 Found 238 hits of ki data for polymerid = 1568,50004998
Found 238 hits of ki data for polymerid = 1568,50004998 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein kinase C (unknown origin) |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated Protein C (unknown origin) |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated Protein C (unknown origin) |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380619
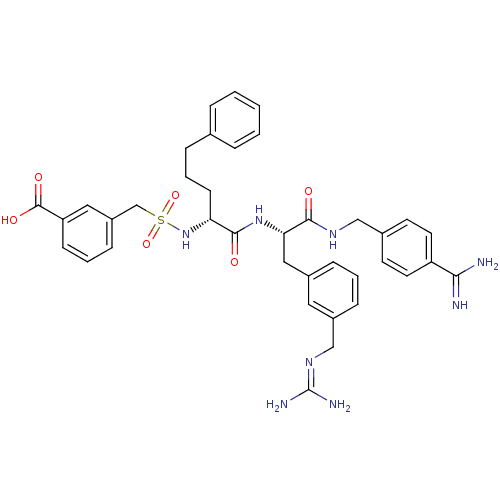
(CHEMBL2016865)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1cccc(-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-c2ccccc2)-[#7]S(=O)(=O)[#6]-c2cccc(c2)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)-[#6](-[#7])=[#7])c1 |r| Show InChI InChI=1S/C38H44N8O6S/c39-34(40)30-17-15-26(16-18-30)22-43-35(47)33(21-27-10-4-11-28(19-27)23-44-38(41)42)45-36(48)32(14-6-9-25-7-2-1-3-8-25)46-53(51,52)24-29-12-5-13-31(20-29)37(49)50/h1-5,7-8,10-13,15-20,32-33,46H,6,9,14,21-24H2,(H3,39,40)(H,43,47)(H,45,48)(H,49,50)(H4,41,42,44)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50541586
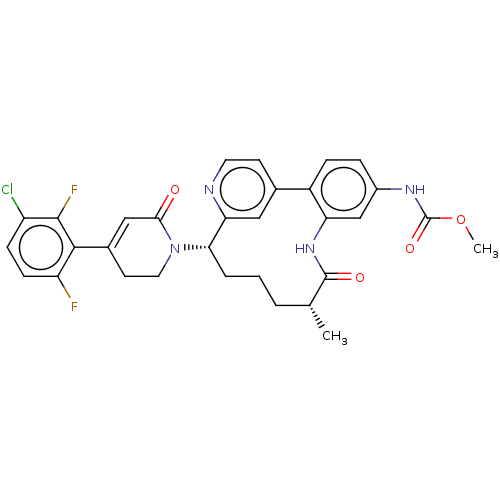
(CHEMBL4638245)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| Show InChI InChI=1S/C31H29ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14-17,26H,3-5,11,13H2,1-2H3,(H,36,41)(H,37,40)/t17-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380620
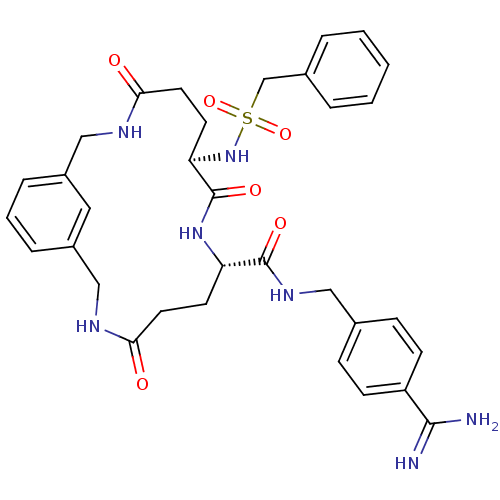
(CHEMBL2016866)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCC(=O)NCc3cccc(CNC(=O)CC[C@@H](NS(=O)(=O)Cc4ccccc4)C(=O)N2)c3)cc1 |r| Show InChI InChI=1S/C33H39N7O6S/c34-31(35)26-11-9-22(10-12-26)18-38-32(43)27-13-15-29(41)36-19-24-7-4-8-25(17-24)20-37-30(42)16-14-28(33(44)39-27)40-47(45,46)21-23-5-2-1-3-6-23/h1-12,17,27-28,40H,13-16,18-21H2,(H3,34,35)(H,36,41)(H,37,42)(H,38,43)(H,39,44)/t27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM47178
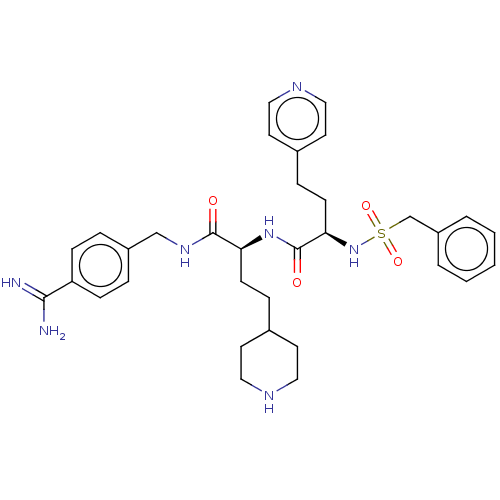
(BDBM108103 | US8598206, Table 6, 12)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCc2ccncc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C33H43N7O4S/c34-31(35)28-10-6-26(7-11-28)22-38-32(41)29(12-8-24-14-18-36-19-15-24)39-33(42)30(13-9-25-16-20-37-21-17-25)40-45(43,44)23-27-4-2-1-3-5-27/h1-7,10-11,16-17,20-21,24,29-30,36,40H,8-9,12-15,18-19,22-23H2,(H3,34,35)(H,38,41)(H,39,42)/t29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50425661
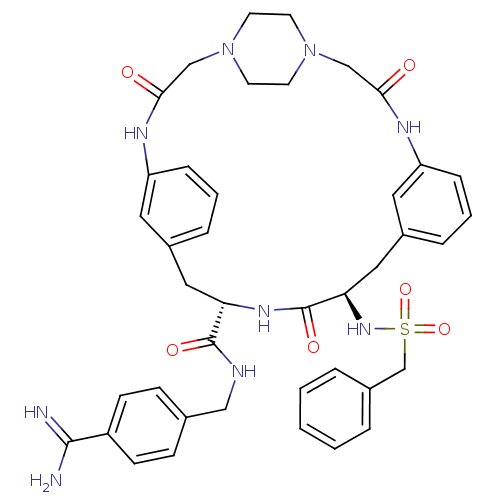
(CHEMBL2315240)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cccc(NC(=O)CN4CCN(CC4)CC(=O)Nc4cccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)c4)c3)cc1 |r,wU:38.39,wD:11.10,(46.63,-3.72,;46.61,-5.26,;47.93,-6.04,;45.27,-6.01,;45.24,-7.55,;43.89,-8.29,;42.58,-7.51,;41.23,-8.26,;39.92,-7.47,;38.57,-8.22,;38.55,-9.76,;37.25,-7.44,;35.9,-8.19,;35.89,-9.73,;34.55,-10.49,;34.54,-12.03,;35.87,-12.8,;37.21,-12.04,;38.54,-12.81,;38.53,-14.34,;39.86,-15.11,;36.03,-17.42,;34.69,-16.66,;33.37,-17.44,;32.04,-16.69,;32.03,-15.16,;33.34,-14.37,;34.68,-15.13,;30.7,-14.41,;30.68,-12.88,;29.34,-12.12,;32,-12.09,;31.98,-10.56,;30.63,-9.8,;30.62,-8.25,;31.95,-7.48,;33.28,-8.24,;34.61,-7.46,;34.59,-5.92,;33.25,-5.16,;31.92,-5.95,;31.15,-7.27,;32.69,-7.27,;30.58,-5.18,;29.26,-5.96,;27.92,-5.19,;26.59,-5.96,;26.59,-7.51,;27.92,-8.28,;29.26,-7.51,;35.92,-5.13,;35.91,-3.59,;37.26,-5.89,;33.3,-9.77,;37.22,-10.5,;42.59,-5.97,;43.93,-5.22,)| Show