

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Homo sapiens (Human)) | BDBM50124947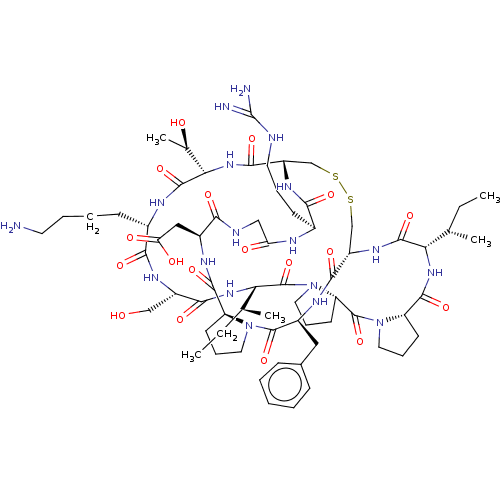 (CHEMBL453539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human cationic trypsin using Boc-VPR-MCA as substrate measured every 30 secs for 10 mins | Eur J Med Chem 155: 695-704 (2018) Article DOI: 10.1016/j.ejmech.2018.06.029 BindingDB Entry DOI: 10.7270/Q29026CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406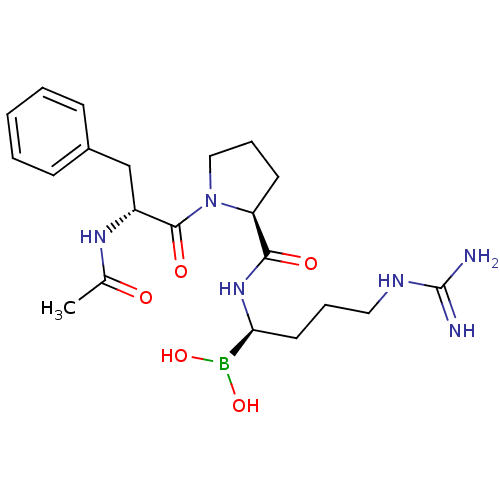 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of trypsin | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50069922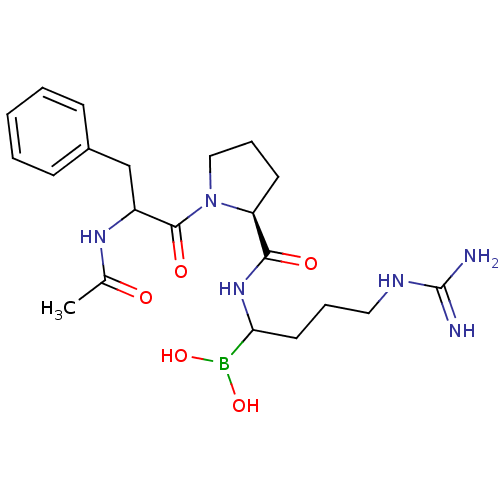 (Boropeptide analogue | CHEMBL102069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for selectivity against trypsin | Bioorg Med Chem Lett 7: 79-84 (1997) Checked by Author Article DOI: 10.1016/S0960-894X(96)00584-7 BindingDB Entry DOI: 10.7270/Q2FN16P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109663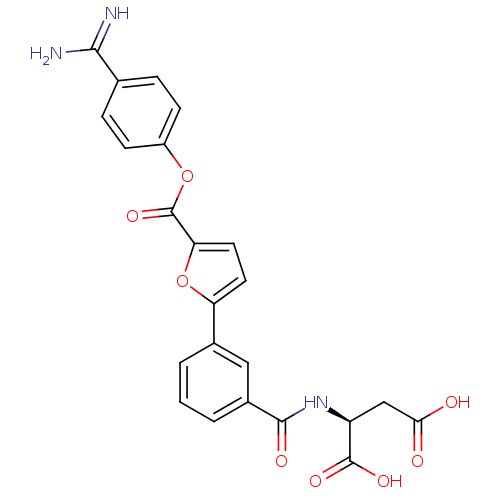 (US8609715, A-71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234238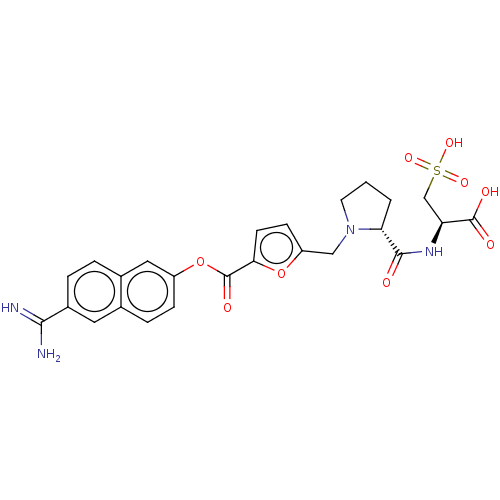 (US9346821, A-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50289586 (3-Methyl-2'-sulfamoyl-biphenyl-4-carboxylic acid [...