 Found 447 hits of ki data for polymerid = 1406,1408,2285,4019,4020
Found 447 hits of ki data for polymerid = 1406,1408,2285,4019,4020 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
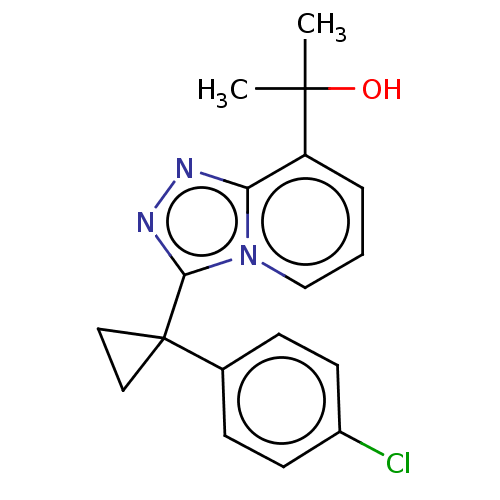
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317217
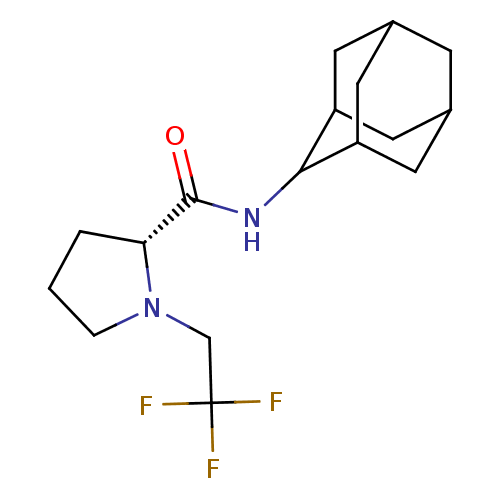
((2R)-N-(adamantan-2-yl)-1-(2,2,2-trifluoroethyl)py...)Show SMILES FC(F)(F)CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:9.10,TLB:19:18:22:15.14.13,19:14:17.18.20:22,THB:12:13:17.18.20:22,13:14:17:20.21.22,13:21:17:15.19.14,(27.97,-20.63,;27.2,-21.96,;25.66,-21.96,;26.42,-20.62,;27.97,-23.29,;27.07,-24.54,;25.53,-24.56,;25.07,-26.02,;26.31,-26.92,;27.52,-25.95,;29.03,-26.46,;30.37,-25.71,;29,-28,;27.66,-28.74,;27.65,-30.27,;26.25,-30.63,;24.91,-30.13,;23.71,-31.41,;25.22,-30.99,;26.63,-31.56,;25.21,-29.4,;26.26,-28.16,;24.9,-28.65,)| Show InChI InChI=1S/C17H25F3N2O/c18-17(19,20)9-22-3-1-2-14(22)16(23)21-15-12-5-10-4-11(7-12)8-13(15)6-10/h10-15H,1-9H2,(H,21,23)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317218
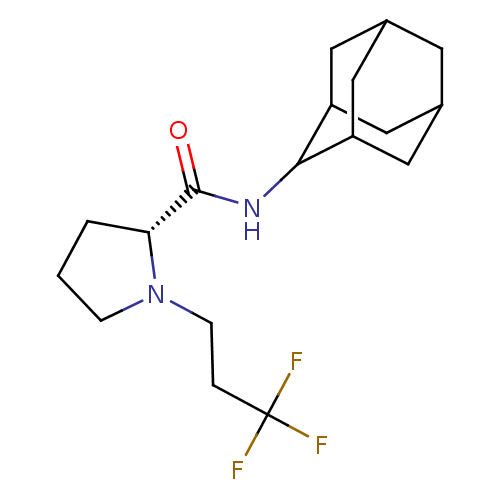
((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317221
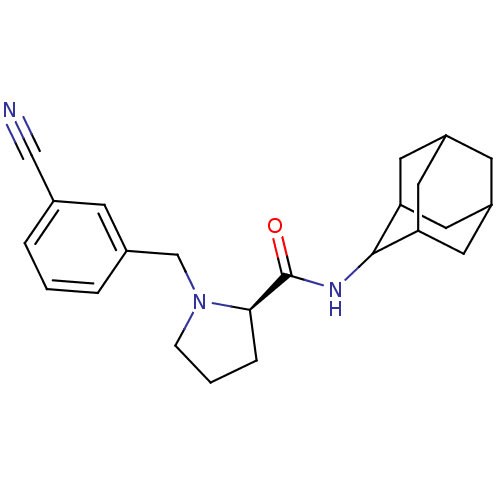
((2R)-N-(adamantan-2-yl)-1-[(3-cyanophenyl)methyl]p...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1Cc1cccc(c1)C#N |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(29.81,-42.16,;28.46,-42.91,;28.44,-44.45,;27.09,-45.2,;27.08,-46.73,;25.68,-47.08,;24.34,-46.59,;23.15,-47.87,;24.65,-47.44,;26.06,-48.01,;24.64,-45.86,;25.69,-44.62,;24.33,-45.1,;26.95,-42.4,;25.74,-43.38,;24.5,-42.47,;24.97,-41.02,;26.5,-41,;27.4,-39.75,;26.63,-38.41,;25.1,-38.41,;24.33,-37.08,;25.11,-35.74,;26.65,-35.75,;27.41,-37.09,;27.43,-34.42,;28.2,-33.09,)| Show InChI InChI=1S/C23H29N3O/c24-13-15-3-1-4-16(7-15)14-26-6-2-5-21(26)23(27)25-22-19-9-17-8-18(11-19)12-20(22)10-17/h1,3-4,7,17-22H,2,5-6,8-12,14H2,(H,25,27)/t17?,18?,19?,20?,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317223
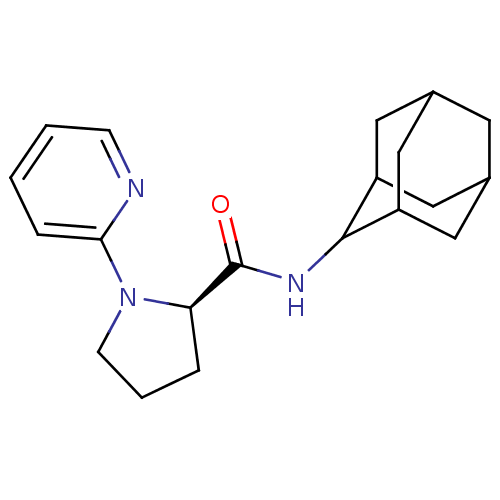
((2R)-N-(adamantan-2-yl)-1-(pyridin-2-yl)pyrrolidin...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1c1ccccn1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(6.04,2.3,;4.69,1.55,;4.66,.01,;3.32,-.73,;3.31,-2.26,;1.91,-2.62,;.57,-2.12,;-.63,-3.4,;.88,-2.98,;2.29,-3.54,;.87,-1.39,;1.92,-.15,;.56,-.63,;3.18,2.06,;1.97,1.09,;.73,1.99,;1.2,3.45,;2.72,3.47,;4.03,4.27,;4,5.82,;5.31,6.62,;6.67,5.87,;6.7,4.35,;5.39,3.54,)| Show InChI InChI=1S/C20H27N3O/c24-20(17-4-3-7-23(17)18-5-1-2-6-21-18)22-19-15-9-13-8-14(11-15)12-16(19)10-13/h1-2,5-6,13-17,19H,3-4,7-12H2,(H,22,24)/t13?,14?,15?,16?,17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
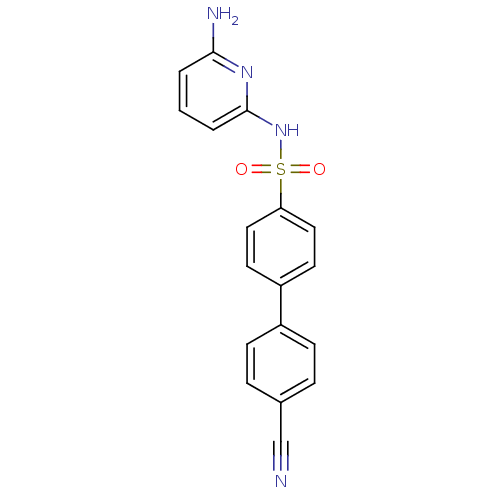
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317211
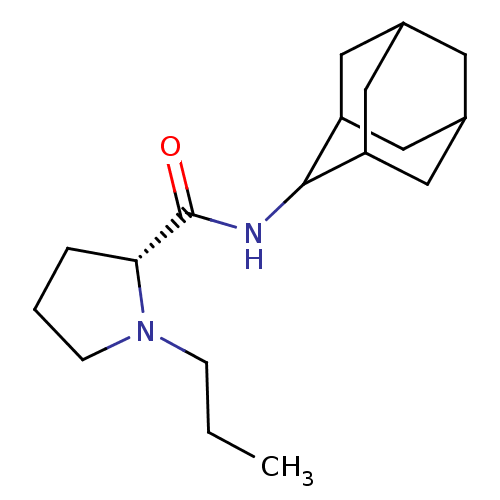
((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317213
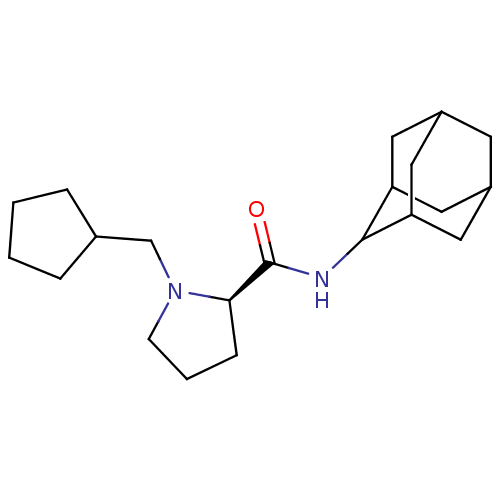
((2R)-N-(adamantan-2-yl)-1-(cyclopentylmethyl)pyrro...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(25.66,-12.64,;24.31,-13.39,;24.29,-14.93,;22.94,-15.67,;22.93,-17.2,;21.53,-17.56,;20.2,-17.06,;19,-18.34,;20.5,-17.92,;21.92,-18.49,;20.5,-16.33,;21.54,-15.09,;20.18,-15.58,;22.8,-12.88,;21.59,-13.85,;20.36,-12.95,;20.82,-11.49,;22.35,-11.47,;23.25,-10.22,;22.62,-8.82,;23.38,-7.49,;22.34,-6.35,;20.94,-6.98,;21.1,-8.51,)| Show InChI InChI=1S/C21H34N2O/c24-21(19-6-3-7-23(19)13-14-4-1-2-5-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317224