InChI InChI=1S/C41H47N9O6S/c42-39(43)32-14-12-28(13-15-32)24-44-40(53)35-22-30-8-4-10-33(20-30)45-37(51)25-49-16-18-50(19-17-49)26-38(52)46-34-11-5-9-31(21-34)23-36(41(54)47-35)48-57(55,56)27-29-6-2-1-3-7-29/h1-15,20-21,35-36,48H,16-19,22-27H2,(H3,42,43)(H,44,53)(H,45,51)(H,46,52)(H,47,54)/t35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C using H-D-Lys(Cbz)-Pro-Arg-pNA as substrate after 5 to 10 mins by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50205841
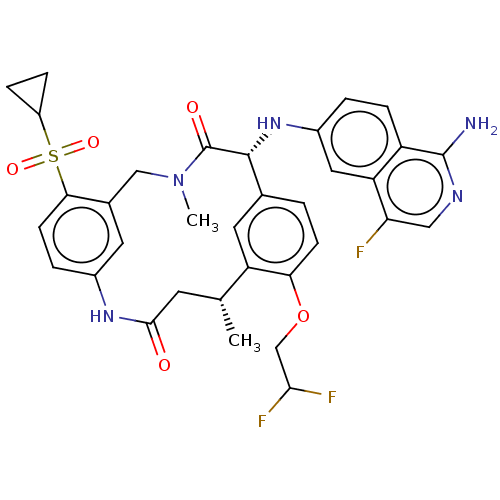
(CHEMBL3898956)Show SMILES C[C@@H]1CC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc(OCC(F)F)c1c3)c2)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C34H34F3N5O5S/c1-18-11-31(43)40-21-5-10-29(48(45,46)23-6-7-23)20(12-21)16-42(2)34(44)32(19-3-9-28(25(18)13-19)47-17-30(36)37)41-22-4-8-24-26(14-22)27(35)15-39-33(24)38/h3-5,8-10,12-15,18,23,30,32,41H,6-7,11,16-17H2,1-2H3,(H2,38,39)(H,40,43)/t18-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) |
ACS Med Chem Lett 8: 67-72 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00375
BindingDB Entry DOI: 10.7270/Q20P120Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447515
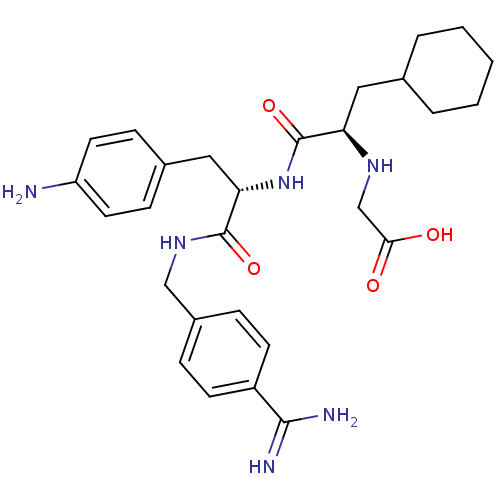
(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50098596
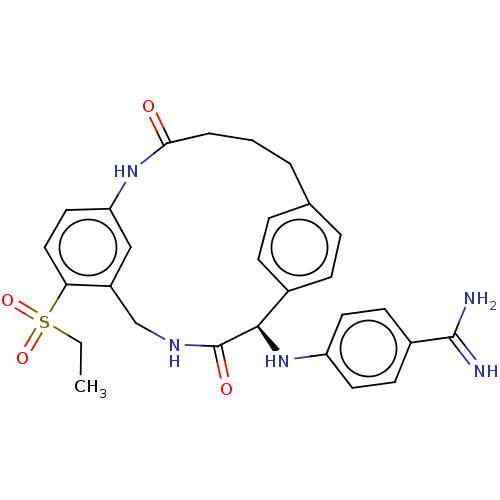
(CHEMBL3594313)Show SMILES CCS(=O)(=O)c1ccc2NC(=O)CCCc3ccc(cc3)[C@@H](Nc3ccc(cc3)C(N)=N)C(=O)NCc1c2 |r| Show InChI InChI=1S/C28H31N5O4S/c1-2-38(36,37)24-15-14-23-16-21(24)17-31-28(35)26(33-22-12-10-20(11-13-22)27(29)30)19-8-6-18(7-9-19)4-3-5-25(34)32-23/h6-16,26,33H,2-5,17H2,1H3,(H3,29,30)(H,31,35)(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human APC measured for 30 mins |
J Med Chem 58: 6225-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00788
BindingDB Entry DOI: 10.7270/Q2DN46TN |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380616
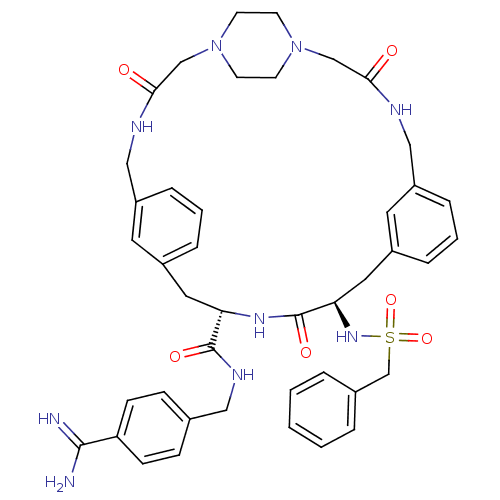
(CHEMBL2016869)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cccc(CNC(=O)CN4CCN(CC4)CC(=O)NCc4cccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)c4)c3)cc1 |r,wU:11.10,wD:40.41,(10.73,-8.05,;9.41,-8.84,;9.42,-10.38,;8.06,-8.08,;6.74,-8.86,;5.4,-8.11,;5.39,-6.57,;4.05,-5.81,;4.03,-4.27,;2.69,-3.51,;1.36,-4.3,;2.68,-1.97,;4,-1.18,;3.98,.36,;2.63,1.11,;2.61,2.65,;3.94,3.43,;5.27,2.67,;6.6,3.46,;6.58,5,;7.91,5.78,;9.25,5.02,;7.89,7.32,;6.55,8.08,;5.22,7.29,;3.88,8.05,;3.87,9.59,;5.19,10.38,;6.53,9.62,;2.53,10.35,;1.21,9.57,;-.13,10.33,;1.22,8.03,;-.11,7.25,;-.09,5.71,;1.25,4.94,;1.26,3.4,;-.07,2.63,;-1.41,3.4,;-2.73,2.62,;-2.73,1.08,;-4.06,.3,;-5.39,1.07,;-6.17,2.41,;-4.63,2.41,;-6.72,.29,;-8.06,1.05,;-8.06,2.59,;-9.4,3.35,;-10.73,2.58,;-10.72,1.03,;-9.38,.27,;-1.39,.32,;-.06,1.09,;-1.38,-1.22,;-1.42,4.93,;5.3,1.14,;6.7,-5.78,;8.04,-6.53,)| Show InChI InChI=1S/C43H51N9O6S/c44-41(45)36-14-12-30(13-15-36)24-48-42(55)37-22-32-8-4-10-34(20-32)25-46-39(53)27-51-16-18-52(19-17-51)28-40(54)47-26-35-11-5-9-33(21-35)23-38(43(56)49-37)50-59(57,58)29-31-6-2-1-3-7-31/h1-15,20-21,37-38,50H,16-19,22-29H2,(H3,44,45)(H,46,53)(H,47,54)(H,48,55)(H,49,56)/t37-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108101
