 Found 36 hits of ki data for polymerid = 1662
Found 36 hits of ki data for polymerid = 1662 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15956
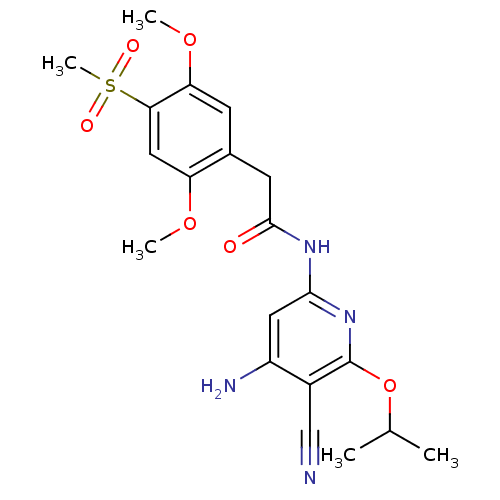
(Aminopyridine-Based Inhibitor 18b | N-(4-Amino-5-c...)Show SMILES COc1cc(c(OC)cc1CC(=O)Nc1cc(N)c(C#N)c(OC(C)C)n1)S(C)(=O)=O Show InChI InChI=1S/C20H24N4O6S/c1-11(2)30-20-13(10-21)14(22)8-18(24-20)23-19(25)7-12-6-16(29-4)17(31(5,26)27)9-15(12)28-3/h6,8-9,11H,7H2,1-5H3,(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... |
J Med Chem 49: 3563-80 (2006)
Article DOI: 10.1021/jm060199b
BindingDB Entry DOI: 10.7270/Q2P26WDX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352629
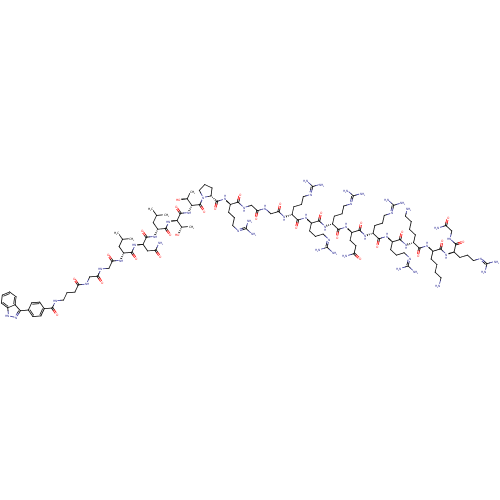
(CHEMBL1822314)Show SMILES CC(C)C[C@@H](NC(=O)CNC(=O)CNC(=O)CCCNC(=O)c1ccc(cc1)-c1n[nH]c2ccccc12)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CC(C)C)C(=O)N[C@H]([C@H](C)O)C(=O)N[C@H]([C@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(N)=O |r,wU:73.77,118.121,48.50,138.141,160.163,4.4,96.99,63.65,178.181,64.68,wD:40.42,107.110,129.132,77.80,56.58,149.152,57.61,169.172,(-33.39,-2.84,;-32.09,-2.03,;-30.72,-2.76,;-32.13,-.49,;-30.83,.31,;-30.84,1.85,;-32.2,2.57,;-33.52,1.77,;-32.25,4.11,;-33.62,4.83,;-33.65,6.37,;-32.34,7.18,;-35.01,7.09,;-35.02,8.63,;-33.67,9.37,;-32.35,8.59,;-33.67,10.92,;-32.31,11.68,;-32.32,13.21,;-30.98,13.98,;-30.95,15.52,;-32.28,16.28,;-29.61,16.28,;-29.61,17.82,;-28.26,18.58,;-26.94,17.79,;-26.94,16.27,;-28.28,15.5,;-25.61,18.57,;-24.2,17.94,;-23.15,19.06,;-23.92,20.39,;-23.44,21.86,;-24.45,23.02,;-25.97,22.7,;-26.45,21.24,;-25.43,20.11,;-29.47,-.41,;-28.16,.39,;-29.42,-1.95,;-28.06,-2.67,;-26.76,-1.87,;-25.41,-2.6,;-24.11,-1.79,;-25.37,-4.14,;-28.03,-4.22,;-29.33,-5.03,;-26.68,-4.95,;-26.65,-6.49,;-27.94,-7.29,;-27.89,-8.81,;-29.22,-9.63,;-26.56,-9.55,;-25.29,-7.2,;-23.99,-6.39,;-25.2,-8.74,;-23.85,-9.49,;-22.54,-8.68,;-22.6,-7.15,;-21.17,-9.42,;-23.79,-11.04,;-25.11,-11.84,;-22.43,-11.75,;-22.38,-13.29,;-23.68,-14.1,;-25.05,-13.36,;-23.63,-15.62,;-21.03,-14.02,;-19.72,-13.2,;-20.97,-15.56,;-22.05,-16.65,;-21.32,-18.02,;-19.82,-17.74,;-19.61,-16.22,;-18.24,-15.51,;-18.28,-13.98,;-16.91,-16.29,;-15.59,-15.51,;-15.61,-13.96,;-14.28,-13.18,;-14.31,-11.63,;-12.99,-10.84,;-12.96,-9.3,;-14.34,-8.56,;-11.69,-8.53,;-14.25,-16.26,;-14.23,-17.8,;-12.92,-15.47,;-11.57,-16.21,;-10.26,-15.41,;-10.27,-13.87,;-8.92,-16.17,;-7.6,-15.38,;-6.24,-16.13,;-6.22,-17.66,;-4.92,-15.34,;-3.58,-16.1,;-3.56,-17.65,;-2.21,-18.4,;-2.2,-19.93,;-.86,-20.68,;-.86,-22.22,;-2.17,-23.02,;.49,-22.98,;-2.25,-15.32,;-2.29,-13.77,;-.93,-16.1,;.4,-15.32,;.38,-13.77,;1.7,-12.99,;1.68,-11.44,;3,-10.66,;2.99,-9.12,;1.65,-8.35,;4.25,-8.34,;1.75,-16.07,;1.76,-17.61,;3.06,-15.26,;4.43,-15.97,;4.43,-17.51,;5.79,-18.26,;5.81,-19.81,;7.13,-20.6,;7.18,-22.14,;5.83,-22.94,;8.49,-22.91,;5.74,-15.18,;5.73,-13.65,;7.06,-15.97,;8.38,-15.18,;8.37,-13.65,;9.68,-12.86,;9.67,-11.32,;8.28,-10.6,;10.99,-10.52,;9.73,-15.94,;9.75,-17.47,;11.