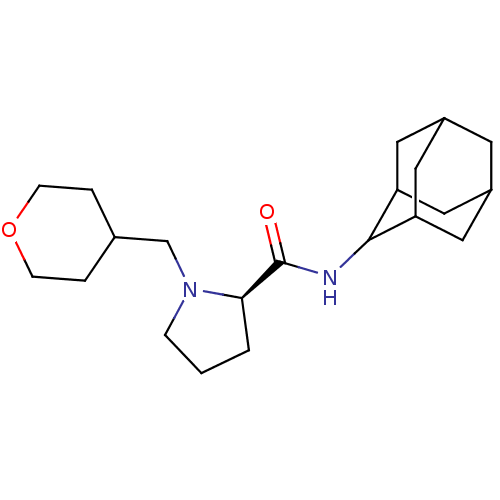
((2R)-N-(adamantan-2-yl)-1-(oxan-4-ylmethyl)pyrroli...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCOCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(16.19,2.36,;14.84,1.61,;14.82,.07,;13.47,-.68,;13.46,-2.21,;12.06,-2.57,;10.72,-2.07,;9.51,-3.36,;11.02,-2.93,;12.44,-3.5,;11.01,-1.34,;12.06,-.09,;10.7,-.58,;13.33,2.12,;12.11,1.14,;10.87,2.05,;11.33,3.52,;12.87,3.53,;13.64,4.87,;15.19,4.87,;15.96,3.52,;17.5,3.52,;18.28,4.86,;17.51,6.2,;15.96,6.21,)| Show InChI InChI=1S/C21H34N2O2/c24-21(19-2-1-5-23(19)13-14-3-6-25-7-4-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50174298
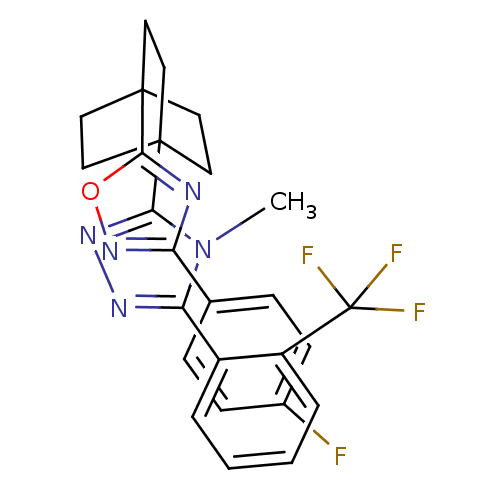
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317211

((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209
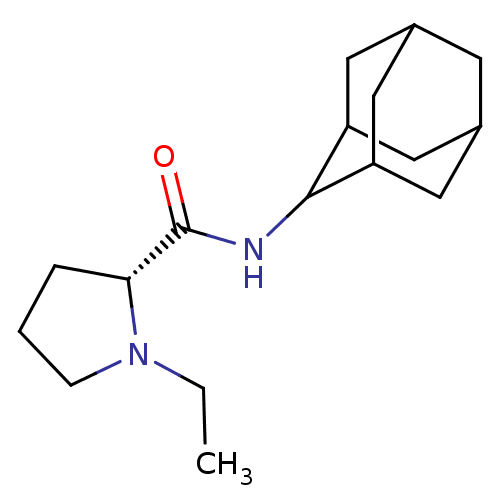
((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317212
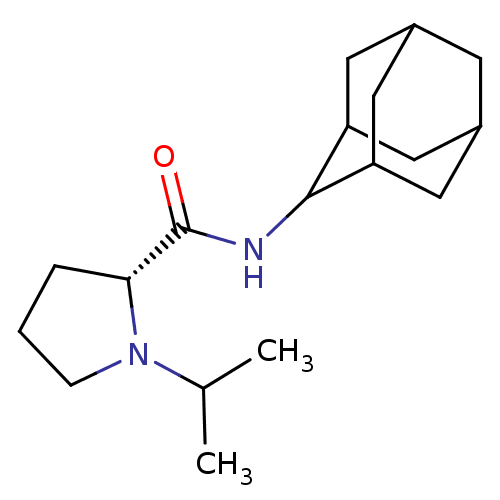
((2R)-N-(adamantan-2-yl)-1-(propan-2-yl)pyrrolidine...)Show SMILES CC(C)N1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(12.99,-6.92,;13.62,-8.33,;15.15,-8.49,;12.71,-9.58,;11.18,-9.59,;10.72,-11.05,;11.95,-11.95,;13.17,-10.98,;14.67,-11.49,;16.02,-10.74,;14.65,-13.03,;13.3,-13.78,;13.3,-15.31,;11.9,-15.66,;10.56,-15.17,;9.36,-16.45,;10.87,-16.02,;12.28,-16.59,;10.86,-14.44,;11.9,-13.19,;10.55,-13.68,)| Show InChI InChI=1S/C18H30N2O/c1-11(2)20-5-3-4-16(20)18(21)19-17-14-7-12-6-13(9-14)10-15(17)8-12/h11-17H,3-10H2,1-2H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29863

(N-(Pyridin-2-yl) arylsulfonamide, 25)Show SMILES CC(C)c1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C21H19N3O2S/c1-15(2)20-4-3-5-21(23-20)24-27(25,26)19-12-10-18(11-13-19)17-8-6-16(14-22)7-9-17/h3-13,15H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29862
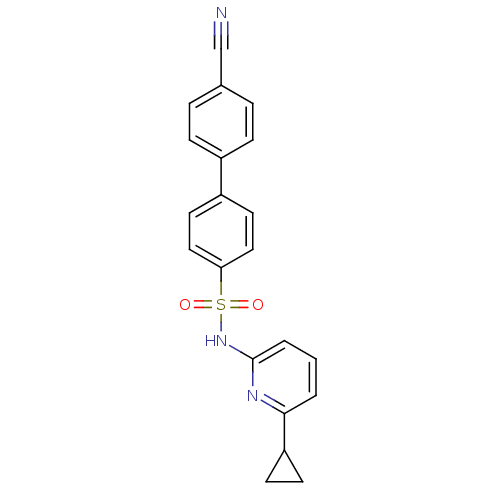
(N-(Pyridin-2-yl) arylsulfonamide, 24)Show SMILES O=S(=O)(Nc1cccc(n1)C1CC1)c1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H17N3O2S/c22-14-15-4-6-16(7-5-15)17-10-12-19(13-11-17)27(25,26)24-21-3-1-2-20(23-21)18-8-9-18/h1-7,10-13,18H,8-9H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317219
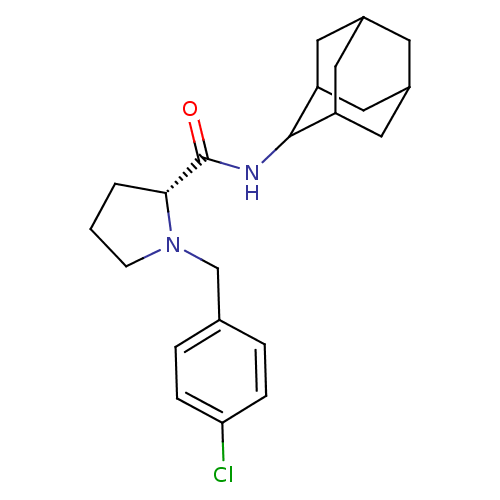
((2R)-N-(adamantan-2-yl)-1-[(4-chlorophenyl)methyl]...)Show SMILES Clc1ccc(CN2CCC[C@@H]2C(=O)NC2C3CC4CC(C3)CC2C4)cc1 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(2.87,-33.68,;3.64,-35.01,;5.18,-35.02,;5.94,-36.36,;5.16,-37.69,;5.93,-39.03,;5.02,-40.28,;3.49,-40.29,;3.03,-41.75,;4.27,-42.66,;5.48,-41.68,;6.99,-42.19,;8.34,-41.44,;6.96,-43.73,;5.62,-44.48,;5.61,-46.01,;4.21,-46.36,;2.87,-45.87,;1.67,-47.15,;3.18,-46.72,;4.59,-47.29,;3.17,-45.14,;4.22,-43.9,;2.86,-44.38,;3.63,-37.69,;2.86,-36.35,)| Show InChI InChI=1S/C22H29ClN2O/c23-19-5-3-14(4-6-19)13-25-7-1-2-20(25)22(26)24-21-17-9-15-8-16(11-17)12-18(21)10-15/h3-6,15-18,20-21H,1-2,7-13H2,(H,24,26)/t15?,16?,17?,18?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197399
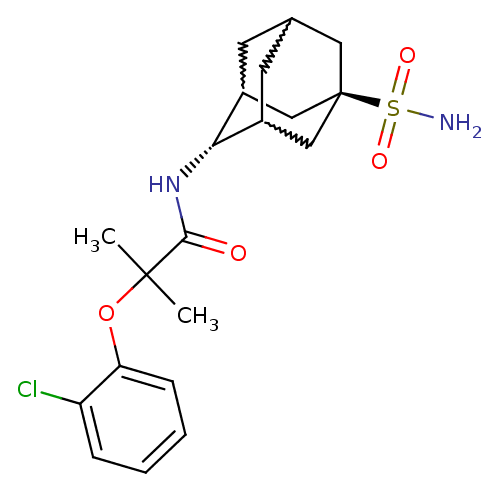