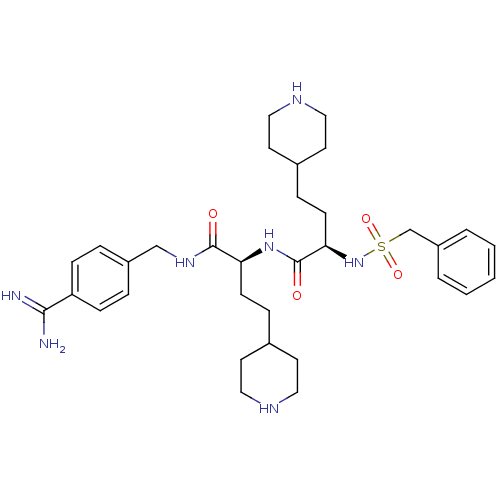
(US8598206, Table 6, 10)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2CCNCC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C33H49N7O4S/c34-31(35)28-10-6-26(7-11-28)22-38-32(41)29(12-8-24-14-18-36-19-15-24)39-33(42)30(13-9-25-16-20-37-21-17-25)40-45(43,44)23-27-4-2-1-3-5-27/h1-7,10-11,24-25,29-30,36-37,40H,8-9,12-23H2,(H3,34,35)(H,38,41)(H,39,42)/t29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50192771

(CHEMBL3898371)Show SMILES [H][C@]1(C)COC(=O)Nc2ccc(c(CN(C)C(=O)[C@]([H])(Nc3ccc4c(c3)c(F)c[nH]c4=N)c3ccc1c(C)c3)c2)C1(CC1)C(O)=O |r| Show InChI InChI=1S/C34H34FN5O5/c1-18-12-20-4-7-24(18)19(2)17-45-33(44)39-22-6-9-27(34(10-11-34)32(42)43)21(13-22)16-40(3)31(41)29(20)38-23-5-8-25-26(14-23)28(35)15-37-30(25)36/h4-9,12-15,19,29,38H,10-11,16-17H2,1-3H3,(H2,36,37)(H,39,44)(H,42,43)/t19-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) at 37 degC by chromogenic substrate assay |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50448581
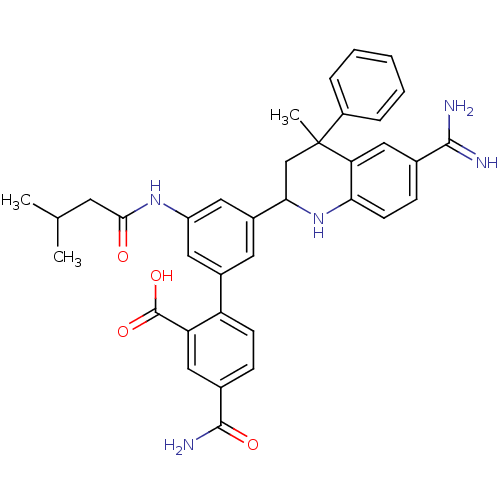
(CHEMBL3127463)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)C1CC(C)(c2ccccc2)c2cc(ccc2N1)C(N)=N Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human activated protein C assessed as release of p-nitroaniline after 10 to 120 mins by spectrophotometric analysis |
J Med Chem 57: 955-69 (2014)
Article DOI: 10.1021/jm401670x
BindingDB Entry DOI: 10.7270/Q2G44RTQ |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM14714
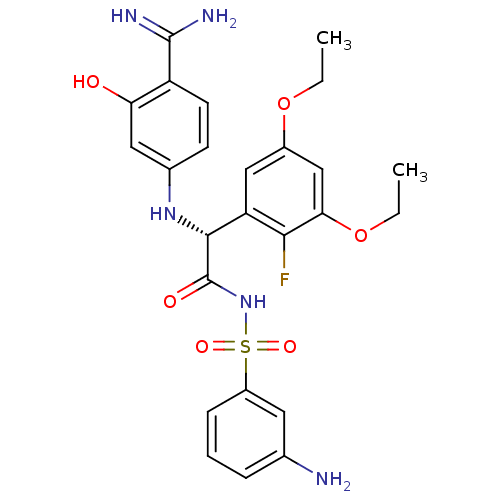
((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 87 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380624
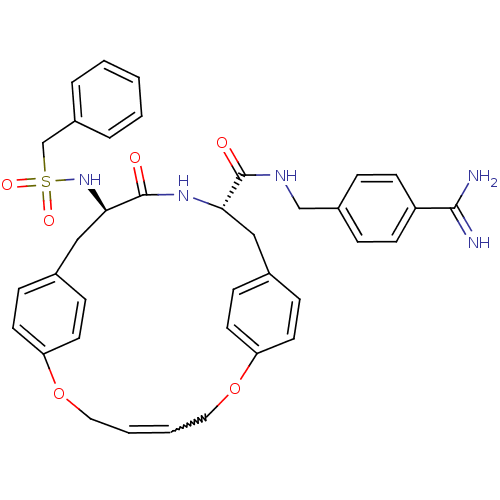
(CHEMBL2016871)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(OCC=CCOc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)cc3)cc1 |r| Show InChI InChI=1S/C37H39N5O6S/c38-35(39)30-14-8-28(9-15-30)24-40-36(43)33-22-26-10-16-31(17-11-26)47-20-4-5-21-48-32-18-12-27(13-19-32)23-34(37(44)41-33)42-49(45,46)25-29-6-2-1-3-7-29/h1-19,33-34,42H,20-25H2,(H3,38,39)(H,40,43)(H,41,44)/t33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447516
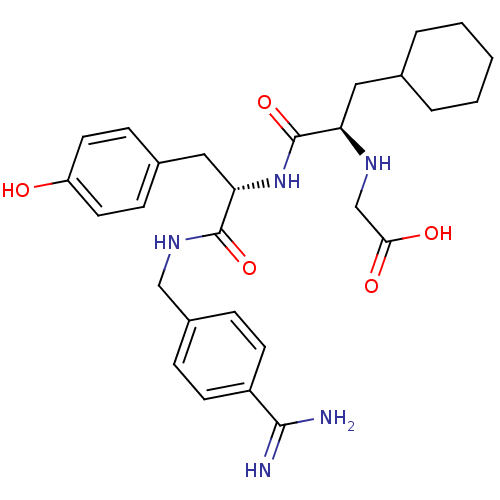
(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447528
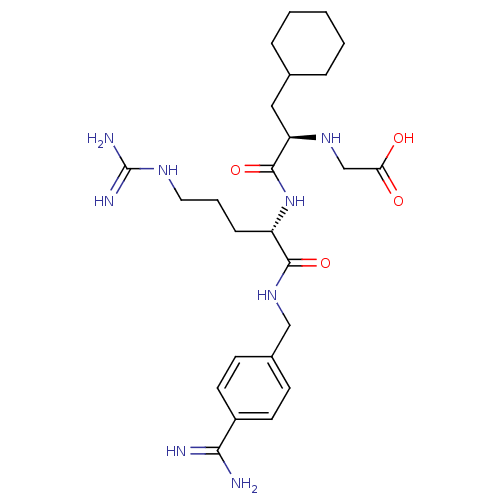
(CHEMBL3115904)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C25H40N8O4/c26-22(27)18-10-8-17(9-11-18)14-32-23(36)19(7-4-12-30-25(28)29)33-24(37)20(31-15-21(34)35)13-16-5-2-1-3-6-16/h8-11,16,19-20,31H,1-7,12-15H2,(H3,26,27)(H,32,36)(H,33,37)(H,34,35)(H4,28,29,30)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein kinase C using H-D-Lys(Cbo)-Pro-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50192770
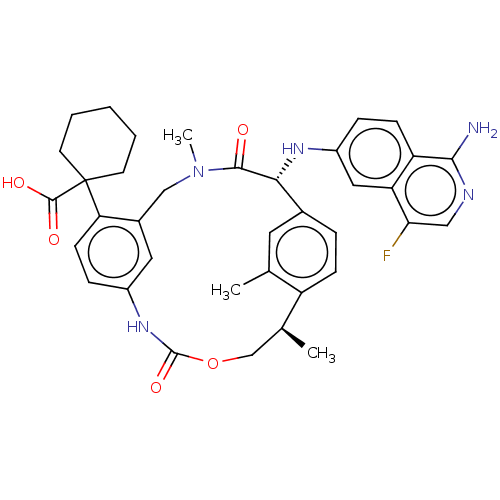
(CHEMBL3956096)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCCCC1)C(O)=O |r| Show InChI InChI=1S/C37H40FN5O5/c1-21-15-23-7-10-27(21)22(2)20-48-36(47)42-25-9-12-30(37(35(45)46)13-5-4-6-14-37)24(16-25)19-43(3)34(44)32(23)41-26-8-11-28-29(17-26)31(38)18-40-33(28)39/h7-12,15-18,22,32,41H,4-6,13-14,19-20H2,1-3H3,(H2,39,40)(H,42,47)(H,45,46)/t22-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) at 37 degC by chromogenic substrate assay |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50192766