04,-15.14,;12.4,-15.88,;12.42,-17.41,;13.77,-18.16,;13.78,-19.69,;15.1,-20.47,;15.16,-22.01,;13.8,-22.83,;16.47,-22.78,;13.72,-15.08,;13.7,-13.55,;15.06,-15.83,;16.38,-15.03,;16.36,-13.5,;17.68,-12.71,;17.65,-11.16,;18.97,-10.38,;18.96,-8.84,;17.62,-8.11,;20.3,-8.06,;17.72,-15.79,;17.74,-17.32,;19.05,-15.01,;20.39,-15.76,;20.42,-17.29,;21.75,-18.04,;21.78,-19.59,;23.13,-20.34,;23.14,-21.9,;21.7,-14.98,;21.7,-13.43,;23.05,-15.73,;24.36,-14.92,;24.34,-13.38,;25.66,-12.59,;25.65,-11.05,;26.98,-10.26,;26.96,-8.74,;25.7,-15.67,;25.73,-17.2,;27.03,-14.89,;28.37,-15.64,;28.38,-17.17,;29.74,-17.92,;29.75,-19.45,;31.1,-20.2,;31.12,-21.74,;29.81,-22.52,;32.46,-22.49,;29.69,-14.85,;29.67,-13.3,;31.03,-15.6,;32.35,-14.8,;33.7,-15.55,;35.02,-14.78,;33.72,-17.09,)| Show InChI InChI=1S/C116H198N50O27/c1-60(2)52-78(150-89(176)59-145-86(173)56-144-85(172)34-20-43-136-93(177)65-37-35-64(36-38-65)92-66-22-7-8-23-67(66)164-165-92)104(188)161-80(54-83(120)170)105(189)160-79(53-61(3)4)106(190)162-90(62(5)167)108(192)163-91(63(6)168)109(193)166-51-21-33-81(166)107(191)159-69(27-14-45-138-111(124)125)95(179)148-57-87(174)146-58-88(175)149-70(28-15-46-139-112(126)127)96(180)154-73(29-16-47-140-113(128)129)100(184)156-76(32-19-50-143-116(134)135)102(186)158-77(39-40-82(119)169)103(187)157-75(31-18-49-142-115(132)133)101(185)155-74(30-17-48-141-114(130)131)99(183)153-72(25-10-12-42-118)98(182)152-71(24-9-11-41-117)97(181)151-68(26-13-44-137-110(122)123)94(178)147-55-84(121)171/h7-8,22-23,35-38,60-63,68-81,90-91,167-168H,9-21,24-34,39-59,117-118H2,1-6H3,(H2,119,169)(H2,120,170)(H2,121,171)(H,136,177)(H,144,172)(H,145,173)(H,146,174)(H,147,178)(H,148,179)(H,149,175)(H,150,176)(H,151,181)(H,152,182)(H,153,183)(H,154,180)(H,155,185)(H,156,184)(H,157,187)(H,158,186)(H,159,191)(H,160,189)(H,161,188)(H,162,190)(H,163,192)(H,164,165)(H4,122,123,137)(H4,124,125,138)(H4,126,127,139)(H4,128,129,140)(H4,130,131,141)(H4,132,133,142)(H4,134,135,143)/t62-,63-,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,79+,80+,81+,90+,91+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
| Assay Description
Competitive inhibition of JNK1 using ATF2 substrate by Lineweaver-Burk analysis |
J Med Chem 54: 6206-14 (2011)
Article DOI: 10.1021/jm200479c
BindingDB Entry DOI: 10.7270/Q2668DMM |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15908
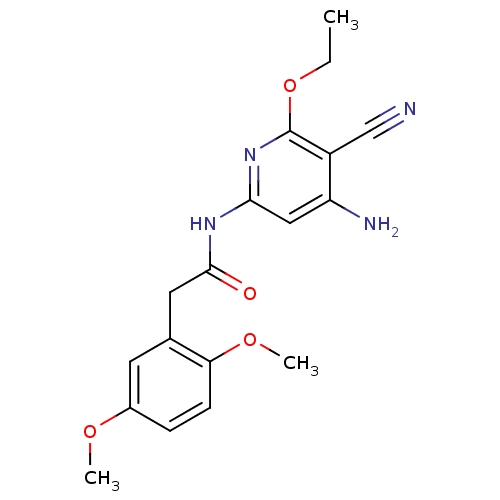
(Aminopyridine-Based Inhibitor 6o | N-(4-Amino-5-cy...)Show InChI InChI=1S/C18H20N4O4/c1-4-26-18-13(10-19)14(20)9-16(22-18)21-17(23)8-11-7-12(24-2)5-6-15(11)25-3/h5-7,9H,4,8H2,1-3H3,(H3,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... |
J Med Chem 49: 3563-80 (2006)
Article DOI: 10.1021/jm060199b
BindingDB Entry DOI: 10.7270/Q2P26WDX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352629