(2-(2-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(20.57,1.97,;21.38,.67,;22.2,-.64,;22.71,1.45,;24.05,.69,;24.05,-.85,;25.39,-1.61,;26.72,-.83,;26.71,.72,;25.37,1.47,;25.35,3.01,;20.1,-.18,;20.19,-1.72,;18.72,.5,;17.44,-.35,;17.42,-1.88,;16.41,-3.16,;15.01,-2.59,;15,-1,;16.04,.23,;14.69,-.25,;14.7,-1.73,;13.5,-3.01,;16.03,-2.22,;13.21,-1.32,;11.71,-.92,;13.61,.16,;12.8,-2.81,)| Show InChI InChI=1S/C20H27ClN2O4S/c1-19(2,27-16-6-4-3-5-15(16)21)18(24)23-17-13-7-12-8-14(17)11-20(9-12,10-13)28(22,25)26/h3-6,12-14,17H,7-11H2,1-2H3,(H,23,24)(H2,22,25,26)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197404
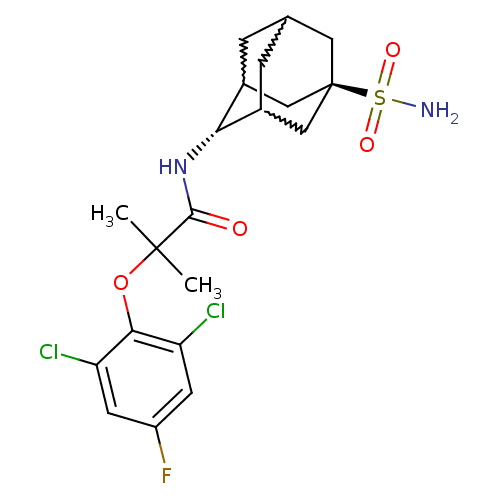
(2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...)Show SMILES CC(C)(Oc1c(Cl)cc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.19,17.28,21.21,wU:16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:17:24.19.20:22,16:21:24:25.18.17,TEB:18:19:22:25.17.16,20:21:25:24.19.18,(24.03,-36.5,;24.84,-37.8,;25.66,-39.11,;26.17,-37.02,;27.51,-37.78,;28.83,-37,;28.81,-35.46,;30.17,-37.75,;30.18,-39.3,;31.52,-40.06,;28.85,-40.08,;27.52,-39.32,;26.19,-40.1,;23.56,-38.65,;23.65,-40.19,;22.18,-37.97,;20.9,-38.82,;20.89,-40.35,;19.87,-41.62,;18.47,-41.06,;18.46,-39.47,;19.5,-38.24,;18.15,-38.72,;18.16,-40.2,;16.97,-41.48,;19.49,-40.69,;16.67,-39.79,;15.17,-39.39,;17.07,-38.31,;16.26,-41.28,)| Show InChI InChI=1S/C20H25Cl2FN2O4S/c1-19(2,29-17-14(21)5-13(23)6-15(17)22)18(26)25-16-11-3-10-4-12(16)9-20(7-10,8-11)30(24,27)28/h5-6,10-12,16H,3-4,7-9H2,1-2H3,(H,25,26)(H2,24,27,28)/t10?,11?,12?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197418
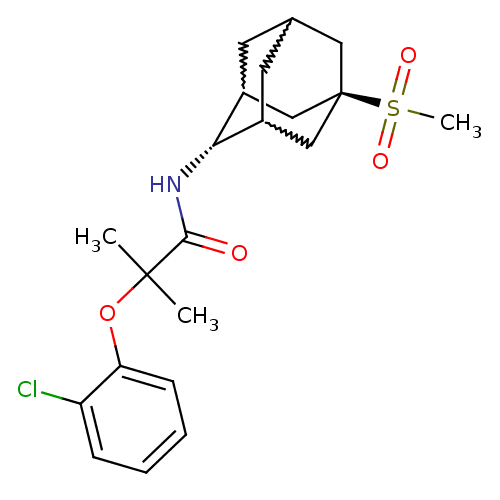
(2-(2-chloro-phenoxy)-N-(5-methanesulfonyl-adamanta...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(C)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(2.61,-15.76,;3.42,-17.07,;4.24,-18.37,;4.75,-16.28,;6.09,-17.05,;6.1,-18.59,;7.44,-19.35,;8.77,-18.57,;8.76,-17.02,;7.41,-16.26,;7.39,-14.72,;2.14,-17.92,;2.23,-19.46,;.76,-17.23,;-.53,-18.08,;-.54,-19.61,;-1.56,-20.89,;-2.97,-20.33,;-2.97,-18.74,;-1.93,-17.5,;-3.28,-17.98,;-3.27,-19.47,;-4.47,-20.75,;-1.94,-19.96,;-4.77,-19.06,;-6.26,-18.66,;-4.36,-17.57,;-5.17,-20.55,)| Show InChI InChI=1S/C21H28ClNO4S/c1-20(2,27-17-7-5-4-6-16(17)22)19(24)23-18-14-8-13-9-15(18)12-21(10-13,11-14)28(3,25)26/h4-7,13-15,18H,8-12H2,1-3H3,(H,23,24)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195289
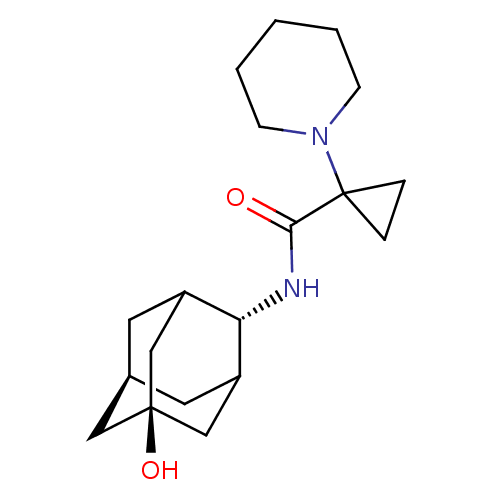
(1-piperidin-1-yl-cyclopropanecarboxylic acid (5-hy...)Show SMILES O[C@@]12C[C@H]3CC(C1)[C@H](NC(=O)C1(CC1)N1CCCCC1)C(C3)C2 |wU:7.8,wD:1.0,3.2,TLB:4:3:22:6.5.7,4:5:2.3.21:22,THB:8:7:2.3.21:22,(1.12,-7.02,;2.66,-7.15,;1.37,-8.33,;2.9,-8.03,;4.25,-8.7,;5.36,-7.5,;3.94,-7.74,;5.49,-5.98,;6.83,-5.23,;8.16,-6.02,;8.13,-7.56,;9.5,-5.27,;10.29,-3.94,;8.75,-3.92,;10.82,-6.06,;10.79,-7.6,;12.11,-8.38,;13.46,-7.64,;13.48,-6.1,;12.16,-5.3,;4.14,-5.3,;3.01,-6.44,;2.76,-5.67,)| Show InChI InChI=1S/C19H30N2O2/c22-17(19(4-5-19)21-6-2-1-3-7-21)20-16-14-8-13-9-15(16)12-18(23,10-13)11-14/h13-16,23H,1-12H2,(H,20,22)/t13-,14?,15?,16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50195289