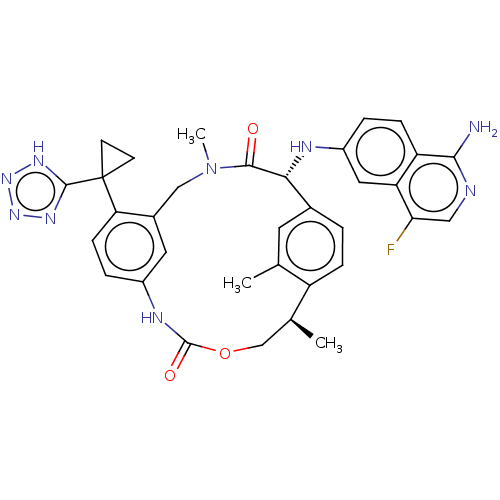
(CHEMBL3967204)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CC1)c1nnn[nH]1 |r| Show InChI InChI=1S/C34H34FN9O3/c1-18-12-20-4-7-24(18)19(2)17-47-33(46)39-22-6-9-27(34(10-11-34)32-40-42-43-41-32)21(13-22)16-44(3)31(45)29(20)38-23-5-8-25-26(14-23)28(35)15-37-30(25)36/h4-9,12-15,19,29,38H,10-11,16-17H2,1-3H3,(H2,36,37)(H,39,46)(H,40,41,42,43)/t19-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) at 37 degC by chromogenic substrate assay |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM189434
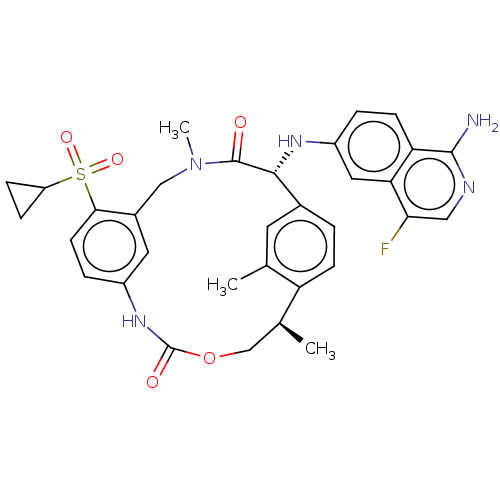
(US9174974, Example 19)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C33H34FN5O5S/c1-18-12-20-4-9-25(18)19(2)17-44-33(41)38-22-6-11-29(45(42,43)24-7-8-24)21(13-22)16-39(3)32(40)30(20)37-23-5-10-26-27(14-23)28(34)15-36-31(26)35/h4-6,9-15,19,24,30,37H,7-8,16-17H2,1-3H3,(H2,35,36)(H,38,41)/t19-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric ... |
J Med Chem 59: 7125-37 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00469
BindingDB Entry DOI: 10.7270/Q2TM7D37 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447523
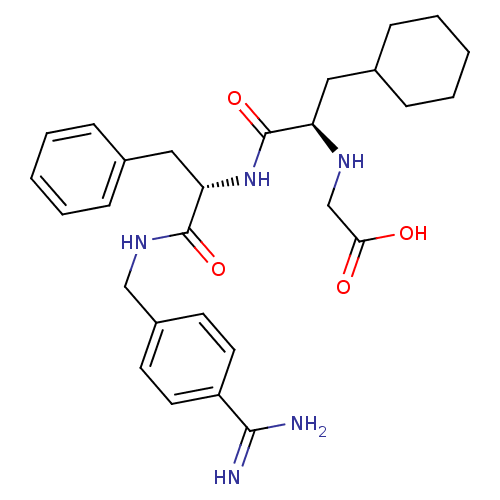
(CHEMBL3115897)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O4/c29-26(30)22-13-11-21(12-14-22)17-32-27(36)24(16-20-9-5-2-6-10-20)33-28(37)23(31-18-25(34)35)15-19-7-3-1-4-8-19/h2,5-6,9-14,19,23-24,31H,1,3-4,7-8,15-18H2,(H3,29,30)(H,32,36)(H,33,37)(H,34,35)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108111
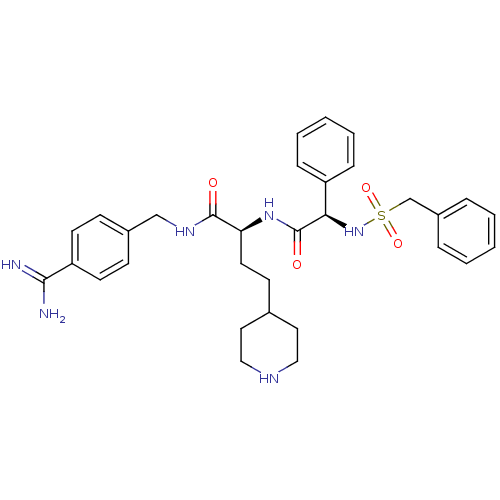
(US8598206, Table 6, 20)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@H](NS(=O)(=O)Cc2ccccc2)c2ccccc2)cc1 |r| Show InChI InChI=1S/C32H40N6O4S/c33-30(34)27-14-11-24(12-15-27)21-36-31(39)28(16-13-23-17-19-35-20-18-23)37-32(40)29(26-9-5-2-6-10-26)38-43(41,42)22-25-7-3-1-4-8-25/h1-12,14-15,23,28-29,35,38H,13,16-22H2,(H3,33,34)(H,36,39)(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50192764
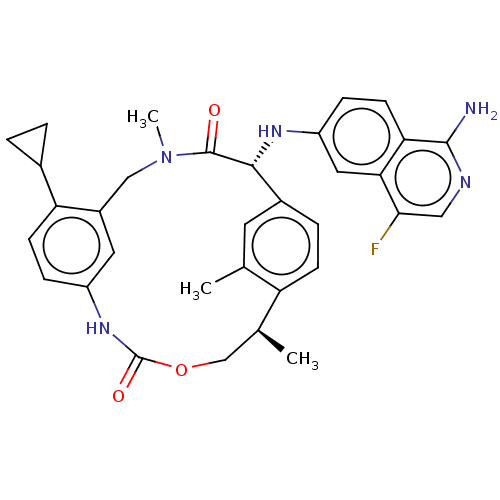
(CHEMBL3934882)Show SMILES C[C@H]1COC(=O)Nc2ccc(C3CC3)c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2 |r| Show InChI InChI=1S/C33H34FN5O3/c1-18-12-21-6-9-25(18)19(2)17-42-33(41)38-23-7-10-26(20-4-5-20)22(13-23)16-39(3)32(40)30(21)37-24-8-11-27-28(14-24)29(34)15-36-31(27)35/h6-15,19-20,30,37H,4-5,16-17H2,1-3H3,(H2,35,36)(H,38,41)/t19-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) at 37 degC by chromogenic substrate assay |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380623
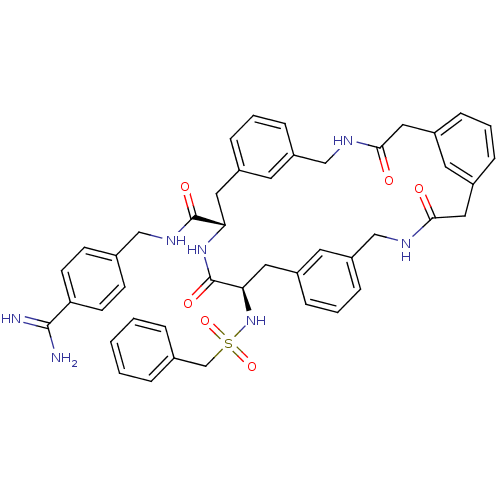
(CHEMBL2016870)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cccc(CNC(=O)Cc4cccc(CC(=O)NCc5cccc(C[C@@H](NS(=O)(=O)Cc6ccccc6)C(=O)N2)c5)c4)c3)cc1 |r| Show InChI InChI=1S/C45H47N7O6S/c46-43(47)38-17-15-30(16-18-38)26-50-44(55)39-22-32-9-5-13-36(20-32)27-48-41(53)24-34-11-4-12-35(19-34)25-42(54)49-28-37-14-6-10-33(21-37)23-40(45(56)51-39)52-59(57,58)29-31-7-2-1-3-8-31/h1-21,39-40,52H,22-29H2,(H3,46,47)(H,48,53)(H,49,54)(H,50,55)(H,51,56)/t39-,40+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108112
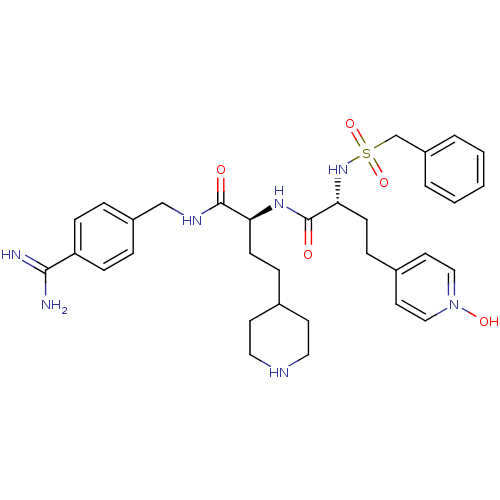