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109651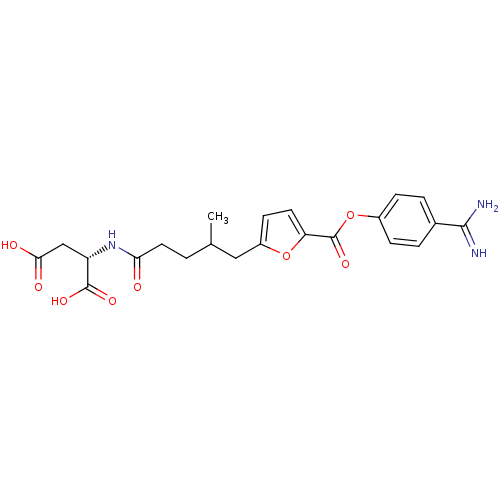 (US8609715, A-59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109630 (US8609715, B-21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158146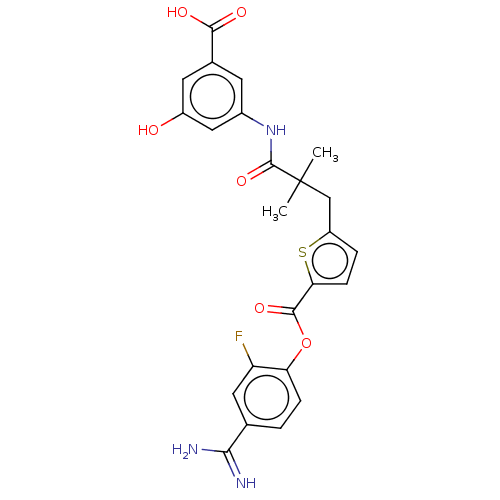 (US9024044, 74 | US9227949, 74 | US9655879, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 uL) was mixed with 20 uM fluorescence enzyme substrate (Boc-Phe-Ser-Arg-AMC, 50 uL) mixed ... | US Patent US9227949 (2016) BindingDB Entry DOI: 10.7270/Q2959GBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158146 (US9024044, 74 | US9227949, 74 | US9655879, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL) was mixed with 20 μM fluorescence enzyme substrate (Boc-Phe-Ser-Arg-AMC, 50 ... | US Patent US9024044 (2015) BindingDB Entry DOI: 10.7270/Q2F47MVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158146 (US9024044, 74 | US9227949, 74 | US9655879, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EA Pharma Co., Ltd. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9655879 (2017) BindingDB Entry DOI: 10.7270/Q22Z17KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109692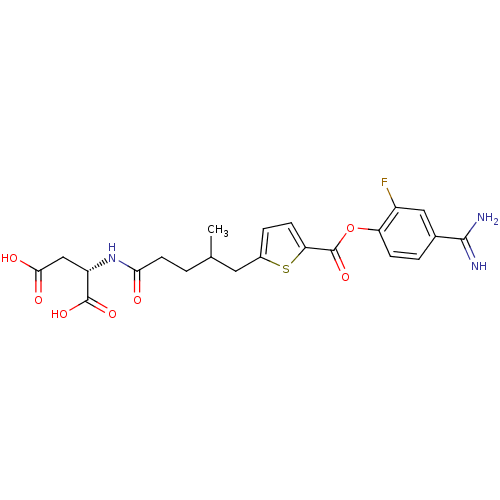 (US8609715, B-49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109629 (US8609715, B-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234247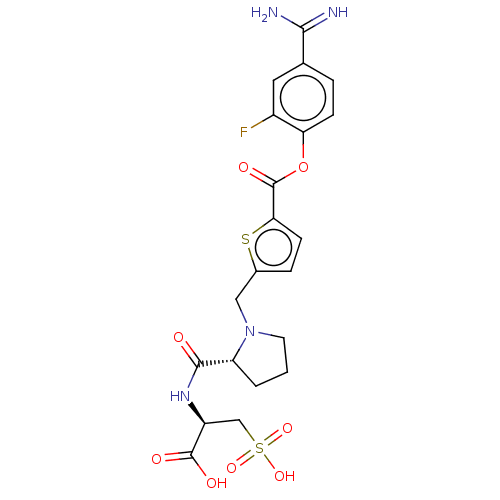 (US9346821, B-14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109668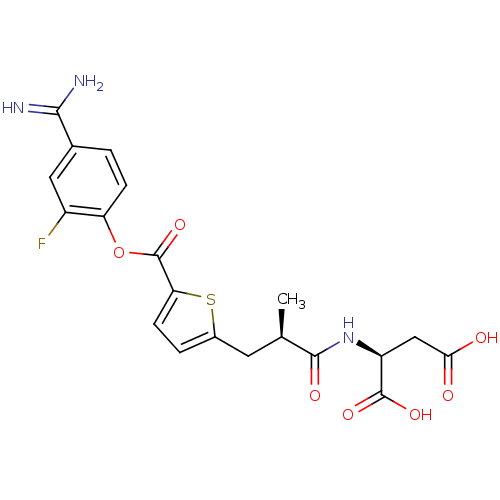 (US8609715, B-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109659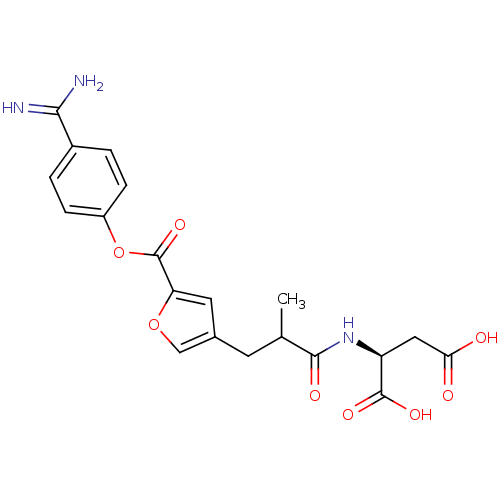 (US8609715, A-67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109710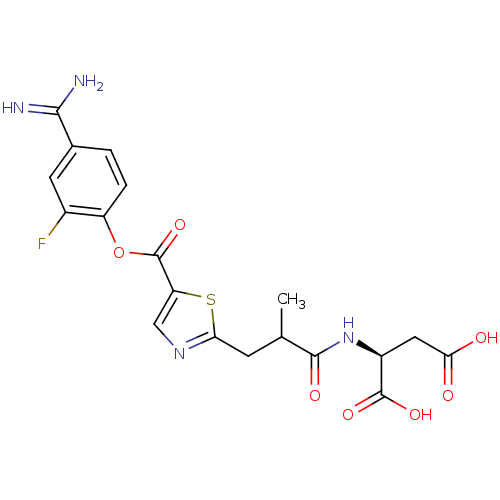 (US8609715, C-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158140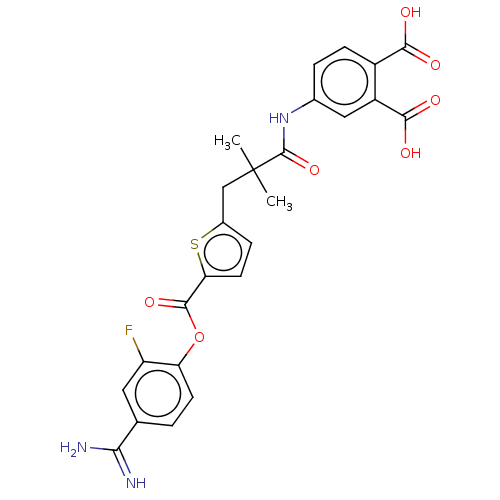 (US9024044, 68 | US9227949, 68 | US9655879, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL) was mixed with 20 μM fluorescence enzyme substrate (Boc-Phe-Ser-Arg-AMC, 50 ... | US Patent US9024044 (2015) BindingDB Entry DOI: 