

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14481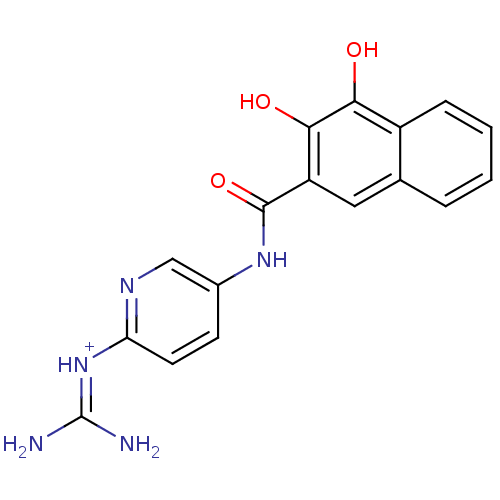 (CA-23 | [amino({5-[(3,4-dihydroxynaphthalene-2-)am...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14142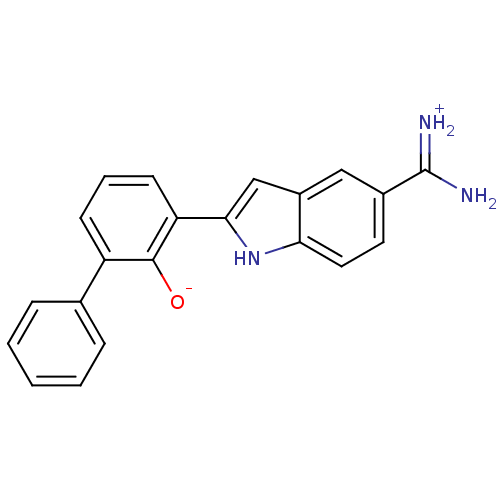 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14479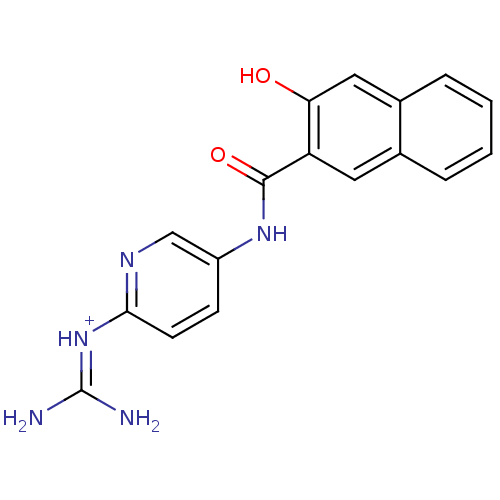 (CA-21 | [amino({5-[(3-hydroxynaphthalene-2-)amido]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14150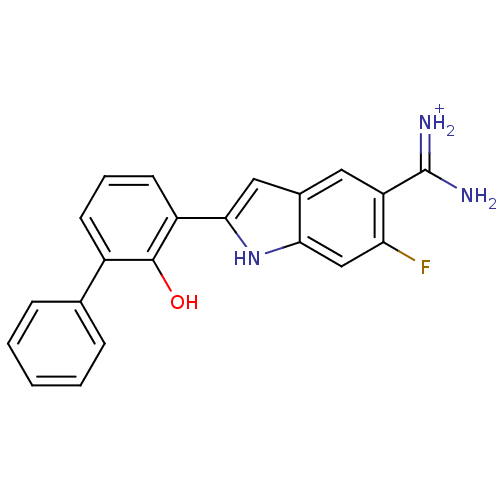 (APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14169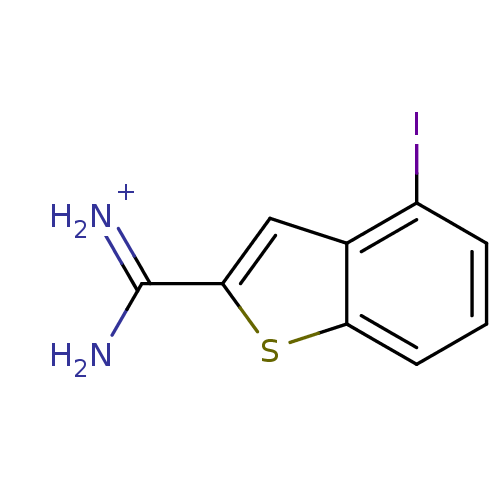 (4-Iodobenzo[b]thiophene-2-carboxamidine | APC-6860...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14152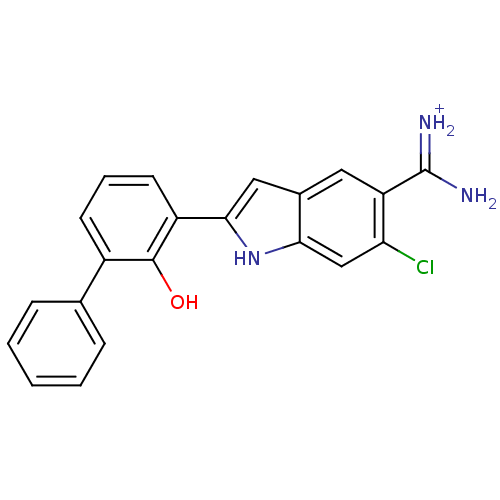 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14145 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14144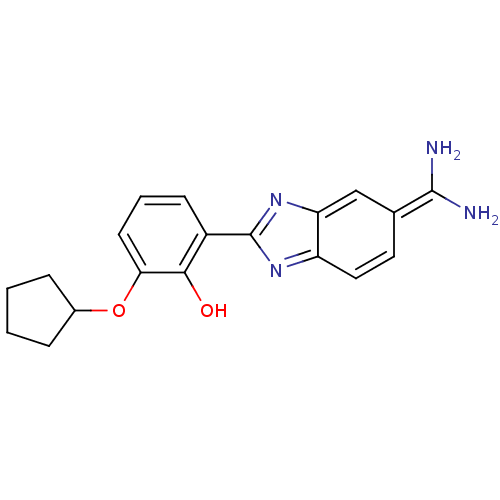 