(US8598206, Table 6, 21)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCC2=CC=[N](O)C=C2)NS(=O)(=O)Cc2ccccc2)cc1 |r,wU:23.34,11.20,c:32,t:27,29,(11.34,2.69,;10,3.47,;10,5,;8.67,2.69,;8.67,1.15,;7.34,.38,;6,1.15,;4.67,.38,;3.33,1.15,;2,.38,;2,-1.15,;.67,1.15,;.67,2.69,;2,3.47,;2,5,;3.33,5.78,;3.33,7.31,;2,8.08,;.67,7.31,;.67,5.78,;-.67,.38,;-2,1.15,;-2,2.69,;-3.33,.38,;-3.33,-1.15,;-2,-1.93,;-2,-3.47,;-3.33,-4.23,;-3.33,-5.78,;-2,-6.54,;-2,-8.08,;-.67,-5.78,;-.67,-4.23,;-4.67,1.15,;-6,.38,;-6.77,-.95,;-5.23,-.95,;-7.34,1.15,;-8.67,.38,;-10,1.15,;-11.34,.38,;-11.34,-1.15,;-10,-1.93,;-8.67,-1.15,;6,2.69,;7.34,3.47,)| Show InChI InChI=1S/C33H44N7O5S/c34-31(35)28-10-6-26(7-11-28)22-37-32(41)29(12-8-24-14-18-36-19-15-24)38-33(42)30(13-9-25-16-20-40(43)21-17-25)39-46(44,45)23-27-4-2-1-3-5-27/h1-7,10-11,16-17,20-21,24,29-30,36,39,43H,8-9,12-15,18-19,22-23H2,(H3,34,35)(H,37,41)(H,38,42)/t29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447517
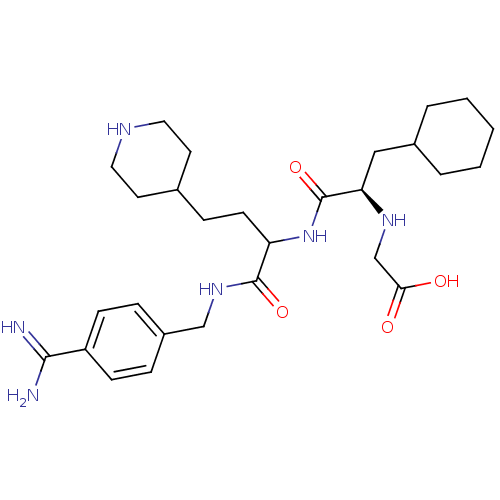
(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447514
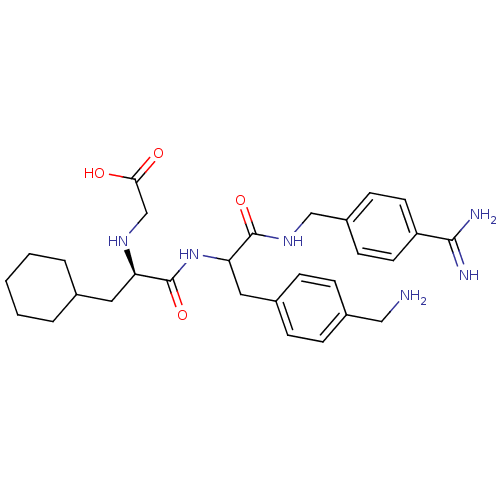
(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447521
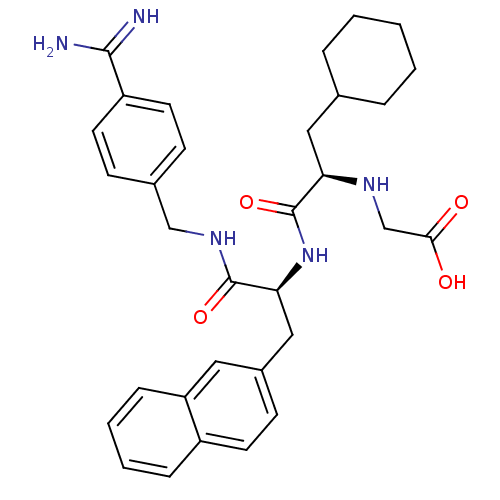
(CHEMBL3115899)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)25-14-10-22(11-15-25)19-36-31(40)28(18-23-12-13-24-8-4-5-9-26(24)16-23)37-32(41)27(35-20-29(38)39)17-21-6-2-1-3-7-21/h4-5,8-16,21,27-28,35H,1-3,6-7,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447522

(CHEMBL3115898)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)24-15-13-22(14-16-24)19-36-31(40)28(18-25-11-6-10-23-9-4-5-12-26(23)25)37-32(41)27(35-20-29(38)39)17-21-7-2-1-3-8-21/h4-6,9-16,21,27-28,35H,1-3,7-8,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108095
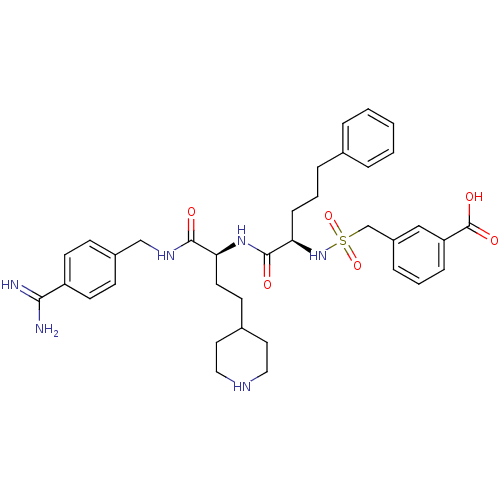
(US8598206, Table 6, 4)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)cc1 |r| Show InChI InChI=1S/C36H46N6O6S/c37-33(38)29-15-12-27(13-16-29)23-40-34(43)31(17-14-26-18-20-39-21-19-26)41-35(44)32(11-5-8-25-6-2-1-3-7-25)42-49(47,48)24-28-9-4-10-30(22-28)36(45)46/h1-4,6-7,9-10,12-13,15-16,22,26,31-32,39,42H,5,8,11,14,17-21,23-24H2,(H3,37,38)(H,40,43)(H,41,44)(H,45,46)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50192767
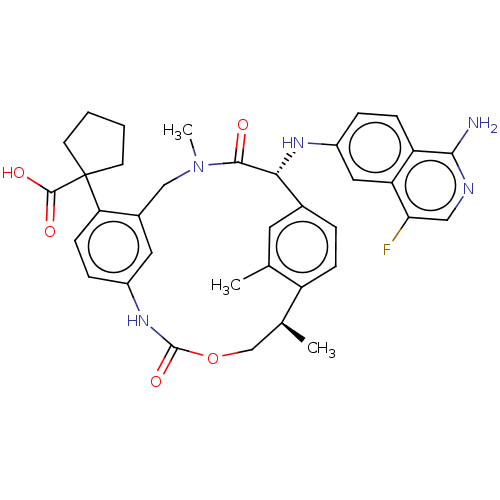
(CHEMBL3984725)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCCC1)C(O)=O |r| Show InChI InChI=1S/C36H38FN5O5/c1-20-14-22-6-9-26(20)21(2)19-47-35(46)41-24-8-11-29(36(34(44)45)12-4-5-13-36)23(15-24)18-42(3)33(43)31(22)40-25-7-10-27-28(16-25)30(37)17-39-32(27)38/h6-11,14-17,21,31,40H,4-5,12-13,18-19H2,1-3H3,(H2,38,39)(H,41,46)(H,44,45)/t21-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) at 37 degC by chromogenic substrate assay |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447525
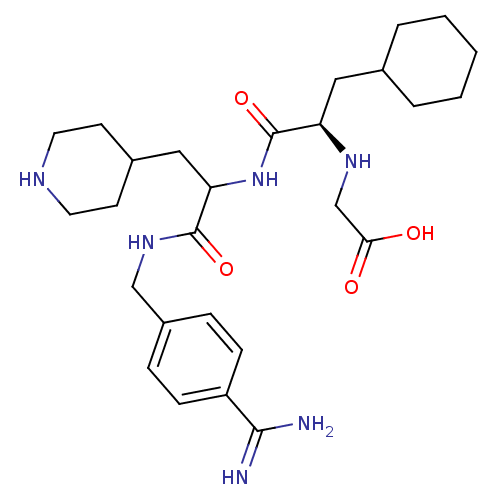
(CHEMBL3115893)Show SMILES NC(=N)c1ccc(CNC(=O)C(CC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C27H42N6O4/c28-25(29)21-8-6-20(7-9-21)16-32-26(36)23(15-19-10-12-30-13-11-19)33-27(37)22(31-17-24(34)35)14-18-4-2-1-3-5-18/h6-9,18-19,22-23,30-31H,1-5,10-17H2,(H3,28,29)(H,32,36)(H,33,37)(H,34,35)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108097
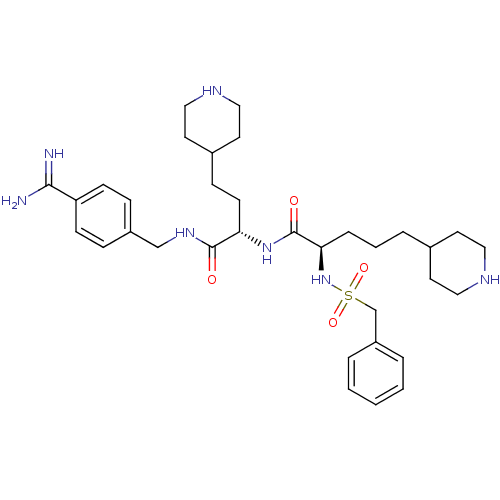