(1-piperidin-1-yl-cyclopropanecarboxylic acid (5-hy...)Show SMILES O[C@@]12C[C@H]3CC(C1)[C@H](NC(=O)C1(CC1)N1CCCCC1)C(C3)C2 |wU:7.8,wD:1.0,3.2,TLB:4:3:22:6.5.7,4:5:2.3.21:22,THB:8:7:2.3.21:22,(1.12,-7.02,;2.66,-7.15,;1.37,-8.33,;2.9,-8.03,;4.25,-8.7,;5.36,-7.5,;3.94,-7.74,;5.49,-5.98,;6.83,-5.23,;8.16,-6.02,;8.13,-7.56,;9.5,-5.27,;10.29,-3.94,;8.75,-3.92,;10.82,-6.06,;10.79,-7.6,;12.11,-8.38,;13.46,-7.64,;13.48,-6.1,;12.16,-5.3,;4.14,-5.3,;3.01,-6.44,;2.76,-5.67,)| Show InChI InChI=1S/C19H30N2O2/c22-17(19(4-5-19)21-6-2-1-3-7-21)20-16-14-8-13-9-15(16)12-18(23,10-13)11-14/h13-16,23H,1-12H2,(H,20,22)/t13-,14?,15?,16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202094
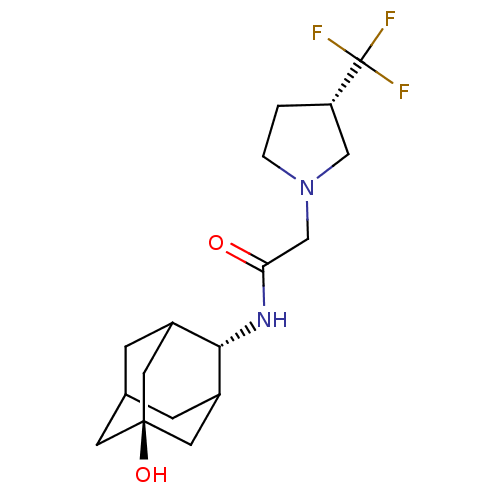
(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317210
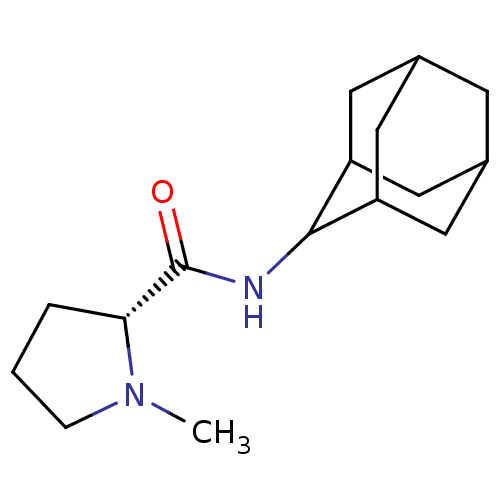
((2R)-N-(adamantan-2-yl)-1-methylpyrrolidine-2-carb...)Show SMILES CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:5.6,TLB:15:14:18:11.10.9,15:10:13.14.16:18,THB:8:9:13.14.16:18,9:10:13:16.17.18,9:17:13:11.15.10,(-4.95,-6.94,;-5.86,-8.19,;-7.4,-8.21,;-7.87,-9.67,;-6.63,-10.58,;-5.41,-9.6,;-3.89,-10.12,;-2.54,-9.36,;-3.91,-11.66,;-5.26,-12.4,;-5.27,-13.94,;-6.68,-14.3,;-8.02,-13.8,;-9.23,-15.09,;-7.71,-14.66,;-6.29,-15.23,;-7.72,-13.07,;-6.67,-11.82,;-8.03,-12.3,)| Show InChI InChI=1S/C16H26N2O/c1-18-4-2-3-14(18)16(19)17-15-12-6-10-5-11(8-12)9-13(15)7-10/h10-15H,2-9H2,1H3,(H,17,19)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317216
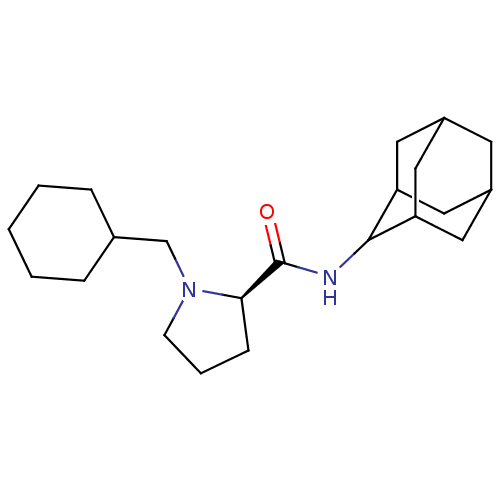
((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(18.88,-25.64,;17.53,-26.39,;17.51,-27.93,;16.16,-28.68,;16.15,-30.21,;14.75,-30.56,;13.41,-30.07,;12.22,-31.35,;13.72,-30.92,;15.13,-31.49,;13.71,-29.34,;14.76,-28.1,;13.4,-28.58,;16.02,-25.88,;14.81,-26.86,;13.57,-25.95,;14.04,-24.49,;15.57,-24.48,;16.47,-23.23,;15.7,-21.89,;16.48,-20.57,;15.71,-19.24,;14.17,-19.23,;13.4,-20.56,;14.17,-21.9,)| Show InChI InChI=1S/C22H36N2O/c25-22(20-7-4-8-24(20)14-15-5-2-1-3-6-15)23-21-18-10-16-9-17(12-18)13-19(21)11-16/h15-21H,1-14H2,(H,23,25)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197416
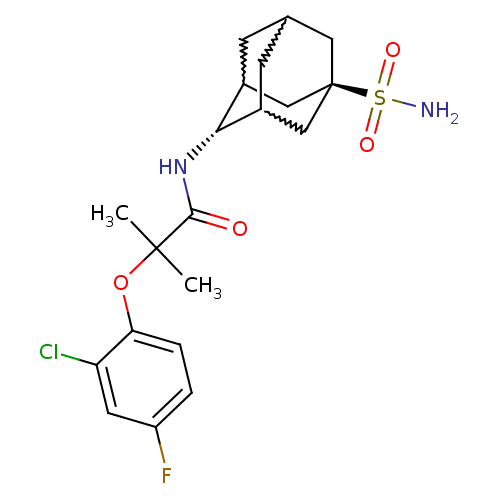
(2-(2-chloro-4-fluoro-phenoxy)-2-methyl-N-(5-sulfam...)Show SMILES CC(C)(Oc1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:18.18,16.27,20.20,wU:15.15,wD:22.28,TLB:15:16:23:19.20.21,14:15:23.18.19:21,THB:17:16:23.18.19:21,15:20:23:24.17.16,TEB:17:18:21:24.16.15,19:20:24:23.18.17,(2.86,-37.78,;3.68,-39.09,;4.49,-40.39,;5.01,-38.31,;6.35,-39.07,;6.35,-40.6,;7.69,-41.36,;9.02,-40.58,;10.36,-41.34,;9,-39.03,;7.67,-38.28,;7.65,-36.74,;2.4,-39.94,;2.49,-41.47,;1.02,-39.25,;-.27,-40.1,;-.28,-41.63,;-1.29,-42.91,;-2.7,-42.34,;-2.7,-40.75,;-1.66,-39.52,;-3.01,-40,;-3,-41.49,;-4.2,-42.76,;-1.67,-41.98,;-4.5,-41.07,;-5.99,-40.67,;-4.09,-39.59,;-4.9,-42.56,)| Show InChI InChI=1S/C20H26ClFN2O4S/c1-19(2,28-16-4-3-14(22)7-15(16)21)18(25)24-17-12-5-11-6-13(17)10-20(8-11,9-12)29(23,26)27/h3-4,7,11-13,17H,5-6,8-10H2,1-2H3,(H,24,25)(H2,23,26,27)/t11?,12?,13?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202094

(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202094

(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50195298
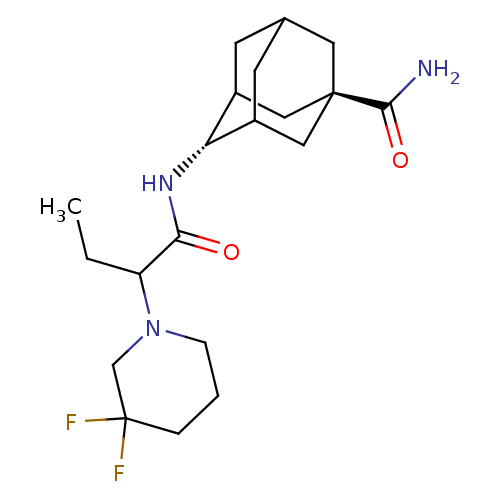
(4-[2-(3,3-difluoro-piperidin-1-yl)-butyrylamino]-a...)Show SMILES CCC(N1CCCC(F)(F)C1)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,14:19:22:23.16.15,(2.47,-8.55,;1.18,-9.41,;1.28,-10.95,;2.66,-11.64,;2.75,-13.17,;4.13,-13.85,;5.41,-13,;5.31,-11.46,;6.8,-11.86,;4.91,-9.99,;3.94,-10.77,;0,-11.8,;.1,-13.34,;-1.38,-11.12,;-2.66,-11.97,;-2.67,-13.5,;-3.69,-14.78,;-5.09,-14.21,;-5.1,-12.62,;-4.06,-11.39,;-5.4,-11.87,;-5.4,-13.35,;-6.59,-14.63,;-4.07,-13.84,;-6.94,-13.35,;-7.71,-14.68,;-7.7,-12.01,)| Show InChI InChI=1S/C20H31F2N3O2/c1-2-15(25-5-3-4-20(21,22)11-25)17(26)24-16-13-6-12-7-14(16)10-19(8-12,9-13)18(23)27/h12-16H,2-11H2,1H3,(H2,23,27)(H,24,26)/t12?,13?,14?,15?,16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50195299