(CHEMBL1822314)Show SMILES CC(C)C[C@@H](NC(=O)CNC(=O)CNC(=O)CCCNC(=O)c1ccc(cc1)-c1n[nH]c2ccccc12)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CC(C)C)C(=O)N[C@H]([C@H](C)O)C(=O)N[C@H]([C@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(N)=O |r,wU:73.77,118.121,48.50,138.141,160.163,4.4,96.99,63.65,178.181,64.68,wD:40.42,107.110,129.132,77.80,56.58,149.152,57.61,169.172,(-33.39,-2.84,;-32.09,-2.03,;-30.72,-2.76,;-32.13,-.49,;-30.83,.31,;-30.84,1.85,;-32.2,2.57,;-33.52,1.77,;-32.25,4.11,;-33.62,4.83,;-33.65,6.37,;-32.34,7.18,;-35.01,7.09,;-35.02,8.63,;-33.67,9.37,;-32.35,8.59,;-33.67,10.92,;-32.31,11.68,;-32.32,13.21,;-30.98,13.98,;-30.95,15.52,;-32.28,16.28,;-29.61,16.28,;-29.61,17.82,;-28.26,18.58,;-26.94,17.79,;-26.94,16.27,;-28.28,15.5,;-25.61,18.57,;-24.2,17.94,;-23.15,19.06,;-23.92,20.39,;-23.44,21.86,;-24.45,23.02,;-25.97,22.7,;-26.45,21.24,;-25.43,20.11,;-29.47,-.41,;-28.16,.39,;-29.42,-1.95,;-28.06,-2.67,;-26.76,-1.87,;-25.41,-2.6,;-24.11,-1.79,;-25.37,-4.14,;-28.03,-4.22,;-29.33,-5.03,;-26.68,-4.95,;-26.65,-6.49,;-27.94,-7.29,;-27.89,-8.81,;-29.22,-9.63,;-26.56,-9.55,;-25.29,-7.2,;-23.99,-6.39,;-25.2,-8.74,;-23.85,-9.49,;-22.54,-8.68,;-22.6,-7.15,;-21.17,-9.42,;-23.79,-11.04,;-25.11,-11.84,;-22.43,-11.75,;-22.38,-13.29,;-23.68,-14.1,;-25.05,-13.36,;-23.63,-15.62,;-21.03,-14.02,;-19.72,-13.2,;-20.97,-15.56,;-22.05,-16.65,;-21.32,-18.02,;-19.82,-17.74,;-19.61,-16.22,;-18.24,-15.51,;-18.28,-13.98,;-16.91,-16.29,;-15.59,-15.51,;-15.61,-13.96,;-14.28,-13.18,;-14.31,-11.63,;-12.99,-10.84,;-12.96,-9.3,;-14.34,-8.56,;-11.69,-8.53,;-14.25,-16.26,;-14.23,-17.8,;-12.92,-15.47,;-11.57,-16.21,;-10.26,-15.41,;-10.27,-13.87,;-8.92,-16.17,;-7.6,-15.38,;-6.24,-16.13,;-6.22,-17.66,;-4.92,-15.34,;-3.58,-16.1,;-3.56,-17.65,;-2.21,-18.4,;-2.2,-19.93,;-.86,-20.68,;-.86,-22.22,;-2.17,-23.02,;.49,-22.98,;-2.25,-15.32,;-2.29,-13.77,;-.93,-16.1,;.4,-15.32,;.38,-13.77,;1.7,-12.99,;1.68,-11.44,;3,-10.66,;2.99,-9.12,;1.65,-8.35,;4.25,-8.34,;1.75,-16.07,;1.76,-17.61,;3.06,-15.26,;4.43,-15.97,;4.43,-17.51,;5.79,-18.26,;5.81,-19.81,;7.13,-20.6,;7.18,-22.14,;5.83,-22.94,;8.49,-22.91,;5.74,-15.18,;5.73,-13.65,;7.06,-15.97,;8.38,-15.18,;8.37,-13.65,;9.68,-12.86,;9.67,-11.32,;8.28,-10.6,;10.99,-10.52,;9.73,-15.94,;9.75,-17.47,;11.04,-15.14,;12.4,-15.88,;12.42,-17.41,;13.77,-18.16,;13.78,-19.69,;15.1,-20.47,;15.16,-22.01,;13.8,-22.83,;16.47,-22.78,;13.72,-15.08,;13.7,-13.55,;15.06,-15.83,;16.38,-15.03,;16.36,-13.5,;17.68,-12.71,;17.65,-11.16,;18.97,-10.38,;18.96,-8.84,;17.62,-8.11,;20.3,-8.06,;17.72,-15.79,;17.74,-17.32,;19.05,-15.01,;20.39,-15.76,;20.42,-17.29,;21.75,-18.04,;21.78,-19.59,;23.13,-20.34,;23.14,-21.9,;21.7,-14.98,;21.7,-13.43,;23.05,-15.73,;24.36,-14.92,;24.34,-13.38,;25.66,-12.59,;25.65,-11.05,;26.98,-10.26,;26.96,-8.74,;25.7,-15.67,;25.73,-17.2,;27.03,-14.89,;28.37,-15.64,;28.38,-17.17,;29.74,-17.92,;29.75,-19.45,;31.1,-20.2,;31.12,-21.74,;29.81,-22.52,;32.46,-22.49,;29.69,-14.85,;29.67,-13.3,;31.03,-15.6,;32.35,-14.8,;33.7,-15.55,;35.02,-14.78,;33.72,-17.09,)| Show InChI