(US8598206, Table 6, 6)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCC2CCNCC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C34H51N7O4S/c35-32(36)29-12-9-27(10-13-29)23-39-33(42)30(14-11-26-17-21-38-22-18-26)40-34(43)31(8-4-7-25-15-19-37-20-16-25)41-46(44,45)24-28-5-2-1-3-6-28/h1-3,5-6,9-10,12-13,25-26,30-31,37-38,41H,4,7-8,11,14-24H2,(H3,35,36)(H,39,42)(H,40,43)/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50425659
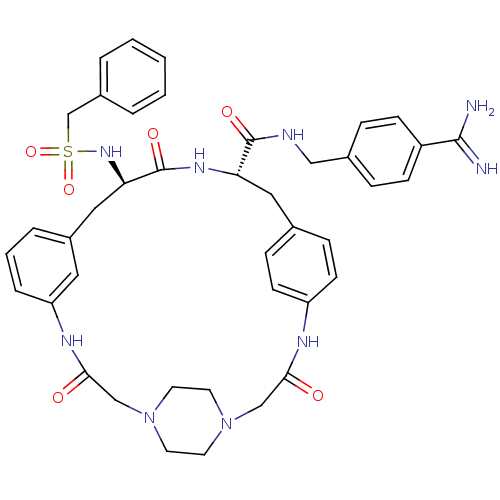
(CHEMBL2315242)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CN4CCN(CC4)CC(=O)Nc4cccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)c4)cc3)cc1 |r,wU:37.38,wD:11.10,(21.45,-20.15,;21.42,-21.69,;22.75,-22.47,;20.08,-22.44,;20.06,-23.98,;18.71,-24.73,;17.4,-23.95,;16.05,-24.69,;14.73,-23.91,;13.39,-24.65,;13.37,-26.19,;12.06,-23.87,;10.72,-24.62,;10.71,-26.16,;9.37,-26.92,;9.36,-28.46,;10.69,-29.24,;10.68,-30.77,;12.01,-31.54,;13.34,-30.78,;12,-33.07,;9.51,-33.1,;8.19,-33.87,;6.86,-33.12,;6.85,-31.59,;8.16,-30.8,;9.35,-31.53,;5.52,-30.84,;5.5,-29.31,;4.16,-28.56,;6.82,-28.53,;6.8,-26.99,;5.45,-26.23,;5.43,-24.69,;6.76,-23.91,;8.1,-24.67,;9.42,-23.89,;9.41,-22.35,;8.07,-21.59,;6.74,-22.38,;5.96,-23.7,;7.5,-23.7,;5.4,-21.61,;4.07,-22.39,;2.74,-21.62,;1.41,-22.4,;1.4,-23.94,;2.74,-24.71,;4.08,-23.94,;10.74,-21.56,;10.73,-20.02,;12.08,-22.33,;8.12,-26.2,;12.03,-28.47,;12.03,-26.93,;17.41,-22.41,;18.75,-21.65,)| Show InChI InChI=1S/C41H47N9O6S/c42-39(43)32-13-9-29(10-14-32)24-44-40(53)35-22-28-11-15-33(16-12-28)45-37(51)25-49-17-19-50(20-18-49)26-38(52)46-34-8-4-7-31(21-34)23-36(41(54)47-35)48-57(55,56)27-30-5-2-1-3-6-30/h1-16,21,35-36,48H,17-20,22-27H2,(H3,42,43)(H,44,53)(H,45,51)(H,46,52)(H,47,54)/t35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C using H-D-Lys(Cbz)-Pro-Arg-pNA as substrate after 5 to 10 mins by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447527
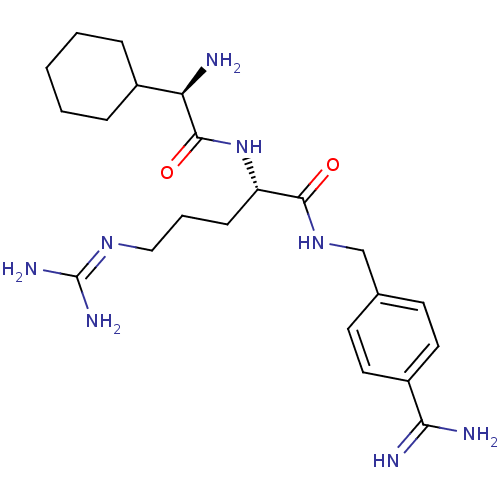
(CHEMBL3115905)Show SMILES [#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C22H36N8O2/c23-18(15-5-2-1-3-6-15)21(32)30-17(7-4-12-28-22(26)27)20(31)29-13-14-8-10-16(11-9-14)19(24)25/h8-11,15,17-18H,1-7,12-13,23H2,(H3,24,25)(H,29,31)(H,30,32)(H4,26,27,28)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50193243
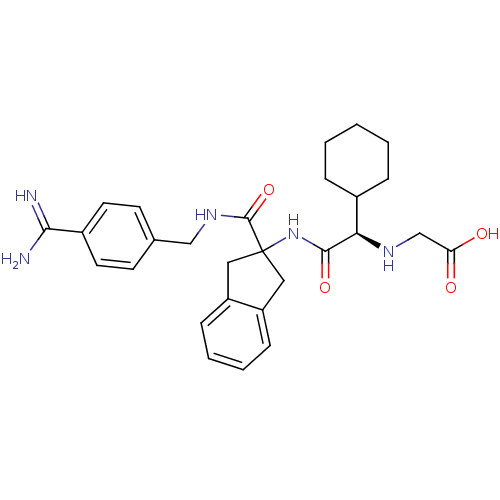
(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 555 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380618
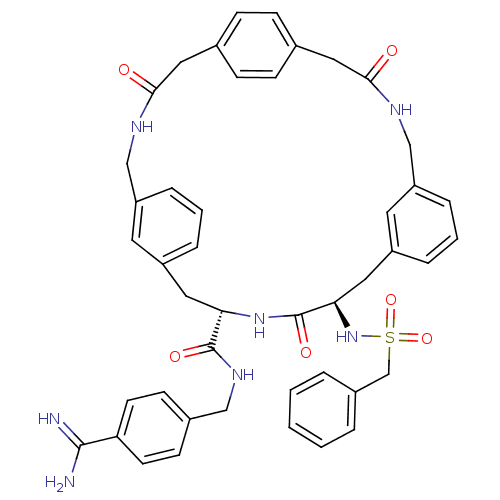
(CHEMBL2016868)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cccc(CNC(=O)Cc4ccc(CC(=O)NCc5cccc(C[C@@H](NS(=O)(=O)Cc6ccccc6)C(=O)N2)c5)cc4)c3)cc1 |r| Show InChI InChI=1S/C45H47N7O6S/c46-43(47)38-18-16-32(17-19-38)26-50-44(55)39-22-34-8-4-10-36(20-34)27-48-41(53)24-30-12-14-31(15-13-30)25-42(54)49-28-37-11-5-9-35(21-37)23-40(45(56)51-39)52-59(57,58)29-33-6-2-1-3-7-33/h1-21,39-40,52H,22-29H2,(H3,46,47)(H,48,53)(H,49,54)(H,50,55)(H,51,56)/t39-,40+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 564 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108116
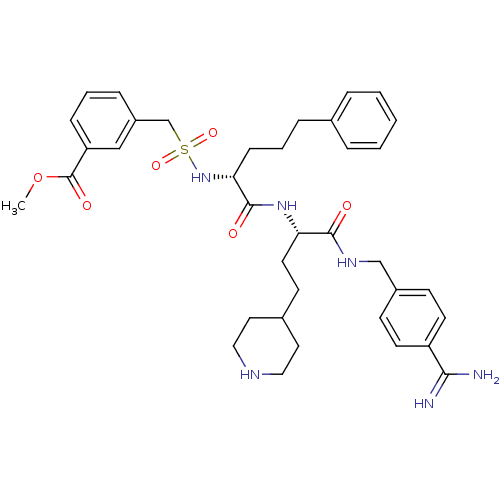