10.7270/Q2F47MVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234250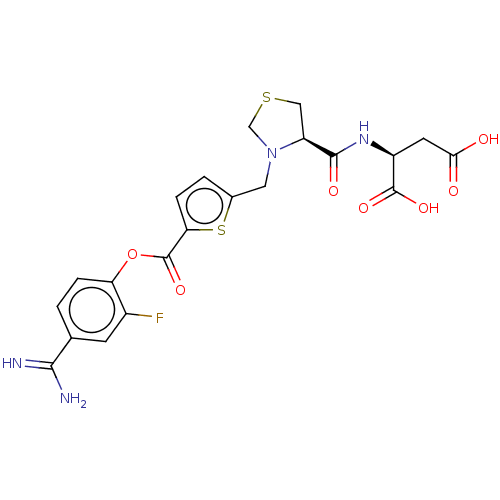 (US9346821, B-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158140 (US9024044, 68 | US9227949, 68 | US9655879, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EA Pharma Co., Ltd. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9655879 (2017) BindingDB Entry DOI: 10.7270/Q22Z17KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158140 (US9024044, 68 | US9227949, 68 | US9655879, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 uL) was mixed with 20 uM fluorescence enzyme substrate (Boc-Phe-Ser-Arg-AMC, 50 uL) mixed ... | US Patent US9227949 (2016) BindingDB Entry DOI: 10.7270/Q2959GBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109684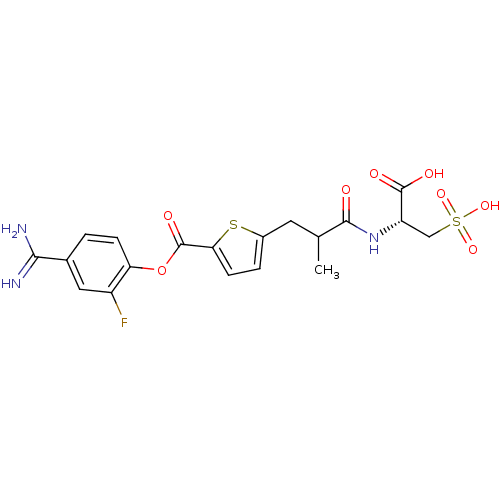 (US8609715, B-41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109622 (US8609715, B-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109685 (US8609715, B-42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109660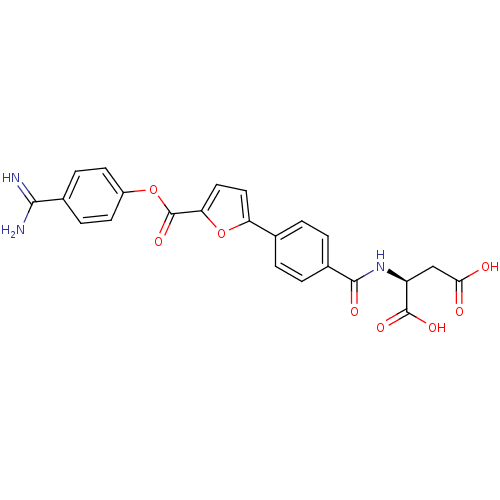 (US8609715, A-68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234237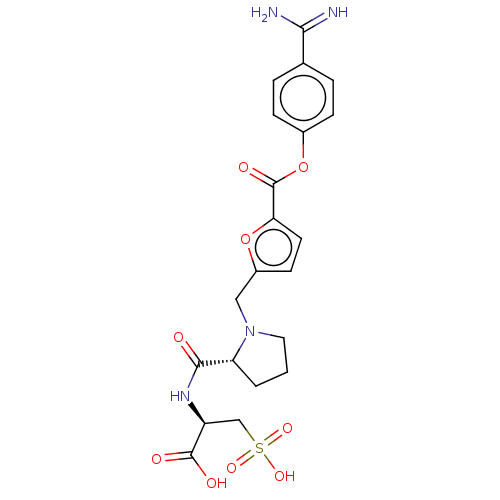 (US9346821, A-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088657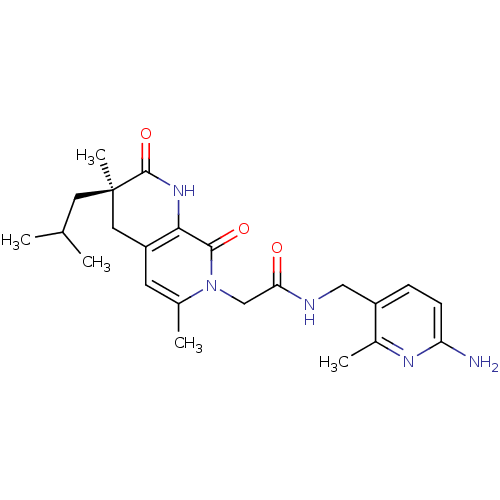 (CHEMBL11181 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234266 (US9346821, B-55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234243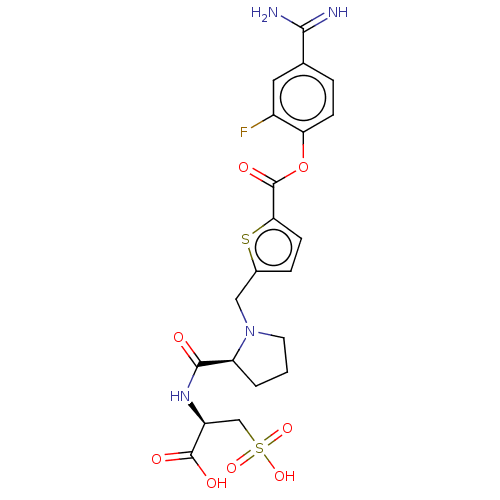 (US9346821, B-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109700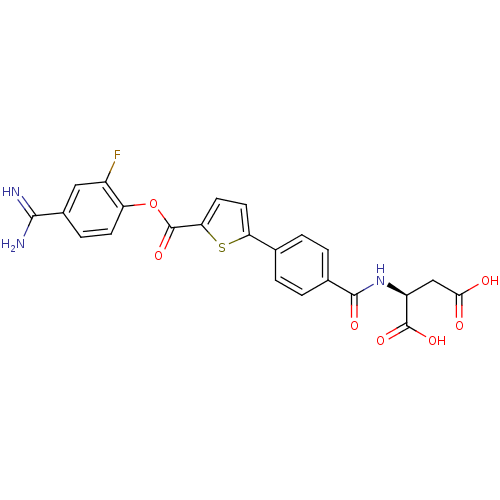 (US8609715, B-57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088659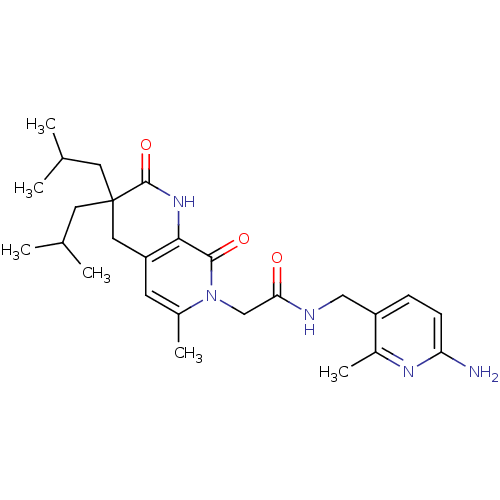 (CHEMBL10346 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071570 (8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234246 (US9346821, B-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109662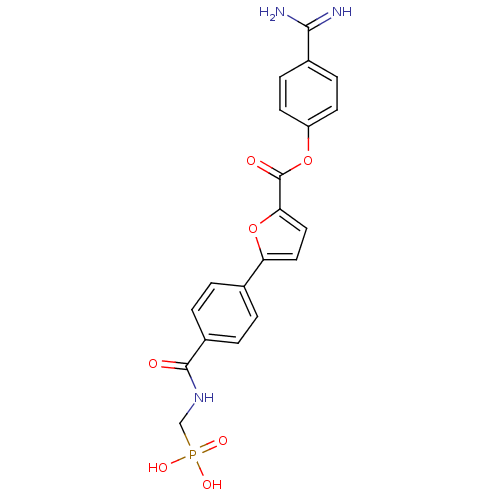 (US8609715, A-70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109673 (US8609715, B-30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109691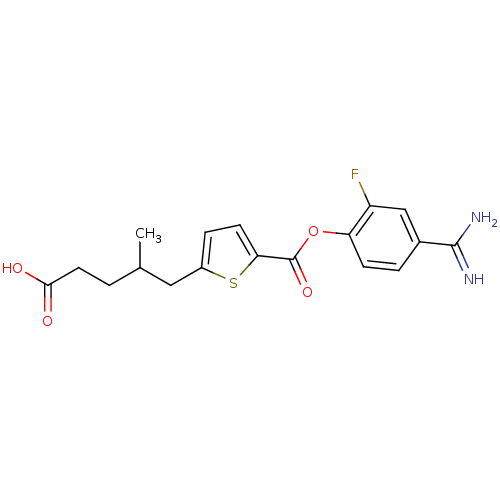 (US8609715, B-48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234251 (US9346821, B-21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109613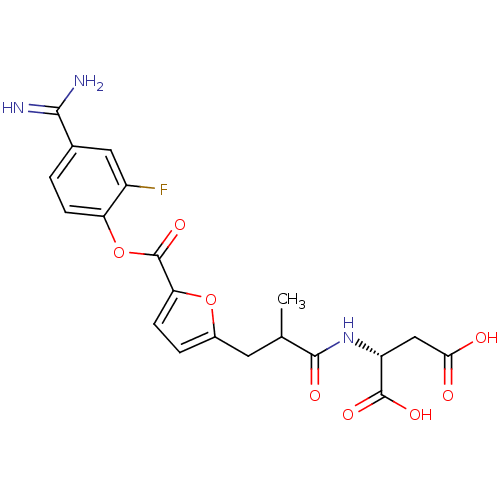 (US8609715, A-35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234253 (US9346821, B-26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109667 (US8609715, C-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109631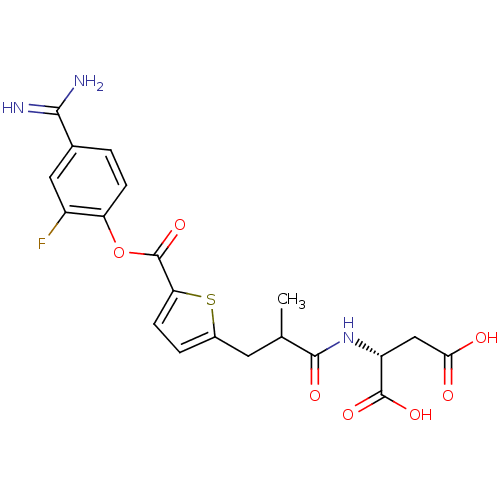 (US8609715, B-22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109705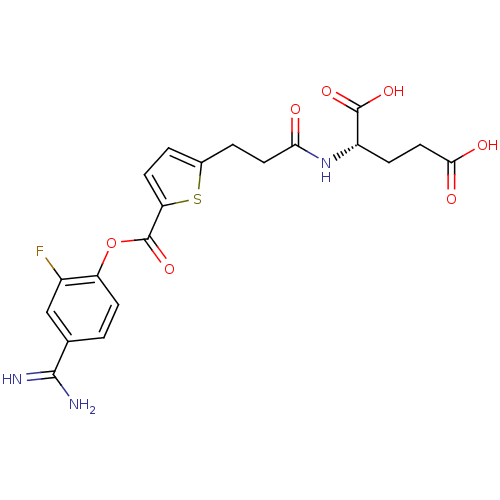 (US8609715, B-62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM308123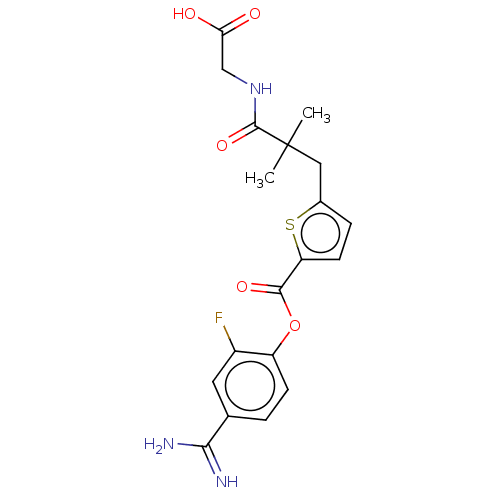 (US9655879, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EA Pharma Co., Ltd. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9655879 (2017) BindingDB Entry DOI: 10.7270/Q22Z17KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158075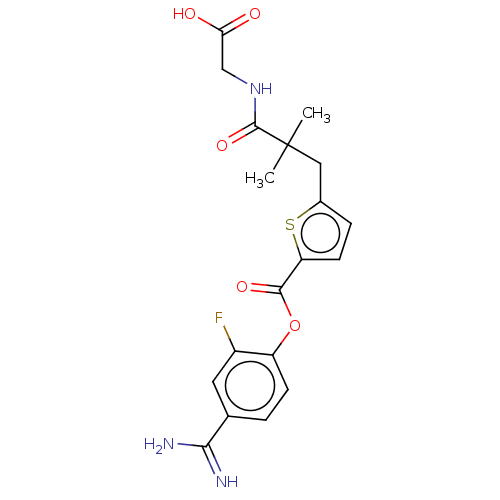 (US9024044, 3 | US9227949, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL) was mixed with 20 μM fluorescence enzyme substrate (Boc-Phe-Ser-Arg-AMC, 50 ... | US Patent US9024044 (2015) BindingDB Entry DOI: 10.7270/Q2F47MVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234269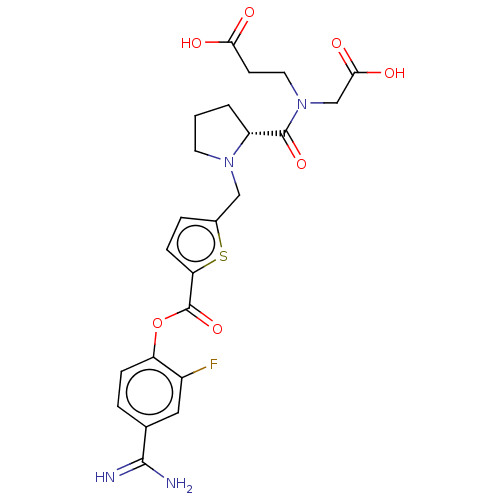 (US9346821, B-60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM158075 (US9024044, 3 | US9227949, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 uL) was mixed with 20 uM fluorescence enzyme substrate (Boc-Phe-Ser-Arg-AMC, 50 uL) mixed ... | US Patent US9227949 (2016) BindingDB Entry DOI: 10.7270/Q2959GBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM234267 (US9346821, B-56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AJINOMOTO CO., INC. US Patent | Assay Description Using a 96 well plate (#3915, Costar), a test compound (25 μL), 400 mM Tris-HCl buffer (pH 8.0, 25 μL) and 0.5 mg/mL fluorescence enzyme su... | US Patent US9346821 (2016) BindingDB Entry DOI: 10.7270/Q2RB73G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM109671 (US8609715, B-28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. US Patent | Assay Description Inhibition assay using human trysin. | US Patent US8609715 (2013) BindingDB Entry DOI: 10.7270/Q2Z31X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071571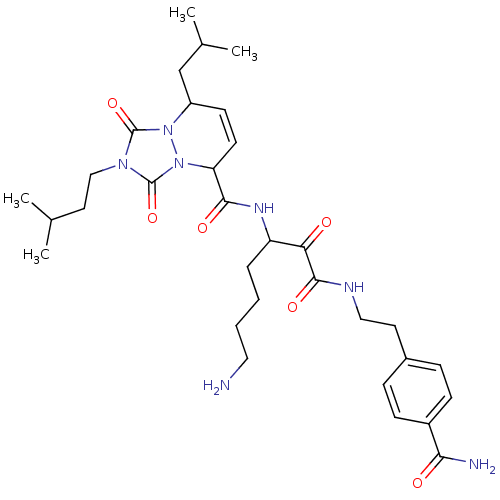 (8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1616 total ) | Next | Last >> |