InChI=1S/C116H198N50O27/c1-60(2)52-78(150-89(176)59-145-86(173)56-144-85(172)34-20-43-136-93(177)65-37-35-64(36-38-65)92-66-22-7-8-23-67(66)164-165-92)104(188)161-80(54-83(120)170)105(189)160-79(53-61(3)4)106(190)162-90(62(5)167)108(192)163-91(63(6)168)109(193)166-51-21-33-81(166)107(191)159-69(27-14-45-138-111(124)125)95(179)148-57-87(174)146-58-88(175)149-70(28-15-46-139-112(126)127)96(180)154-73(29-16-47-140-113(128)129)100(184)156-76(32-19-50-143-116(134)135)102(186)158-77(39-40-82(119)169)103(187)157-75(31-18-49-142-115(132)133)101(185)155-74(30-17-48-141-114(130)131)99(183)153-72(25-10-12-42-118)98(182)152-71(24-9-11-41-117)97(181)151-68(26-13-44-137-110(122)123)94(178)147-55-84(121)171/h7-8,22-23,35-38,60-63,68-81,90-91,167-168H,9-21,24-34,39-59,117-118H2,1-6H3,(H2,119,169)(H2,120,170)(H2,121,171)(H,136,177)(H,144,172)(H,145,173)(H,146,174)(H,147,178)(H,148,179)(H,149,175)(H,150,176)(H,151,181)(H,152,182)(H,153,183)(H,154,180)(H,155,185)(H,156,184)(H,157,187)(H,158,186)(H,159,191)(H,160,189)(H,161,188)(H,162,190)(H,163,192)(H,164,165)(H4,122,123,137)(H4,124,125,138)(H4,126,127,139)(H4,128,129,140)(H4,130,131,141)(H4,132,133,142)(H4,134,135,143)/t62-,63-,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,79+,80+,81+,90+,91+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
| Assay Description
Competitive inhibition of JNK1 using ATP by Lineweaver-Burk analysis |
J Med Chem 54: 6206-14 (2011)
Article DOI: 10.1021/jm200479c
BindingDB Entry DOI: 10.7270/Q2668DMM |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15908

(Aminopyridine-Based Inhibitor 6o | N-(4-Amino-5-cy...)Show InChI InChI=1S/C18H20N4O4/c1-4-26-18-13(10-19)14(20)9-16(22-18)21-17(23)8-11-7-12(24-2)5-6-15(11)25-3/h5-7,9H,4,8H2,1-3H3,(H3,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1 |
Proc Natl Acad Sci USA 104: 20523-8 (2007)
Checked by Author
Article DOI: 10.1073/pnas.0708800104
BindingDB Entry DOI: 10.7270/Q2DB82RH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15976
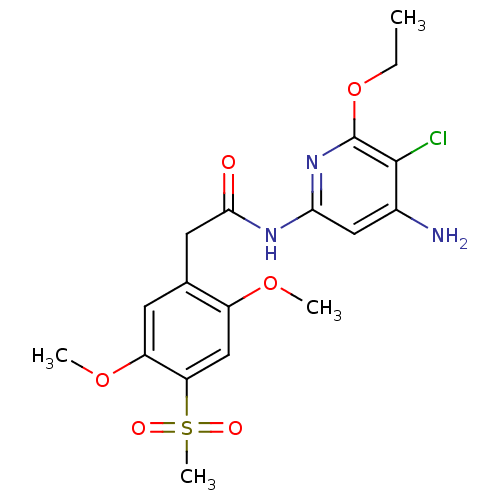
(Aminopyridine-Based Inhibitor 35 | N-(4-Amino-5-ch...)Show SMILES CCOc1nc(NC(=O)Cc2cc(OC)c(cc2OC)S(C)(=O)=O)cc(N)c1Cl Show InChI InChI=1S/C18H22ClN3O6S/c1-5-28-18-17(19)11(20)8-15(22-18)21-16(23)7-10-6-13(27-3)14(29(4,24)25)9-12(10)26-2/h6,8-9H,5,7H2,1-4H3,(H3,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... |
J Med Chem 49: 3563-80 (2006)
Article DOI: 10.1021/jm060199b
BindingDB Entry DOI: 10.7270/Q2P26WDX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50314147
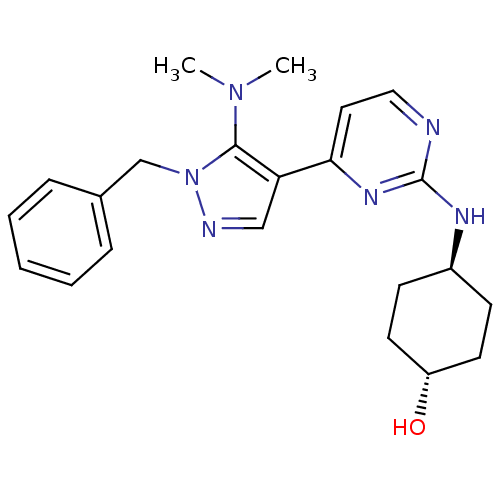
(CHEMBL1094014 | trans-4-(4-(1-benzyl-5-(dimethylam...)Show SMILES CN(C)c1c(cnn1Cc1ccccc1)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.26,wD:21.22,(-.4,-38.39,;-1.54,-37.36,;-1.22,-35.86,;-3.01,-37.84,;-4.26,-36.93,;-5.5,-37.84,;-5.03,-39.31,;-3.49,-39.3,;-2.7,-40.63,;-3.46,-41.97,;-5,-41.98,;-5.75,-43.32,;-4.97,-44.65,;-3.42,-44.62,;-2.67,-43.29,;-4.26,-35.39,;-5.59,-34.62,;-5.59,-33.07,;-4.26,-32.3,;-2.92,-33.06,;-1.6,-32.29,;-.26,-33.06,;-.26,-34.6,;1.07,-35.36,;2.4,-34.59,;3.74,-35.36,;2.4,-33.05,;1.07,-32.28,;-2.92,-34.62,)| Show InChI InChI=1S/C22H28N6O/c1-27(2)21-19(14-24-28(21)15-16-6-4-3-5-7-16)20-12-13-23-22(26-20)25-17-8-10-18(29)11-9-17/h3-7,12-14,17-18,29H,8-11,15H2,1-2H3,(H,23,25,26)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