(US8598206, Table 6, 3)Show SMILES COC(=O)c1cccc(CS(=O)(=O)N[C@H](CCCc2ccccc2)C(=O)N[C@@H](CCC2CCNCC2)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H48N6O6S/c1-49-37(46)31-11-5-10-29(23-31)25-50(47,48)43-33(12-6-9-26-7-3-2-4-8-26)36(45)42-32(18-15-27-19-21-40-22-20-27)35(44)41-24-28-13-16-30(17-14-28)34(38)39/h2-5,7-8,10-11,13-14,16-17,23,27,32-33,40,43H,6,9,12,15,18-22,24-25H2,1H3,(H3,38,39)(H,41,44)(H,42,45)/t32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108117
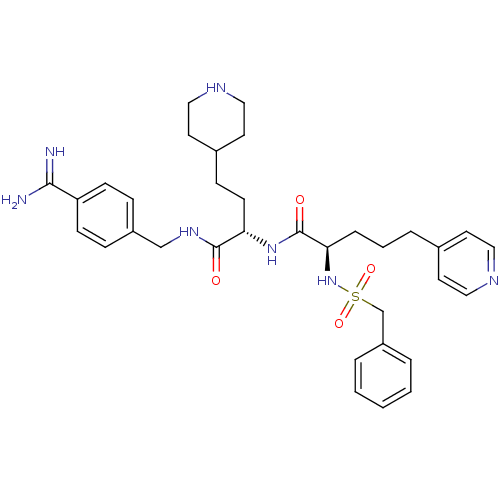
(US8598206, Table 6, 2)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccncc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C34H45N7O4S/c35-32(36)29-12-9-27(10-13-29)23-39-33(42)30(14-11-26-17-21-38-22-18-26)40-34(43)31(8-4-7-25-15-19-37-20-16-25)41-46(44,45)24-28-5-2-1-3-6-28/h1-3,5-6,9-10,12-13,15-16,19-20,26,30-31,38,41H,4,7-8,11,14,17-18,21-24H2,(H3,35,36)(H,39,42)(H,40,43)/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50192768
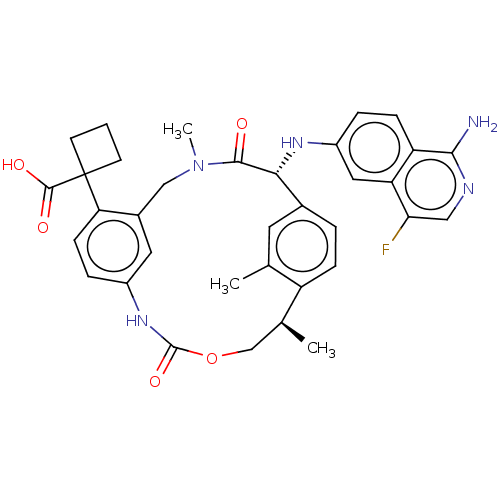
(CHEMBL3900166)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCC1)C(O)=O |r| Show InChI InChI=1S/C35H36FN5O5/c1-19-13-21-5-8-25(19)20(2)18-46-34(45)40-23-7-10-28(35(33(43)44)11-4-12-35)22(14-23)17-41(3)32(42)30(21)39-24-6-9-26-27(15-24)29(36)16-38-31(26)37/h5-10,13-16,20,30,39H,4,11-12,17-18H2,1-3H3,(H2,37,38)(H,40,45)(H,43,44)/t20-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 725 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C (unknown origin) at 37 degC by chromogenic substrate assay |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108115
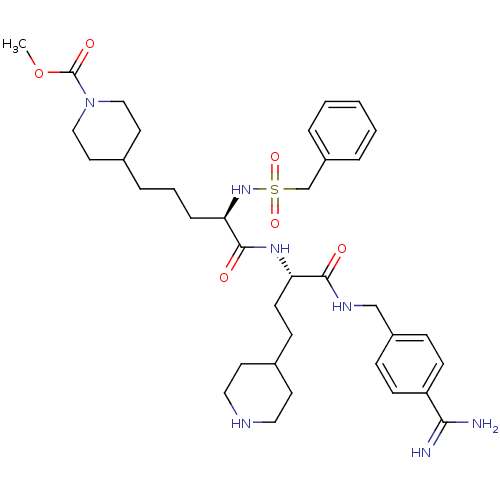
(US8598206, Table 6, 24)Show SMILES COC(=O)N1CCC(CCC[C@@H](NS(=O)(=O)Cc2ccccc2)C(=O)N[C@@H](CCC2CCNCC2)C(=O)NCc2ccc(cc2)C(N)=N)CC1 |r| Show InChI InChI=1S/C36H53N7O6S/c1-49-36(46)43-22-18-26(19-23-43)8-5-9-32(42-50(47,48)25-29-6-3-2-4-7-29)35(45)41-31(15-12-27-16-20-39-21-17-27)34(44)40-24-28-10-13-30(14-11-28)33(37)38/h2-4,6-7,10-11,13-14,26-27,31-32,39,42H,5,8-9,12,15-25H2,1H3,(H3,37,38)(H,40,44)(H,41,45)/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447519
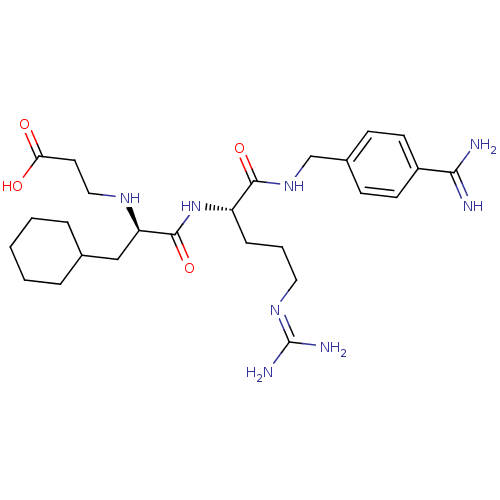
(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50098595
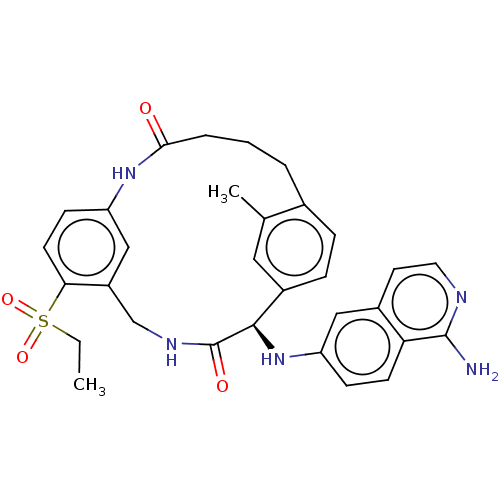
(CHEMBL3594314)Show SMILES CCS(=O)(=O)c1ccc2NC(=O)CCCc3ccc(cc3C)[C@@H](Nc3ccc4c(N)nccc4c3)C(=O)NCc1c2 |r| Show InChI InChI=1S/C31H33N5O4S/c1-3-41(39,40)27-12-10-24-17-23(27)18-34-31(38)29(36-25-9-11-26-21(16-25)13-14-33-30(26)32)22-8-7-20(19(2)15-22)5-4-6-28(37)35-24/h7-17,29,36H,3-6,18H2,1-2H3,(H2,32,33)(H,34,38)(H,35,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human APC measured for 30 mins |
J Med Chem 58: 6225-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00788
BindingDB Entry DOI: 10.7270/Q2DN46TN |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108108
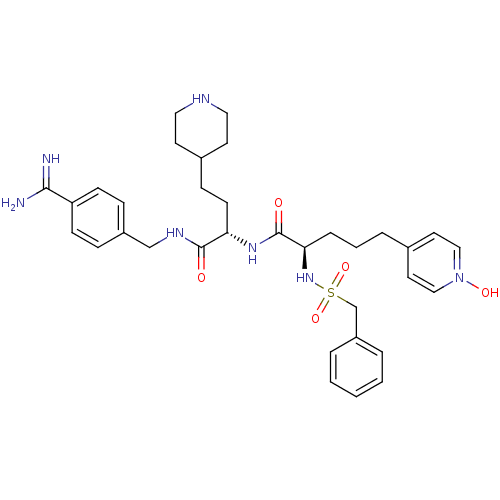
(US8598206, 117 | US8598206, 123)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCC2=CC=[N](O)C=C2)NS(=O)(=O)Cc2ccccc2)cc1 |r,wU:23.35,11.20,c:33,t:28,30,(11.34,1.93,;10,2.7,;10,4.23,;8.67,1.93,;8.67,.38,;7.34,-.38,;6,.38,;4.67,-.38,;3.33,.38,;2,-.38,;2,-1.93,;.67,.38,;.67,1.93,;2,2.69,;2,4.23,;3.33,5,;3.33,6.54,;2,7.31,;.67,6.54,;.67,5,;-.67,-.38,;-2,.38,;-2,1.93,;-3.33,-.38,;-3.33,-1.93,;-2,-2.69,;-2,-4.23,;-.67,-5,;-.67,-6.54,;.67,-7.31,;2,-6.54,;3.33,-7.31,;2,-5,;.67,-4.23,;-4.67,.38,;-6,-.38,;-6.77,-1.72,;-5.23,-1.72,;-7.34,.38,;-8.67,-.38,;-10,.38,;-11.34,-.38,;-11.34,-1.93,;-10,-2.69,;-8.67,-1.93,;6,1.93,;7.34,2.69,)| Show InChI InChI=1S/C34H46N7O5S/c35-32(36)29-12-9-27(10-13-29)23-38-33(42)30(14-11-26-15-19-37-20-16-26)39-34(43)31(8-4-7-25-17-21-41(44)22-18-25)40-47(45,46)24-28-5-2-1-3-6-28/h1-3,5-6,9-10,12-13,17-18,21-22,26,30-31,37,40,44H,4,7-8,11,14-16,19-20,23-24H2,(H3,35,36)(H,38,42)(H,39,43)/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM108108