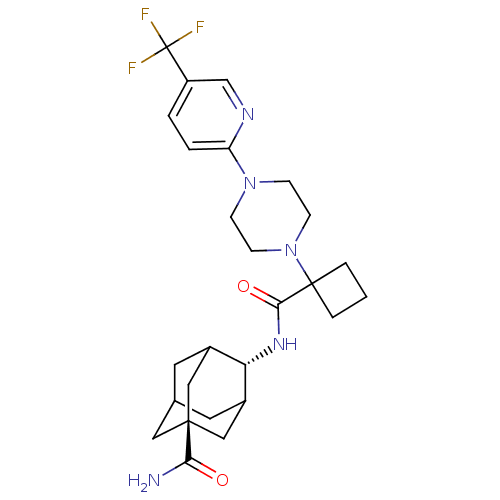
(4-({1-[4-(5-trifluoromethyl-pyridin-2-yl)-piperazi...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)C1(CCC1)N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |wU:9.10,wD:3.2,TLB:6:5:35:8.7.9,6:7:4.5.34:35,THB:9:7:4:34.33.35,9:33:4:8.6.7,10:9:4.5.34:35,(11.1,-46.36,;11.88,-45.03,;11.11,-43.69,;13.42,-45.04,;12.22,-46.31,;13.72,-45.89,;15.12,-46.46,;16.14,-45.18,;14.74,-45.53,;16.15,-43.65,;17.43,-42.8,;18.81,-43.49,;18.9,-45.03,;20.09,-42.63,;21.18,-41.54,;20.08,-40.45,;18.99,-41.55,;21.47,-43.32,;21.56,-44.85,;22.93,-45.53,;24.22,-44.69,;24.12,-43.15,;22.74,-42.46,;25.59,-45.38,;25.68,-46.91,;27.05,-47.6,;28.34,-46.75,;28.24,-45.21,;26.87,-44.53,;29.72,-47.44,;31.04,-48.2,;28.94,-48.76,;30.47,-46.1,;14.75,-43.08,;13.72,-44.3,;13.41,-43.55,)| Show InChI InChI=1S/C26H34F3N5O2/c27-26(28,29)19-2-3-20(31-15-19)33-6-8-34(9-7-33)25(4-1-5-25)23(36)32-21-17-10-16-11-18(21)14-24(12-16,13-17)22(30)35/h2-3,15-18,21H,1,4-14H2,(H2,30,35)(H,32,36)/t16?,17?,18?,21-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197404

(2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...)Show SMILES CC(C)(Oc1c(Cl)cc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.19,17.28,21.21,wU:16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:17:24.19.20:22,16:21:24:25.18.17,TEB:18:19:22:25.17.16,20:21:25:24.19.18,(24.03,-36.5,;24.84,-37.8,;25.66,-39.11,;26.17,-37.02,;27.51,-37.78,;28.83,-37,;28.81,-35.46,;30.17,-37.75,;30.18,-39.3,;31.52,-40.06,;28.85,-40.08,;27.52,-39.32,;26.19,-40.1,;23.56,-38.65,;23.65,-40.19,;22.18,-37.97,;20.9,-38.82,;20.89,-40.35,;19.87,-41.62,;18.47,-41.06,;18.46,-39.47,;19.5,-38.24,;18.15,-38.72,;18.16,-40.2,;16.97,-41.48,;19.49,-40.69,;16.67,-39.79,;15.17,-39.39,;17.07,-38.31,;16.26,-41.28,)| Show InChI InChI=1S/C20H25Cl2FN2O4S/c1-19(2,29-17-14(21)5-13(23)6-15(17)22)18(26)25-16-11-3-10-4-12(16)9-20(7-10,8-11)30(24,27)28/h5-6,10-12,16H,3-4,7-9H2,1-2H3,(H,25,26)(H2,24,27,28)/t10?,11?,12?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197410
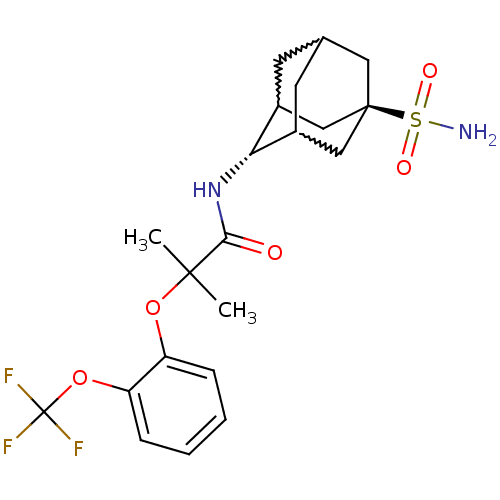
(2-methyl-N-(5-sulfamoyl-adamantan-2-yl)-2-(2-trifl...)Show SMILES CC(C)(Oc1ccccc1OC(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.30,23.23,21.22,wU:18.18,wD:25.31,TLB:18:19:26:22.23.24,17:18:26.21.22:24,THB:20:21:24:27.19.18,20:19:26.21.22:24,18:23:26:27.20.19,TEB:22:21:27:23.24.18,22:23:27:26.21.20,(22.5,-25.65,;23.31,-26.95,;24.12,-28.26,;24.64,-26.17,;25.98,-26.93,;25.98,-28.47,;27.32,-29.23,;28.65,-28.45,;28.64,-26.9,;27.3,-26.15,;27.28,-24.61,;28.6,-23.82,;29.93,-23.04,;27.82,-22.5,;29.39,-25.15,;22.03,-27.8,;22.12,-29.34,;20.65,-27.12,;19.36,-27.97,;19.35,-29.5,;18.34,-30.77,;16.93,-30.21,;16.93,-28.62,;17.97,-27.39,;16.62,-27.87,;16.63,-29.35,;15.43,-30.63,;17.96,-29.84,;15.14,-28.94,;13.64,-28.54,;15.54,-27.46,;14.73,-30.43,)| Show InChI InChI=1S/C21H27F3N2O5S/c1-19(2,30-15-5-3-4-6-16(15)31-21(22,23)24)18(27)26-17-13-7-12-8-14(17)11-20(9-12,10-13)32(25,28)29/h3-6,12-14,17H,7-11H2,1-2H3,(H,26,27)(H2,25,28,29)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197403