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14350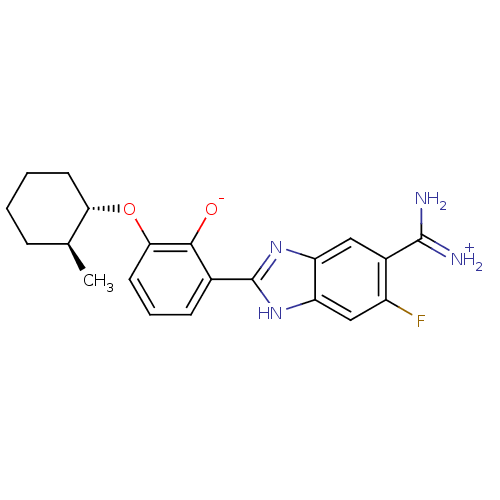 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14480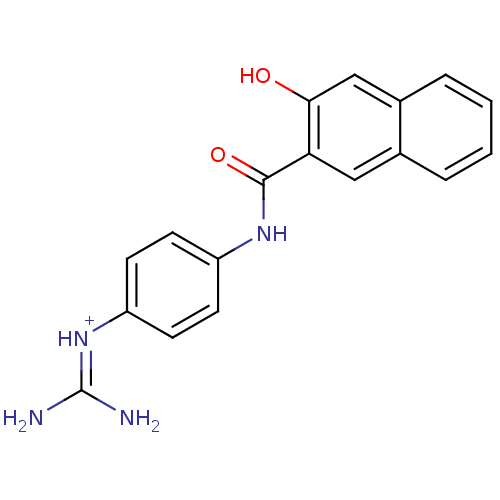 (CA-22 | [amino({4-[(3-hydroxynaphthalene-2-)amido]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14148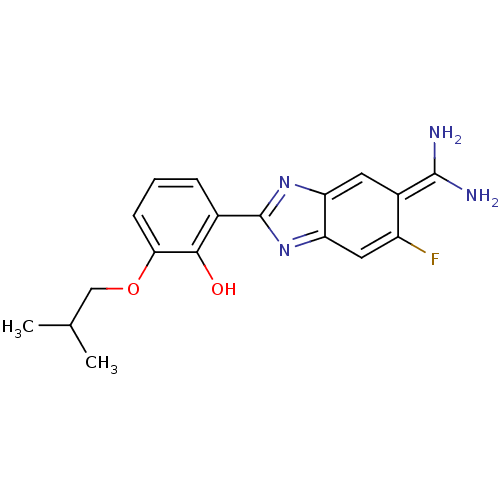 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14333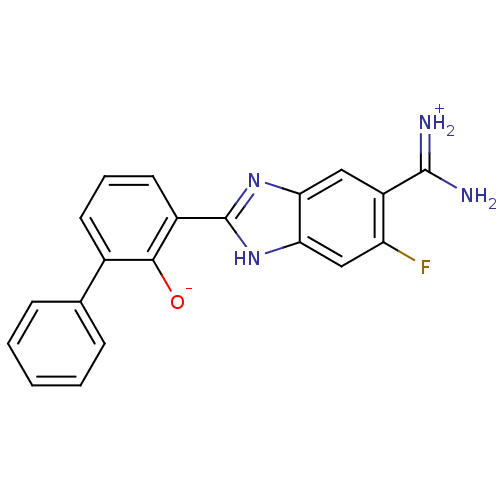 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM14482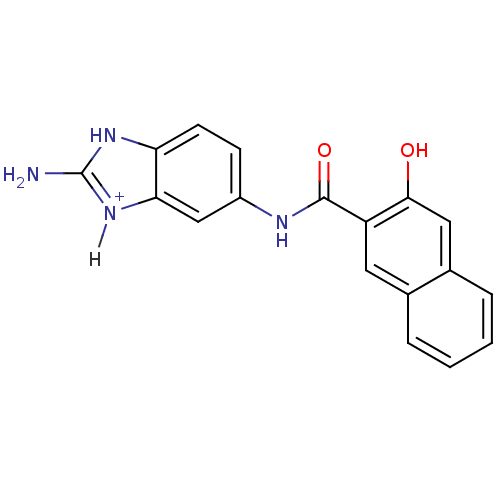 (2-amino-5-[(3-hydroxynaphthalene-2-)amido]-1H-1,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator [179-431,I214M,N322A,S371A] (Homo sapiens (Human)) | BDBM772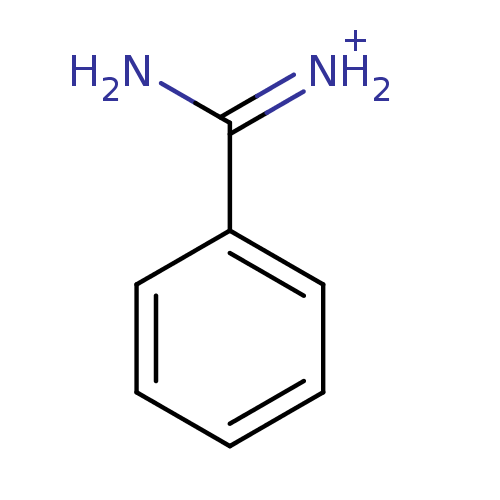 (Benzamidine | CHEMBL79897 | [amino(phenyl)methylid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||