

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16292 (3-N-[(2S,3S,5R)-3-amino-5-[(4-fluorophenyl)carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16291 (3-N-[(2S,3S,5R)-3-amino-5-{[(1S)-1-(benzylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16286 (3-N-[(1R,3S,4S)-1-{[(1S)-1-(benzylcarbamoyl)-2-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 71 | -40.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16287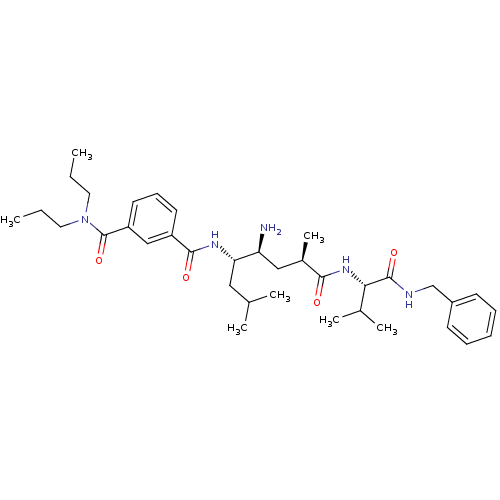 (3-N-[(1R,3S,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16289 (3-N-[(1R,3S,4S)-1-[(4-fluorophenyl)carbamoyl]-3-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16290 (3-N-[(1R,3S,4S)-3-amino-1-[(4-fluorophenyl)carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16288 (3-N-[(1R,3R,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.13E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-457] (Homo sapiens (Human)) | BDBM32389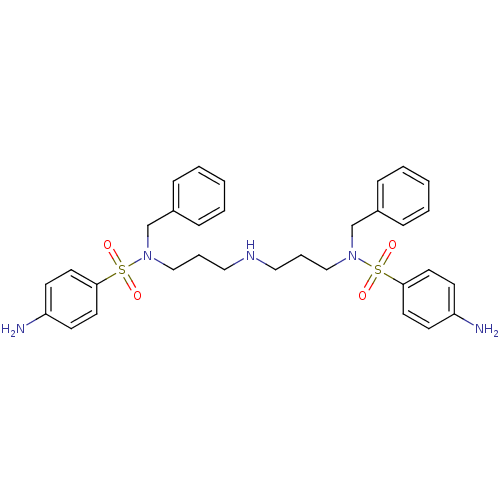 (phenyl-substituted sulfonamides, 6f) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | -23.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Philipps-University Marburg | Assay Description Enzyme assay was performed at room temperature on a microplate reader (Safire2TM) using black 96-well microtiter plates purchased from Nunc. Inhibito... | Bioorg Med Chem 16: 8574-86 (2008) Article DOI: 10.1016/j.bmc.2008.08.012 BindingDB Entry DOI: 10.7270/Q2639N2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-457] (Homo sapiens (Human)) | BDBM32386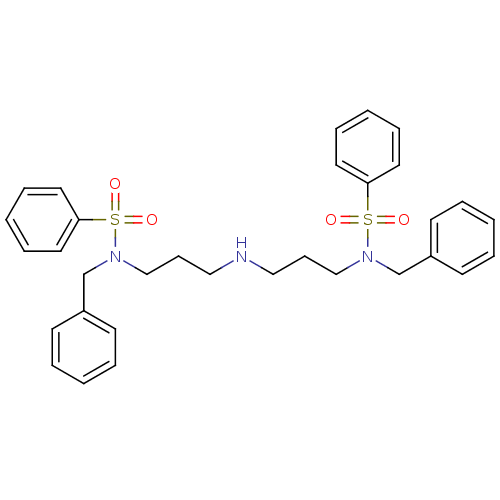 (phenyl-substituted sulfonamides, 6c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | -21.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Philipps-University Marburg | Assay Description Enzyme assay was performed at room temperature on a microplate reader (Safire2TM) using black 96-well microtiter plates purchased from Nunc. Inhibito... | Bioorg Med Chem 16: 8574-86 (2008) Article DOI: 10.1016/j.bmc.2008.08.012 BindingDB Entry DOI: 10.7270/Q2639N2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||