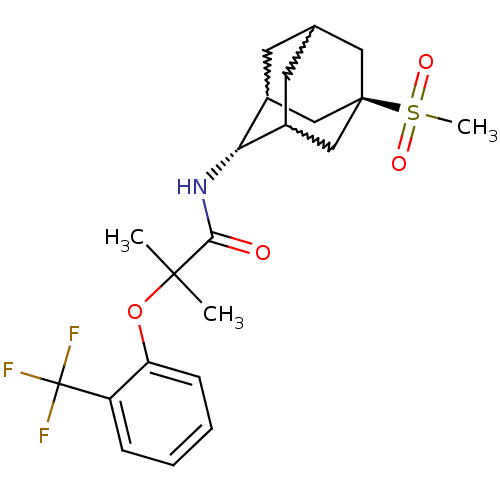
(CHEMBL393167 | N-(5-methanesulfonyl-adamantan-2-yl...)Show SMILES CC(C)(Oc1ccccc1C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(C)(=O)=O |w:20.20,18.29,22.22,wU:17.17,wD:24.30,TLB:17:18:25:21.22.23,16:17:25.20.21:23,THB:19:18:25.20.21:23,17:22:25:26.19.18,TEB:19:20:23:26.18.17,21:22:26:25.20.19,(22.98,-26.47,;23.79,-27.78,;24.61,-29.08,;25.12,-27,;26.46,-27.76,;26.47,-29.29,;27.8,-30.05,;29.13,-29.27,;29.12,-27.73,;27.78,-26.97,;27.76,-25.43,;27.75,-23.89,;26.22,-25.45,;29.3,-25.43,;22.51,-28.63,;22.6,-30.16,;21.13,-27.94,;19.85,-28.79,;19.84,-30.32,;18.82,-31.6,;17.42,-31.03,;17.41,-29.44,;18.45,-28.21,;17.1,-28.69,;17.11,-30.18,;15.92,-31.45,;18.44,-30.67,;15.62,-29.77,;14.12,-29.36,;16.02,-28.28,;15.21,-31.25,)| Show InChI InChI=1S/C22H28F3NO4S/c1-20(2,30-17-7-5-4-6-16(17)22(23,24)25)19(27)26-18-14-8-13-9-15(18)12-21(10-13,11-14)31(3,28)29/h4-7,13-15,18H,8-12H2,1-3H3,(H,26,27)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM22473
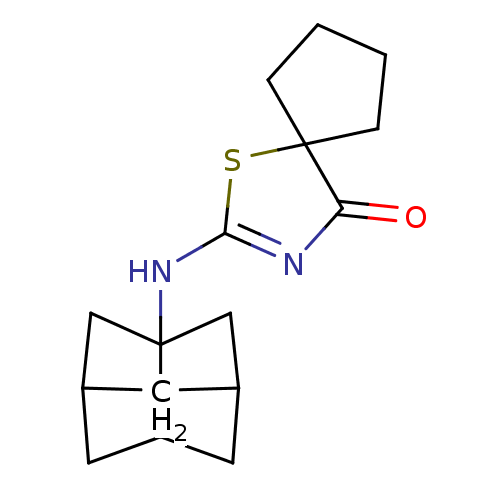
(2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...)Show SMILES O=C1N=C(NC23CC4CC2CC(C3)C4)SC11CCCC1 |t:2,TLB:12:11:5.6:8,4:5:8:11.10.13,4:5:10:7.8.13,THB:6:5:10:7.8.13,6:7:5.12:10,12:5:8:11.10.13| Show InChI InChI=1S/C16H22N2OS/c19-13-16(3-1-2-4-16)20-14(17-13)18-15-8-10-5-11(9-15)7-12(15)6-10/h10-12H,1-9H2,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13746
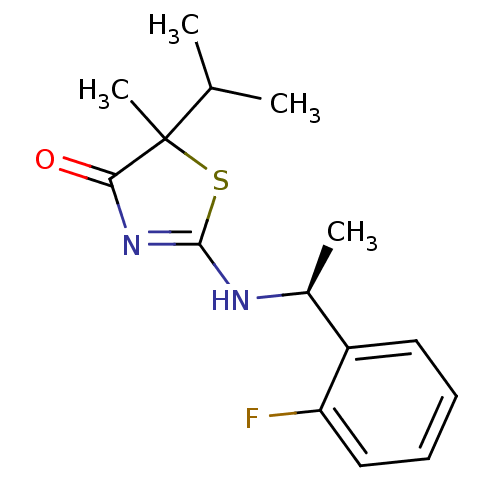
(2-((S)-1-(2-fluorophenyl)ethylamino)-5-isopropyl-5...)Show SMILES CC(C)C1(C)SC(N[C@@H](C)c2ccccc2F)=NC1=O |r,c:17| Show InChI InChI=1S/C15H19FN2OS/c1-9(2)15(4)13(19)18-14(20-15)17-10(3)11-7-5-6-8-12(11)16/h5-10H,1-4H3,(H,17,18,19)/t10-,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Amgen
| Assay Description
Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... |
J Med Chem 50: 429-32 (2007)
Article DOI: 10.1021/jm061214f
BindingDB Entry DOI: 10.7270/Q20Z71JH |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317208
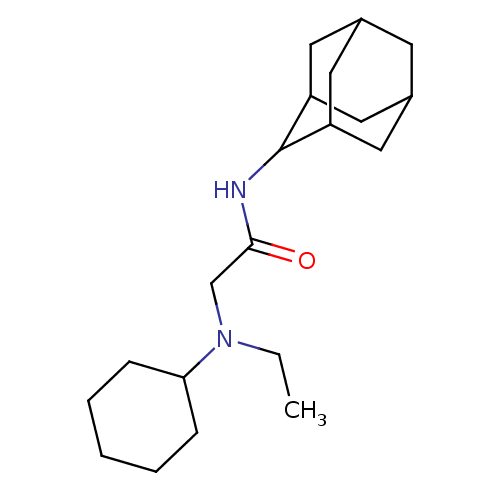
(CHEMBL1088115 | N-(adamantan-2-yl)-2-[cyclohexyl(e...)Show SMILES CCN(CC(=O)NC1C2CC3CC(C2)CC1C3)C1CCCCC1 |TLB:13:12:16:9.8.7,13:8:11.12.14:16,THB:6:7:11.12.14:16,7:8:11:14.15.16,7:15:11:9.13.8,(27.37,5.32,;27.37,3.78,;28.7,3,;28.71,1.46,;30.04,.69,;31.38,1.46,;30.04,-.85,;28.7,-1.62,;28.7,-3.14,;27.29,-3.49,;25.97,-3.01,;24.77,-4.28,;26.26,-3.86,;27.67,-4.42,;26.25,-2.27,;27.3,-1.05,;25.96,-1.52,;30.04,3.78,;31.38,3.01,;32.71,3.78,;32.71,5.31,;31.38,6.08,;30.04,5.31,)| Show InChI InChI=1S/C20H34N2O/c1-2-22(18-6-4-3-5-7-18)13-19(23)21-20-16-9-14-8-15(11-16)12-17(20)10-14/h14-18,20H,2-13H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317216

((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(18.88,-25.64,;17.53,-26.39,;17.51,-27.93,;16.16,-28.68,;16.15,-30.21,;14.75,-30.56,;13.41,-30.07,;12.22,-31.35,;13.72,-30.92,;15.13,-31.49,;13.71,-29.34,;14.76,-28.1,;13.4,-28.58,;16.02,-25.88,;14.81,-26.86,;13.57,-25.95,;14.04,-24.49,;15.57,-24.48,;16.47,-23.23,;15.7,-21.89,;16.48,-20.57,;15.71,-19.24,;14.17,-19.23,;13.4,-20.56,;14.17,-21.9,)| Show InChI InChI=1S/C22H36N2O/c25-22(20-7-4-8-24(20)14-15-5-2-1-3-6-15)23-21-18-10-16-9-17(12-18)13-19(21)11-16/h15-21H,1-14H2,(H,23,25)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317222