J Med Chem 53: 3005-12 (2010)
Article DOI: 10.1021/jm9003279
BindingDB Entry DOI: 10.7270/Q2KS6RP5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15939
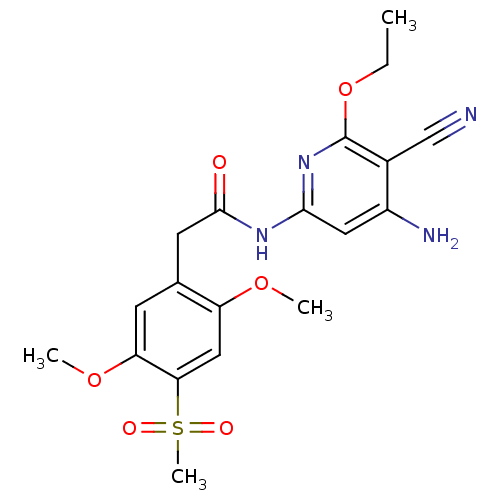
(Aminopyridine-Based Inhibitor 6s | N-(4-amino-5-cy...)Show SMILES CCOc1nc(NC(=O)Cc2cc(OC)c(cc2OC)S(C)(=O)=O)cc(N)c1C#N Show InChI InChI=1S/C19H22N4O6S/c1-5-29-19-12(10-20)13(21)8-17(23-19)22-18(24)7-11-6-15(28-3)16(30(4,25)26)9-14(11)27-2/h6,8-9H,5,7H2,1-4H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... |
J Med Chem 49: 3563-80 (2006)
Article DOI: 10.1021/jm060199b
BindingDB Entry DOI: 10.7270/Q2P26WDX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277947
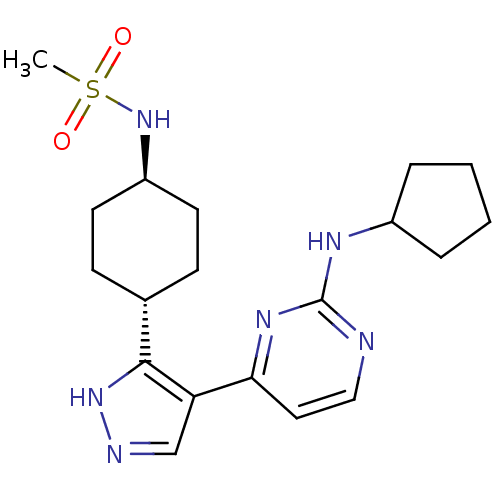
(CHEMBL482559 | N-(4-(4-(2-(cyclopentylamino)pyrimi...)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)c1[nH]ncc1-c1ccnc(NC2CCCC2)n1 |r,wU:8.11,wD:5.4,(22.41,-2.51,;22.42,-.98,;20.88,-.96,;23.96,-1,;22.44,.56,;23.79,1.31,;25.11,.52,;26.46,1.27,;26.48,2.81,;25.16,3.6,;23.82,2.85,;27.94,3.29,;28.41,4.76,;29.95,4.76,;30.43,3.3,;29.2,2.4,;29.2,.86,;27.87,.09,;27.87,-1.46,;29.21,-2.23,;30.54,-1.46,;31.88,-2.23,;31.88,-3.77,;30.64,-4.66,;31.11,-6.13,;32.66,-6.12,;33.13,-4.66,;30.54,.09,)| Show InChI InChI=1S/C19H28N6O2S/c1-28(26,27)25-15-8-6-13(7-9-15)18-16(12-21-24-18)17-10-11-20-19(23-17)22-14-4-2-3-5-14/h10-15,25H,2-9H2,1H3,(H,21,24)(H,20,22,23)/t13-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50314148

(CHEMBL1089007 | trans-4-(4-(3-(tetrahydro-2H-pyran...)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-c1cn[nH]c1C1CCCOC1 |r,wU:1.0,wD:4.7,(27.93,-38.08,;26.61,-37.31,;25.27,-38.08,;23.94,-37.31,;23.94,-35.78,;25.26,-34.99,;26.6,-35.77,;22.6,-35.02,;21.27,-35.79,;19.93,-35.03,;18.61,-35.8,;18.61,-37.34,;19.94,-38.11,;21.28,-37.34,;19.94,-39.66,;21.19,-40.56,;20.71,-42.02,;19.17,-42.02,;18.7,-40.56,;17.37,-39.78,;16.04,-40.55,;14.72,-39.77,;14.72,-38.23,;16.07,-37.47,;17.38,-38.24,)| Show InChI InChI=1S/C18H25N5O2/c24-14-5-3-13(4-6-14)21-18-19-8-7-16(22-18)15-10-20-23-17(15)12-2-1-9-25-11-12/h7-8,10,12-14,24H,1-6,9,11H2,(H,20,23)(H,19,21,22)/t12?,13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
J Med Chem 53: 3005-12 (2010)
Article DOI: 10.1021/jm9003279
BindingDB Entry DOI: 10.7270/Q2KS6RP5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277909
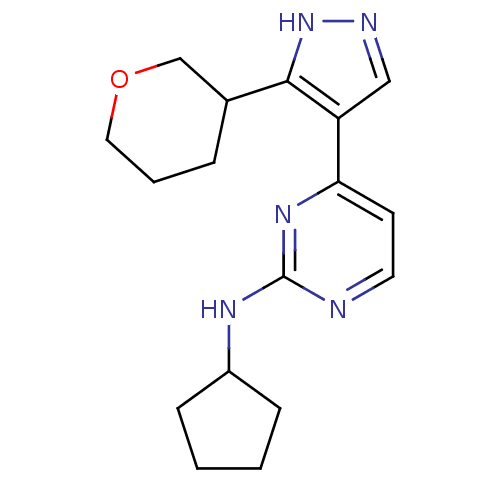
(CHEMBL483741 | N-cyclopentyl-4-(3-(tetrahydro-2H-p...)Show InChI InChI=1S/C17H23N5O/c1-2-6-13(5-1)20-17-18-8-7-15(21-17)14-10-19-22-16(14)12-4-3-9-23-11-12/h7-8,10,12-13H,1-6,9,11H2,(H,19,22)(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277910
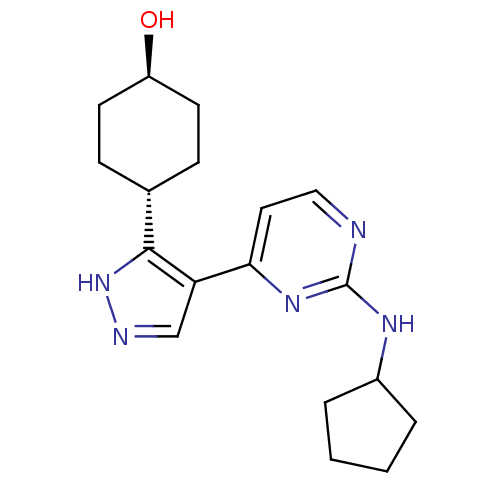
