13,26-28,35,43H,2-9,14-23H2,1H3,(H,33,39)(H,34,42)(H,36,41)(H,37,40)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283715
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(morpholine-4-sulf...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C29H47N5O7S/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(35)33-38)28(36)32-26(20-24-6-3-2-4-7-24)29(37)30-14-15-31-42(39,40)34-16-18-41-19-17-34/h10-13,24-26,31,38H,2-9,14-21H2,1H3,(H,30,37)(H,32,36)(H,33,35)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50102631
(8-Isobutyl-9-oxo-6-oxa-1,10-diaza-tricyclo[11.6.1....)Show SMILES CNC(=O)[C@@H]1Cc2cn(CCCCO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)c1ccccc21 Show InChI InChI=1S/C24H34N4O5/c1-15(2)12-18-21(24(31)27-32)33-11-7-6-10-28-14-16(17-8-4-5-9-20(17)28)13-19(23(30)25-3)26-22(18)29/h4-5,8-9,14-15,18-19,21,32H,6-7,10-13H2,1-3H3,(H,25,30)(H,26,29)(H,27,31)/t18-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-3. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109630
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show InChI InChI=1S/C18H21N2O5P/c1-24-15-7-9-16(10-8-15)26(23,25-2)20-12-14-6-4-3-5-13(14)11-17(20)18(21)19-22/h3-10,17,22H,11-12H2,1-2H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101499
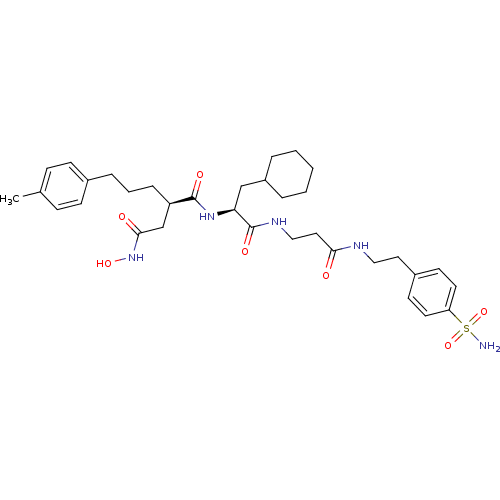
(CHEMBL74040 | N*1*-(2-Cyclohexyl-1-{2-[2-(4-sulfam...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C34H49N5O7S/c1-24-10-12-25(13-11-24)8-5-9-28(23-32(41)39-44)33(42)38-30(22-27-6-3-2-4-7-27)34(43)37-21-19-31(40)36-20-18-26-14-16-29(17-15-26)47(35,45)46/h10-17,27-28,30,44H,2-9,18-23H2,1H3,(H,36,40)(H,37,43)(H,38,42)(H,39,41)(H2,35,45,46)/t28-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076995
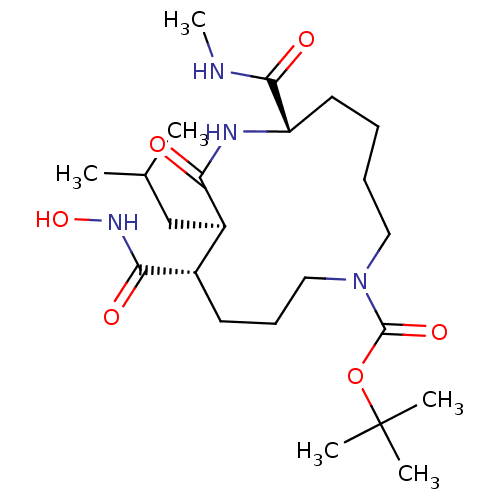
((6S,9R,10S)-10-Hydroxycarbamoyl-9-isobutyl-6-methy...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H42N4O6/c1-15(2)14-17-16(20(29)26-32)10-9-13-27(22(31)33-23(3,4)5)12-8-7-11-18(21(30)24-6)25-19(17)28/h15-18,32H,7-14H2,1-6H3,(H,24,30)(H,25,28)(H,26,29)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-3. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50069228
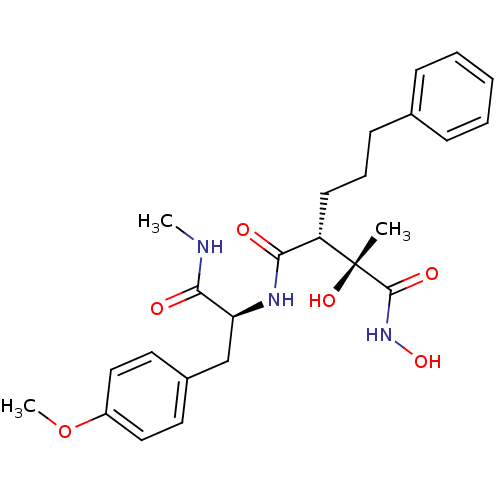
((2R,3R)-2,N*1*-Dihydroxy-N*4*-[(S)-2-(4-methoxy-ph...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CCCc1ccccc1)[C@@](C)(O)C(=O)NO Show InChI InChI=1S/C25H33N3O6/c1-25(32,24(31)28-33)20(11-7-10-17-8-5-4-6-9-17)22(29)27-21(23(30)26-2)16-18-12-14-19(34-3)15-13-18/h4-6,8-9,12-15,20-21,32-33H,7,10-11,16H2,1-3H3,(H,26,30)(H,27,29)(H,28,31)/t20-,21-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity against human matrix metalloproteinase-3. |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data