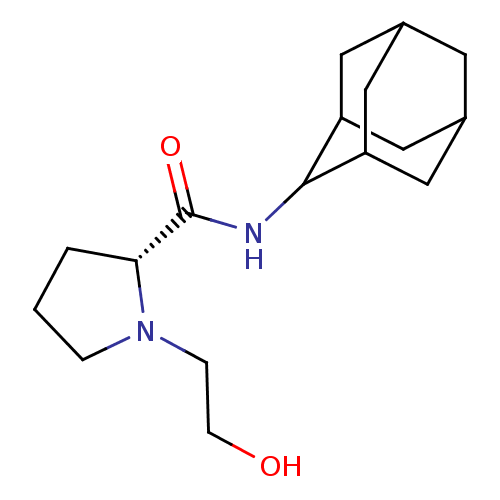
((2R)-N-(adamantan-2-yl)-1-(2-hydroxyethyl)pyrrolid...)Show SMILES OCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(-3.11,5.86,;-3.88,4.52,;-5.43,4.52,;-6.21,3.18,;-7.75,3.17,;-8.21,1.7,;-6.97,.79,;-5.75,1.77,;-4.23,1.26,;-2.88,2.01,;-4.26,-.28,;-5.6,-1.03,;-5.61,-2.57,;-7.02,-2.92,;-8.36,-2.43,;-9.57,-3.71,;-8.06,-3.29,;-6.63,-3.86,;-8.06,-1.69,;-7.01,-.44,;-8.38,-.93,)| Show InChI InChI=1S/C17H28N2O2/c20-5-4-19-3-1-2-15(19)17(21)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16,20H,1-10H2,(H,18,21)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317218

((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50340378
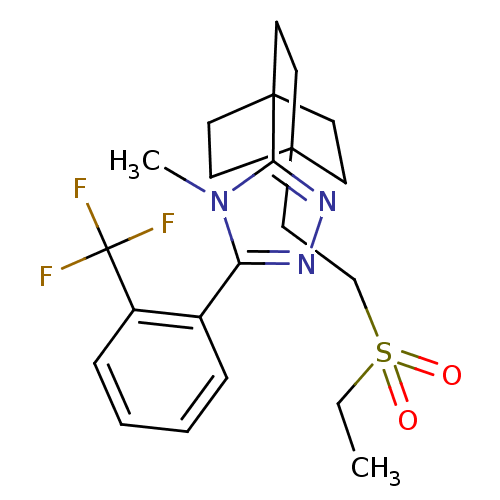
(3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...)Show SMILES CCS(=O)(=O)CCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C22H28F3N3O2S/c1-3-31(29,30)15-14-20-8-11-21(12-9-20,13-10-20)19-27-26-18(28(19)2)16-6-4-5-7-17(16)22(23,24)25/h4-7H,3,8-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197414
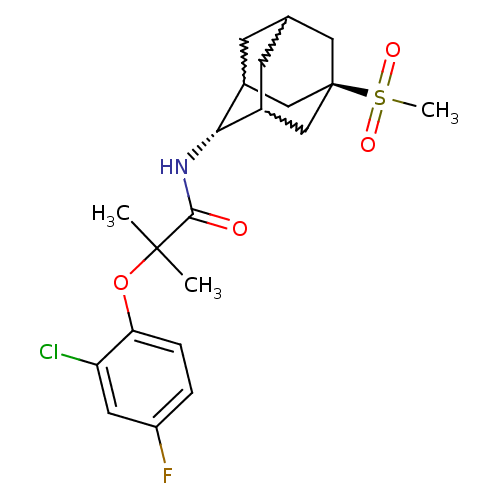
(2-(2-chloro-4-fluoro-phenoxy)-N-(5-methanesulfonyl...)Show SMILES CC(C)(Oc1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(C)(=O)=O |w:18.18,16.27,20.20,wU:15.15,wD:22.28,TLB:15:16:23:19.20.21,14:15:23.18.19:21,THB:17:16:23.18.19:21,15:20:23:24.17.16,TEB:17:18:21:24.16.15,19:20:24:23.18.17,(2.51,4.26,;3.34,2.95,;4.16,1.64,;4.67,3.73,;6.01,2.96,;6.02,1.43,;7.36,.67,;8.69,1.44,;10.03,.69,;8.67,3,;7.33,3.75,;7.32,5.3,;2.05,2.1,;2.14,.56,;.67,2.78,;-.62,1.93,;-.63,.4,;-1.64,-.88,;-3.05,-.32,;-3.06,1.28,;-2.02,2.51,;-3.37,2.03,;-3.36,.54,;-4.56,-.74,;-2.03,.05,;-4.86,.95,;-6.35,1.36,;-4.45,2.44,;-5.27,-.53,)| Show InChI InChI=1S/C21H27ClFNO4S/c1-20(2,28-17-5-4-15(23)8-16(17)22)19(25)24-18-13-6-12-7-14(18)11-21(9-12,10-13)29(3,26)27/h4-5,8,12-14,18H,6-7,9-11H2,1-3H3,(H,24,25)/t12?,13?,14?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197412
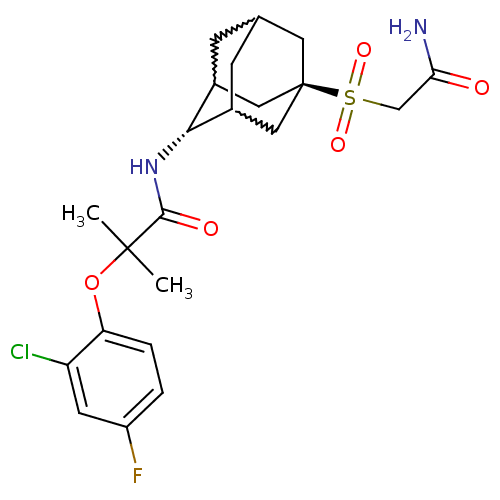
(CHEMBL241711 | N-(5-carbamoylmethanesulfonyl-adama...)Show SMILES CC(C)(Oc1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(=O)(=O)CC(N)=O |w:16.27,20.20,18.19,wU:15.15,wD:22.28,TLB:15:16:23:19.20.21,14:15:23.18.19:21,THB:17:18:21:24.16.15,17:16:23.18.19:21,15:20:23:24.17.16,TEB:19:18:24:20.21.15,19:20:24:23.18.17,(4.92,-5.99,;5.74,-7.3,;6.55,-8.6,;7.06,-6.51,;8.4,-7.28,;8.41,-8.82,;9.75,-9.57,;11.08,-8.8,;12.42,-9.55,;11.06,-7.25,;9.72,-6.49,;9.7,-4.95,;4.45,-8.15,;4.55,-9.68,;3.07,-7.46,;1.79,-8.31,;1.78,-9.84,;.76,-11.12,;-.65,-10.56,;-.65,-8.96,;.39,-7.73,;-.96,-8.21,;-.95,-9.7,;-2.15,-10.97,;.38,-10.18,;-2.45,-9.29,;-2.04,-7.8,;-2.85,-10.77,;-3.94,-8.88,;-4.33,-7.39,;-5.82,-6.98,;-3.24,-6.3,)| Show InChI InChI=1S/C22H28ClFN2O5S/c1-21(2,31-17-4-3-15(24)7-16(17)23)20(28)26-19-13-5-12-6-14(19)10-22(8-12,9-13)32(29,30)11-18(25)27/h3-4,7,12-14,19H,5-6,8-11H2,1-2H3,(H2,25,27)(H,26,28)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50195506
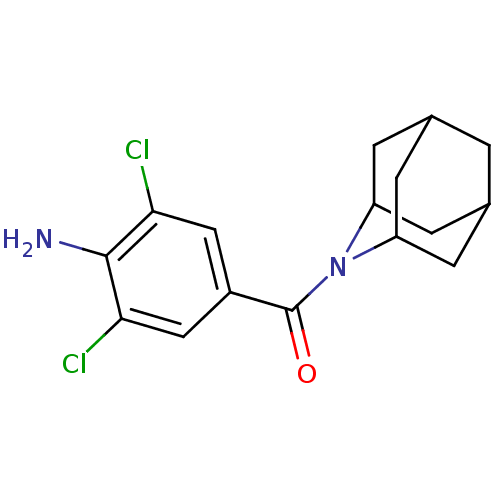