(4-(4-(2-(cyclopentylamino)pyrimidin-4-yl)-1H-pyraz...)Show SMILES O[C@H]1CC[C@@H](CC1)c1[nH]ncc1-c1ccnc(NC2CCCC2)n1 |r,wU:4.7,wD:1.0,(-.62,.56,;.73,1.31,;2.05,.52,;3.4,1.27,;3.42,2.81,;2.09,3.6,;.76,2.85,;4.88,3.29,;5.35,4.76,;6.89,4.76,;7.37,3.3,;6.14,2.4,;6.14,.86,;4.81,.09,;4.81,-1.46,;6.14,-2.23,;7.48,-1.46,;8.82,-2.23,;8.82,-3.77,;7.58,-4.66,;8.05,-6.13,;9.59,-6.12,;10.07,-4.66,;7.48,.09,)| Show InChI InChI=1S/C18H25N5O/c24-14-7-5-12(6-8-14)17-15(11-20-23-17)16-9-10-19-18(22-16)21-13-3-1-2-4-13/h9-14,24H,1-8H2,(H,20,23)(H,19,21,22)/t12-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277869
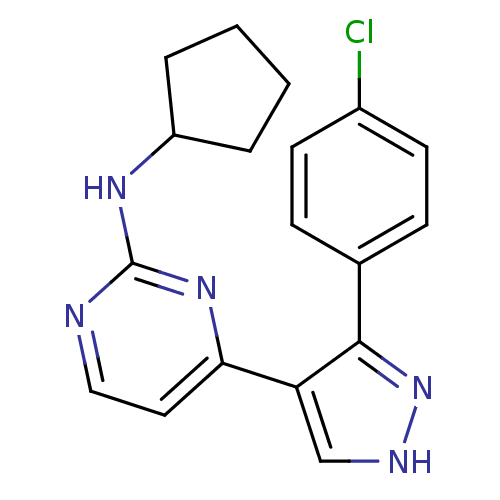
(4-(3-(4-chlorophenyl)-1H-pyrazol-4-yl)-N-cyclopent...)Show InChI InChI=1S/C18H18ClN5/c19-13-7-5-12(6-8-13)17-15(11-21-24-17)16-9-10-20-18(23-16)22-14-3-1-2-4-14/h5-11,14H,1-4H2,(H,21,24)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277838

(4-(3-(4-chlorophenyl)-1H-pyrazol-4-yl)-N-cyclohexy...)Show SMILES Clc1ccc(cc1)-c1n[nH]cc1-c1ccnc(NC2CCCCC2)n1 Show InChI InChI=1S/C19H20ClN5/c20-14-8-6-13(7-9-14)18-16(12-22-25-18)17-10-11-21-19(24-17)23-15-4-2-1-3-5-15/h6-12,15H,1-5H2,(H,22,25)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277948

(CHEMBL482753 | N-(4-(4-(2-(cyclopentylamino)pyrimi...)Show SMILES CC(=O)N[C@H]1CC[C@@H](CC1)c1[nH]ncc1-c1ccnc(NC2CCCC2)n1 |r,wU:7.10,wD:4.3,(-8.79,-14.61,;-7.44,-13.86,;-7.42,-12.32,;-6.12,-14.65,;-4.78,-13.9,;-3.45,-14.69,;-2.1,-13.94,;-2.09,-12.4,;-3.41,-11.62,;-4.75,-12.36,;-.63,-11.92,;-.15,-10.45,;1.39,-10.45,;1.87,-11.91,;.63,-12.82,;.63,-14.36,;-.69,-15.13,;-.7,-16.67,;.64,-17.44,;1.97,-16.67,;3.31,-17.44,;3.31,-18.98,;2.07,-19.88,;2.55,-21.34,;4.09,-21.34,;4.56,-19.87,;1.97,-15.12,)| Show InChI InChI=1S/C20H28N6O/c1-13(27)23-16-8-6-14(7-9-16)19-17(12-22-26-19)18-10-11-21-20(25-18)24-15-4-2-3-5-15/h10-12,14-16H,2-9H2,1H3,(H,22,26)(H,23,27)(H,21,24,25)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277946
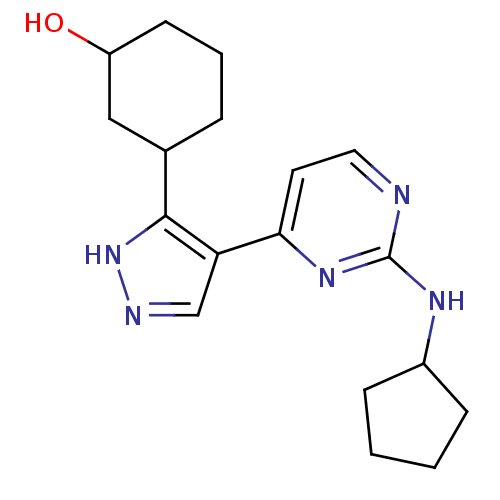
(3-(4-(2-(cyclopentylamino)pyrimidin-4-yl)-1H-pyraz...)Show InChI InChI=1S/C18H25N5O/c24-14-7-3-4-12(10-14)17-15(11-20-23-17)16-8-9-19-18(22-16)21-13-5-1-2-6-13/h8-9,11-14,24H,1-7,10H2,(H,20,23)(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277873

(CHEMBL485365 | N-isopropyl-4-(3-(tetrahydro-2H-pyr...)Show InChI InChI=1S/C15H21N5O/c1-10(2)18-15-16-6-5-13(19-15)12-8-17-20-14(12)11-4-3-7-21-9-11/h5-6,8,10-11H,3-4,7,9H2,1-2H3,(H,17,20)(H,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50364378
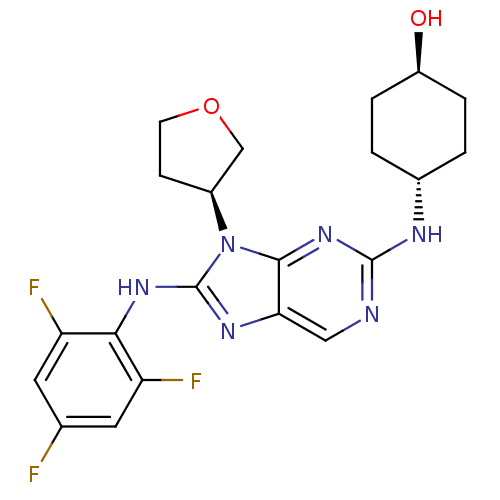
(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK1 expressed in baculoviral system using GST-tagged c-Jun as substrate preincubated for 15 mins prior ATP addition me... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277836
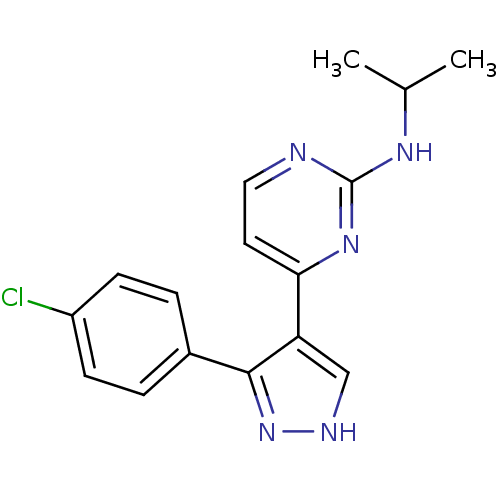
(4-(3-(4-chlorophenyl)-1H-pyrazol-4-yl)-N-isopropyl...)Show InChI InChI=1S/C16H16ClN5/c1-10(2)20-16-18-8-7-14(21-16)13-9-19-22-15(13)11-3-5-12(17)6-4-11/h3-10H,1-2H3,(H,19,22)(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277907