(US8598206, 117 | US8598206, 123)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCC2=CC=[N](O)C=C2)NS(=O)(=O)Cc2ccccc2)cc1 |r,wU:23.35,11.20,c:33,t:28,30,(11.34,1.93,;10,2.7,;10,4.23,;8.67,1.93,;8.67,.38,;7.34,-.38,;6,.38,;4.67,-.38,;3.33,.38,;2,-.38,;2,-1.93,;.67,.38,;.67,1.93,;2,2.69,;2,4.23,;3.33,5,;3.33,6.54,;2,7.31,;.67,6.54,;.67,5,;-.67,-.38,;-2,.38,;-2,1.93,;-3.33,-.38,;-3.33,-1.93,;-2,-2.69,;-2,-4.23,;-.67,-5,;-.67,-6.54,;.67,-7.31,;2,-6.54,;3.33,-7.31,;2,-5,;.67,-4.23,;-4.67,.38,;-6,-.38,;-6.77,-1.72,;-5.23,-1.72,;-7.34,.38,;-8.67,-.38,;-10,.38,;-11.34,-.38,;-11.34,-1.93,;-10,-2.69,;-8.67,-1.93,;6,1.93,;7.34,2.69,)| Show InChI InChI=1S/C34H46N7O5S/c35-32(36)29-12-9-27(10-13-29)23-38-33(42)30(14-11-26-15-19-37-20-16-26)39-34(43)31(8-4-7-25-17-21-41(44)22-18-25)40-47(45,46)24-28-5-2-1-3-6-28/h1-3,5-6,9-10,12-13,17-18,21-22,26,30-31,37,40,44H,4,7-8,11,14-16,19-20,23-24H2,(H3,35,36)(H,38,42)(H,39,43)/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
Inhibition of human aPC was determined by the method described in [0092]-[0098] using human activated protein C from Enzyme Research Laboratories at ... |
US Patent US8598206 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J5F |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50425658
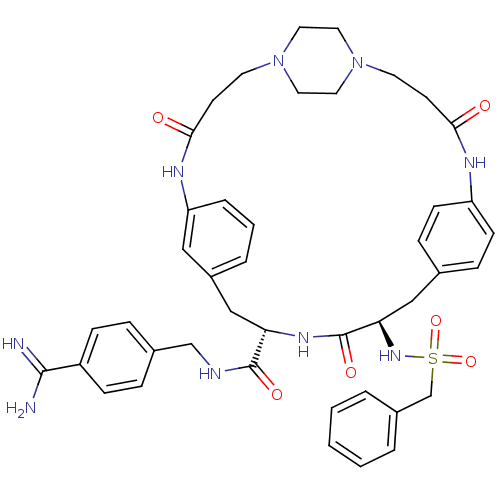
(CHEMBL2315244)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cccc(NC(=O)CCN4CCN(CC4)CCC(=O)Nc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)c3)cc1 |r,wU:39.40,wD:11.10,(72.19,-20.4,;72.17,-21.94,;73.49,-22.72,;70.83,-22.69,;70.8,-24.23,;69.45,-24.97,;68.14,-24.19,;66.79,-24.94,;65.48,-24.16,;64.13,-24.9,;64.11,-26.44,;62.81,-24.12,;61.46,-24.87,;61.45,-26.41,;60.11,-27.17,;60.1,-28.71,;61.43,-29.49,;62.77,-28.72,;64.19,-31.03,;62.87,-31.8,;61.53,-31.04,;62.87,-33.33,;61.55,-34.11,;60.22,-33.35,;58.89,-34.12,;57.57,-33.36,;57.56,-31.83,;58.87,-31.05,;60.21,-31.81,;56.23,-31.08,;56.21,-29.55,;54.88,-28.79,;53.56,-29.57,;54.87,-27.26,;56.19,-26.48,;56.18,-24.94,;57.5,-24.16,;58.84,-24.92,;60.17,-24.14,;60.15,-22.6,;58.81,-21.84,;57.48,-22.63,;56.7,-23.95,;58.25,-23.95,;56.14,-21.86,;54.82,-22.64,;53.48,-21.87,;52.15,-22.64,;52.15,-24.19,;53.48,-24.96,;54.82,-24.19,;61.48,-21.81,;61.47,-20.27,;62.82,-22.58,;58.86,-26.45,;57.54,-27.24,;62.78,-27.18,;68.15,-22.66,;69.49,-21.9,)| Show InChI InChI=1S/C43H51N9O6S/c44-41(45)34-13-9-31(10-14-34)28-46-42(55)37-27-33-7-4-8-36(25-33)48-40(54)18-20-52-23-21-51(22-24-52)19-17-39(53)47-35-15-11-30(12-16-35)26-38(43(56)49-37)50-59(57,58)29-32-5-2-1-3-6-32/h1-16,25,37-38,50H,17-24,26-29H2,(H3,44,45)(H,46,55)(H,47,53)(H,48,54)(H,49,56)/t37-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C using H-D-Lys(Cbz)-Pro-Arg-pNA as substrate after 5 to 10 mins by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data