((4-amino-3,5-dichloro-phenyl)-(2-aza-tricyclo[3.3....)Show SMILES Nc1c(Cl)cc(cc1Cl)C(=O)N1C2CC3CC(C2)CC1C3 |TLB:20:19:15.14.13:17,THB:9:11:15.14.13:17,20:14:17:11.18.19,15:14:11:16.17.18,15:16:11:14.13.20| Show InChI InChI=1S/C16H18Cl2N2O/c17-13-6-10(7-14(18)15(13)19)16(21)20-11-2-8-1-9(4-11)5-12(20)3-8/h6-9,11-12H,1-5,19H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 16: 6241-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.035
BindingDB Entry DOI: 10.7270/Q2B857R4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13749
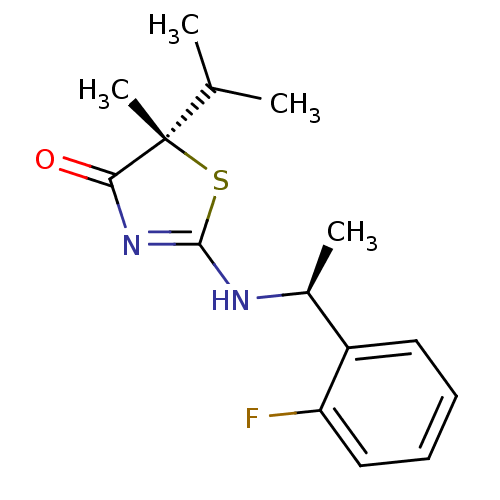
((5S)-2-{[(1S)-1-(2-fluorophenyl)ethyl]amino}-5-met...)Show SMILES CC(C)[C@]1(C)SC(N[C@@H](C)c2ccccc2F)=NC1=O |r,c:17| Show InChI InChI=1S/C15H19FN2OS/c1-9(2)15(4)13(19)18-14(20-15)17-10(3)11-7-5-6-8-12(11)16/h5-10H,1-4H3,(H,17,18,19)/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Amgen
| Assay Description
Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... |
J Med Chem 50: 429-32 (2007)
Article DOI: 10.1021/jm061214f
BindingDB Entry DOI: 10.7270/Q20Z71JH |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197399

(2-(2-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(20.57,1.97,;21.38,.67,;22.2,-.64,;22.71,1.45,;24.05,.69,;24.05,-.85,;25.39,-1.61,;26.72,-.83,;26.71,.72,;25.37,1.47,;25.35,3.01,;20.1,-.18,;20.19,-1.72,;18.72,.5,;17.44,-.35,;17.42,-1.88,;16.41,-3.16,;15.01,-2.59,;15,-1,;16.04,.23,;14.69,-.25,;14.7,-1.73,;13.5,-3.01,;16.03,-2.22,;13.21,-1.32,;11.71,-.92,;13.61,.16,;12.8,-2.81,)| Show InChI InChI=1S/C20H27ClN2O4S/c1-19(2,27-16-6-4-3-5-15(16)21)18(24)23-17-13-7-12-8-14(17)11-20(9-12,10-13)28(22,25)26/h3-6,12-14,17H,7-11H2,1-2H3,(H,23,24)(H2,22,25,26)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195504
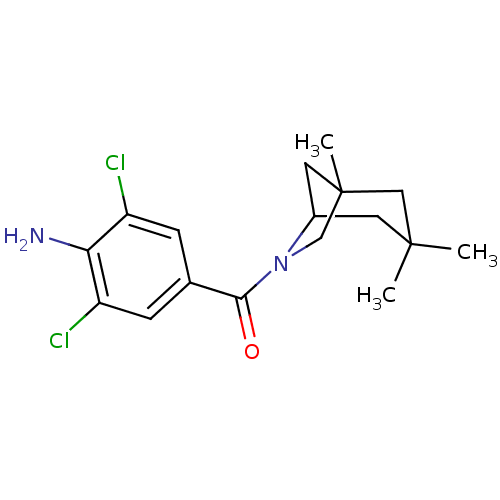
((4-amino-3,5-dichlorophenyl)(1,3,3-trimethyl-6-aza...)Show SMILES CC12CC(CC(C)(C)C1)N(C2)C(=O)c1cc(Cl)c(N)c(Cl)c1 Show InChI InChI=1S/C17H22Cl2N2O/c1-16(2)6-11-7-17(3,8-16)9-21(11)15(22)10-4-12(18)14(20)13(19)5-10/h4-5,11H,6-9,20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli by SPA |
Bioorg Med Chem Lett 16: 6241-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.035
BindingDB Entry DOI: 10.7270/Q2B857R4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195506

((4-amino-3,5-dichloro-phenyl)-(2-aza-tricyclo[3.3....)Show SMILES Nc1c(Cl)cc(cc1Cl)C(=O)N1C2CC3CC(C2)CC1C3 |TLB:20:19:15.14.13:17,THB:9:11:15.14.13:17,20:14:17:11.18.19,15:14:11:16.17.18,15:16:11:14.13.20| Show InChI InChI=1S/C16H18Cl2N2O/c17-13-6-10(7-14(18)15(13)19)16(21)20-11-2-8-1-9(4-11)5-12(20)3-8/h6-9,11-12H,1-5,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli by SPA |
Bioorg Med Chem Lett 16: 6241-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.035
BindingDB Entry DOI: 10.7270/Q2B857R4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202102
(CHEMBL375156 | N-(5-Hydroxy-adamantan-2-yl)-2-[4-(...)Show SMILES CC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(-1.15,-33.95,;-1.14,-35.49,;.19,-36.26,;.19,-37.8,;1.52,-38.56,;2.85,-37.8,;2.85,-36.25,;1.52,-35.48,;4.19,-38.57,;4.18,-40.11,;5.51,-40.88,;6.84,-40.11,;6.84,-38.56,;5.51,-37.8,;8.18,-40.88,;9.46,-41.72,;8.99,-39.57,;7.38,-42.19,;-2.48,-36.27,;-2.47,-37.81,;-3.81,-35.5,;-5.14,-36.28,;-5.24,-37.8,;-6.33,-39.02,;-7.7,-38.37,;-7.61,-36.79,;-6.5,-35.62,;-7.88,-36.01,;-7.95,-37.5,;-9.42,-37,;-9.22,-38.7,;-6.66,-38.06,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14?,15?,16?,17?,20-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data