(CHEMBL520488 | N-isopropyl-4-(3-(1-(methylsulfonyl...)Show SMILES CC(C)Nc1nccc(n1)-c1cn[nH]c1C1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C16H24N6O2S/c1-11(2)19-16-17-7-4-14(20-16)13-10-18-21-15(13)12-5-8-22(9-6-12)25(3,23)24/h4,7,10-12H,5-6,8-9H2,1-3H3,(H,18,21)(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277837
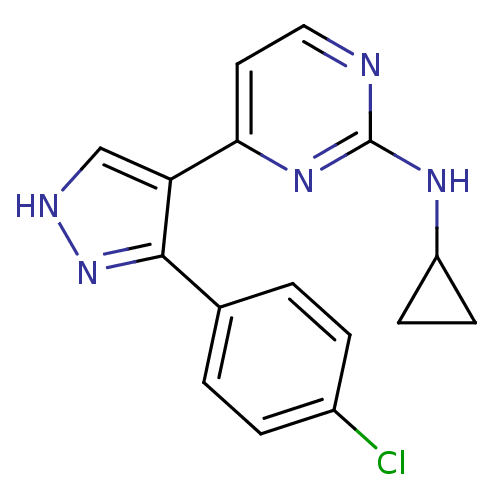
(4-(3-(4-chlorophenyl)-1H-pyrazol-4-yl)-N-cycloprop...)Show InChI InChI=1S/C16H14ClN5/c17-11-3-1-10(2-4-11)15-13(9-19-22-15)14-7-8-18-16(21-14)20-12-5-6-12/h1-4,7-9,12H,5-6H2,(H,19,22)(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277908
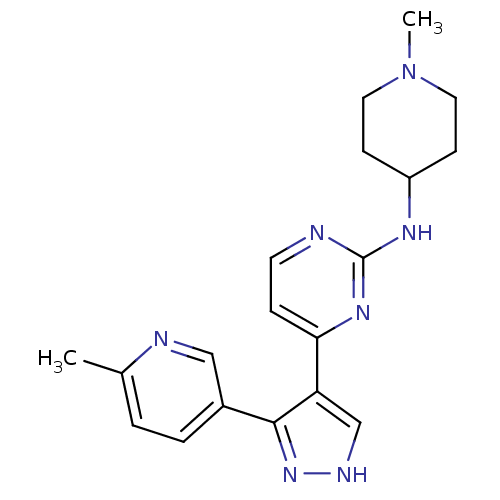
(CHEMBL518996 | N-(1-methylpiperidin-4-yl)-4-(3-(6-...)Show SMILES CN1CCC(CC1)Nc1nccc(n1)-c1c[nH]nc1-c1ccc(C)nc1 Show InChI InChI=1S/C19H23N7/c1-13-3-4-14(11-21-13)18-16(12-22-25-18)17-5-8-20-19(24-17)23-15-6-9-26(2)10-7-15/h3-5,8,11-12,15H,6-7,9-10H2,1-2H3,(H,22,25)(H,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277905

(CHEMBL520332 | N-isopropyl-4-(3-(tetrahydro-2H-pyr...)Show InChI InChI=1S/C15H21N5O/c1-10(2)18-15-16-6-3-13(19-15)12-9-17-20-14(12)11-4-7-21-8-5-11/h3,6,9-11H,4-5,7-8H2,1-2H3,(H,17,20)(H,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15907
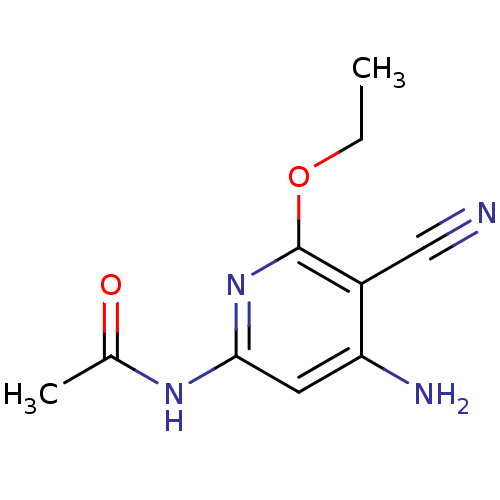
(Aminopyridine-Based Inhibitor 6a | N-(4-Amino-5-cy...)Show InChI InChI=1S/C10H12N4O2/c1-3-16-10-7(5-11)8(12)4-9(14-10)13-6(2)15/h4H,3H2,1-2H3,(H3,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... |
J Med Chem 49: 3563-80 (2006)
Article DOI: 10.1021/jm060199b
BindingDB Entry DOI: 10.7270/Q2P26WDX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277868
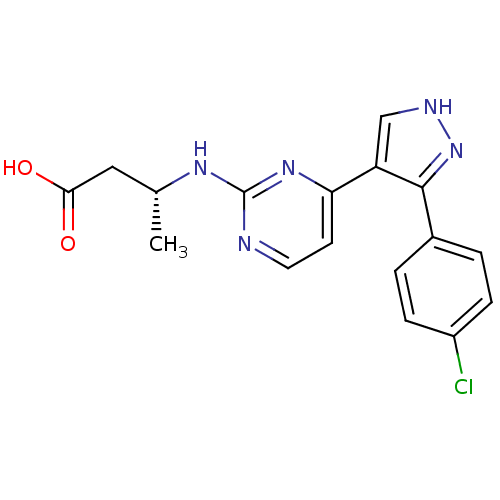
((R)-3-(4-(3-(4-chlorophenyl)-1H-pyrazol-4-yl)pyrim...)Show SMILES C[C@H](CC(O)=O)Nc1nccc(n1)-c1c[nH]nc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H16ClN5O2/c1-10(8-15(24)25)21-17-19-7-6-14(22-17)13-9-20-23-16(13)11-2-4-12(18)5-3-11/h2-7,9-10H,8H2,1H3,(H,20,23)(H,24,25)(H,19,21,22)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277872
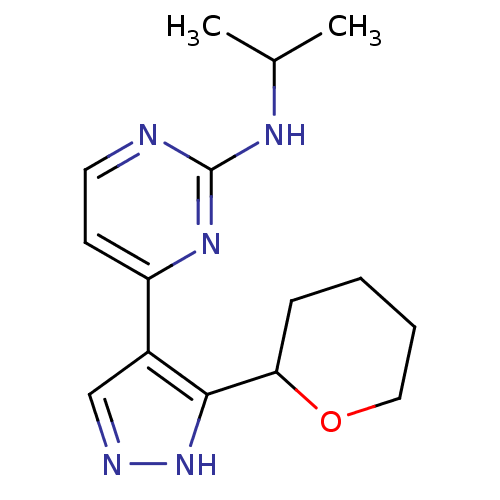
(CHEMBL485364 | N-isopropyl-4-(3-(tetrahydro-2H-pyr...)Show InChI InChI=1S/C15H21N5O/c1-10(2)18-15-16-7-6-12(19-15)11-9-17-20-14(11)13-5-3-4-8-21-13/h6-7,9-10,13H,3-5,8H2,1-2H3,(H,17,20)(H,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 381 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50297455
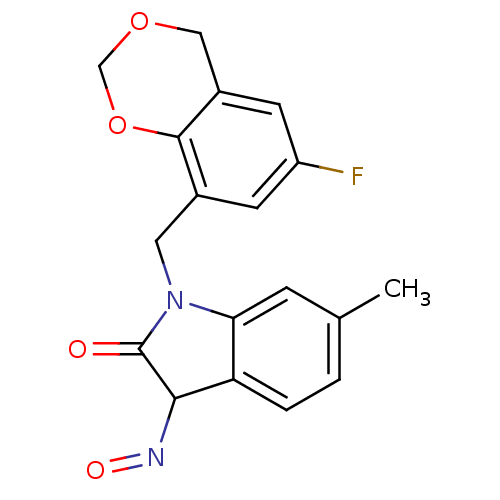
(1-((6-fluoro-4H-benzo[d][1,3]dioxin-8-yl)methyl)-3...)Show SMILES Cc1ccc2C(N=O)C(=O)N(Cc3cc(F)cc4COCOc34)c2c1 Show InChI InChI=1S/C18H15FN2O4/c1-10-2-3-14-15(4-10)21(18(22)16(14)20-23)7-11-5-13(19)6-12-8-24-9-25-17(11)12/h2-6,16H,7-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2891-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.043
BindingDB Entry DOI: 10.7270/Q2F76CMZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277906
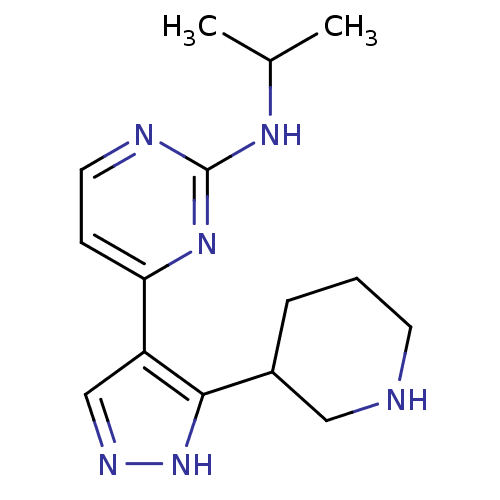
(CHEMBL484326 | N-isopropyl-4-(3-(piperidin-3-yl)-1...)Show InChI InChI=1S/C15H22N6/c1-10(2)19-15-17-7-5-13(20-15)12-9-18-21-14(12)11-4-3-6-16-8-11/h5,7,9-11,16H,3-4,6,8H2,1-2H3,(H,18,21)(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM15968
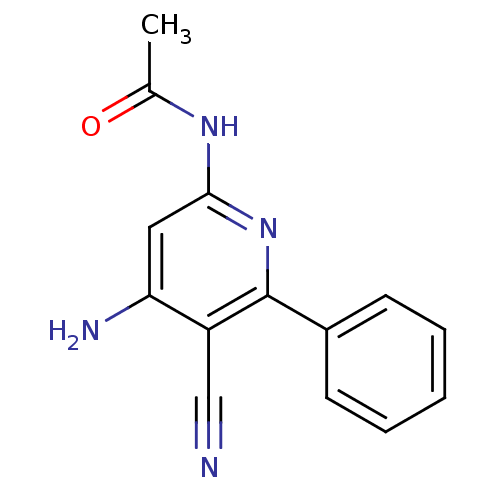
(Aminopyridine-Based Inhibitor 24 | N-(4-Amino-5-cy...)Show InChI InChI=1S/C14H12N4O/c1-9(19)17-13-7-12(16)11(8-15)14(18-13)10-5-3-2-4-6-10/h2-7H,1H3,(H3,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... |
J Med Chem 49: 3563-80 (2006)
Article DOI: 10.1021/jm060199b
BindingDB Entry DOI: 10.7270/Q2P26WDX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50341519
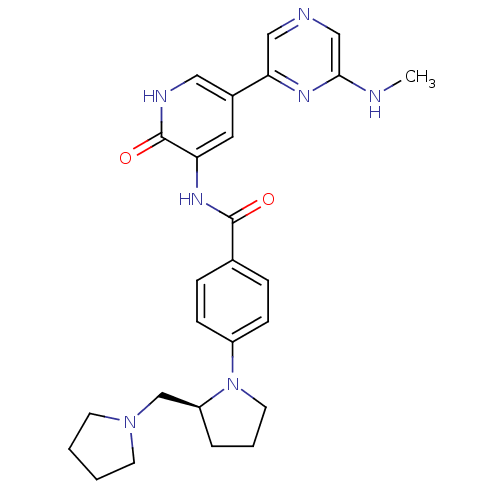
((S)-3-(4-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl...)Show SMILES CNc1cncc(n1)-c1c[nH]c(=O)c(NC(=O)c2ccc(cc2)N2CCC[C@H]2CN2CCCC2)c1 |r| Show InChI InChI=1S/C26H31N7O2/c1-27-24-16-28-15-23(30-24)19-13-22(26(35)29-14-19)31-25(34)18-6-8-20(9-7-18)33-12-4-5-21(33)17-32-10-2-3-11-32/h6-9,13-16,21H,2-5,10-12,17H2,1H3,(H,27,30)(H,29,35)(H,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
J Med Chem 54: 2341-50 (2011)
Article DOI: 10.1021/jm101499u
BindingDB Entry DOI: 10.7270/Q2KH0NPW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277870

(4-(3-(4-chlorophenyl)-1H-pyrazol-4-yl)-N-(1-methyl...)Show SMILES CN1CCC(CC1)Nc1nccc(n1)-c1c[nH]nc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C19H21ClN6/c1-26-10-7-15(8-11-26)23-19-21-9-6-17(24-19)16-12-22-25-18(16)13-2-4-14(20)5-3-13/h2-6,9,12,15H,7-8,10-11H2,1H3,(H,22,25)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277835

(4-methylpyrimidine-2-thiol | CHEMBL455772)Show InChI InChI=1S/C5H6N2S/c1-4-2-3-6-5(8)7-4/h2-3H,1H3,(H,6,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50277871
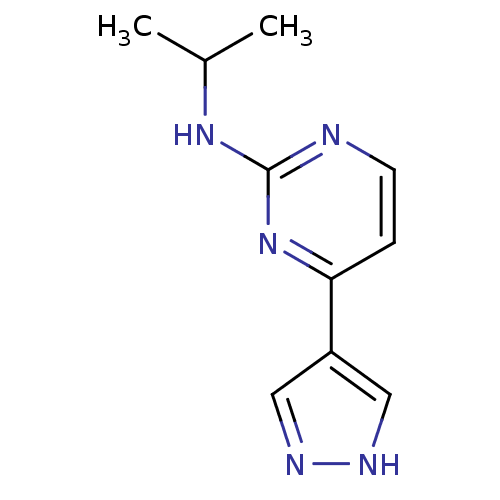
(CHEMBL485193 | N-isopropyl-4-(1H-pyrazol-4-yl)pyri...)Show InChI InChI=1S/C10H13N5/c1-7(2)14-10-11-4-3-9(15-10)8-5-12-13-6-8/h3-7H,1-2H3,(H,12,13)(H,11,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 19: 2099-102 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.023
BindingDB Entry DOI: 10.7270/Q26H4H9D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50402020
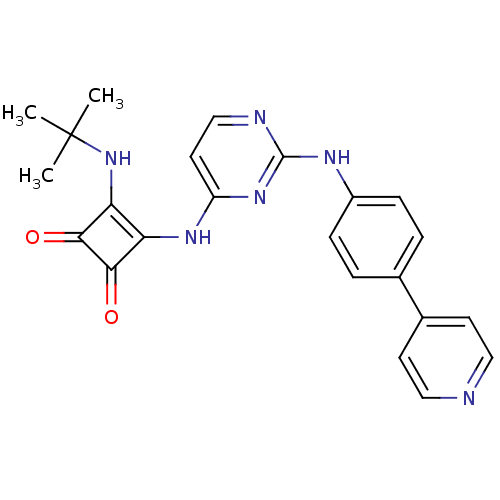
(CHEMBL2205426)Show SMILES CC(C)(C)Nc1c(Nc2ccnc(Nc3ccc(cc3)-c3ccncc3)n2)c(=O)c1=O Show InChI InChI=1S/C23H22N6O2/c1-23(2,3)29-19-18(20(30)21(19)31)27-17-10-13-25-22(28-17)26-16-6-4-14(5-7-16)15-8-11-24-12-9-15/h4-13,29H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1A1 after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP |
Bioorg Med Chem Lett 22: 7615-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.009
BindingDB Entry DOI: 10.7270/Q2XK8GQ3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50463484
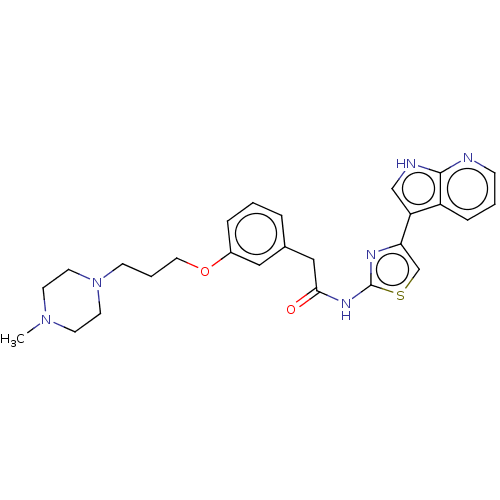
(CHEMBL4248525)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C26H30N6O2S/c1-31-10-12-32(13-11-31)9-4-14-34-20-6-2-5-19(15-20)16-24(33)30-26-29-23(18-35-26)22-17-28-25-21(22)7-3-8-27-25/h2-3,5-8,15,17-18H,4,9-14,16H2,1H3,(H,27,28)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data