 Found 1446 hits of ki data for polymerid = 1924,50002415,10718,5978
Found 1446 hits of ki data for polymerid = 1924,50002415,10718,5978 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50157823
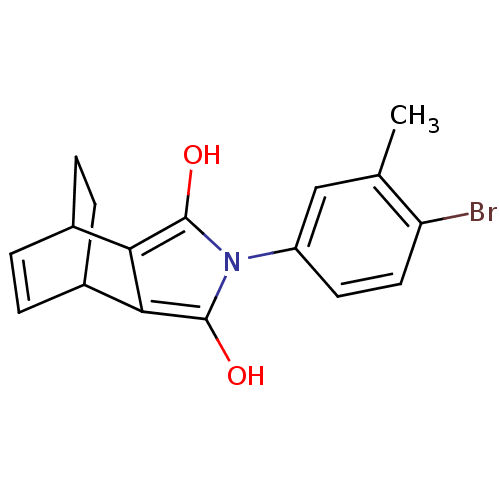
((2R,6S)-4-(4-Bromo-3-methyl-phenyl)-4-aza-tricyclo...)Show SMILES Cc1cc(ccc1Br)-n1c(O)c2C3CCC(C=C3)c2c1O |c:17,THB:9:11:16.17:13.14| Show InChI InChI=1S/C17H16BrNO2/c1-9-8-12(6-7-13(9)18)19-16(20)14-10-2-3-11(5-4-10)15(14)17(19)21/h2-3,6-8,10-11,20-21H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for mutant T877A Androgen receptor in human LNCaP cells |
Bioorg Med Chem Lett 15: 271-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.085
BindingDB Entry DOI: 10.7270/Q2N58KVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29321
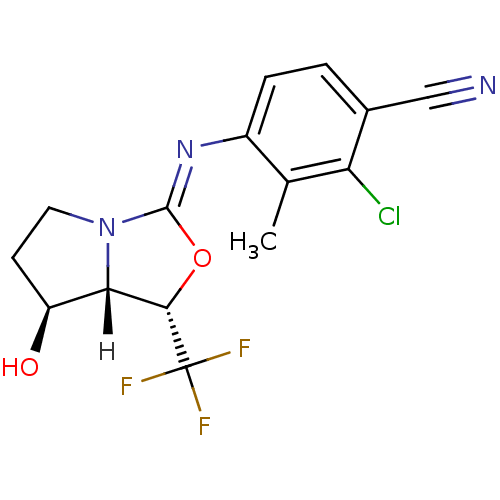
(oxazolidin-2-imine, 6d)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
Bioorg Med Chem Lett 17: 1523-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.007
BindingDB Entry DOI: 10.7270/Q22J6950 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 49: 6143-6 (2006)
Article DOI: 10.1021/jm060792t
BindingDB Entry DOI: 10.7270/Q26971VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 5049-52 (2007)
Article DOI: 10.1021/jm070231h
BindingDB Entry DOI: 10.7270/Q29Z935Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor expressed in monkey COS7 cells by whole cell binding assay |
Bioorg Med Chem Lett 18: 3431-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.085
BindingDB Entry DOI: 10.7270/Q25M66J9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215709
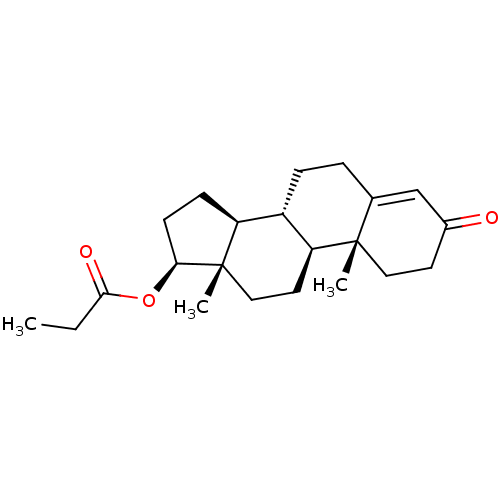
(CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...)Show SMILES CCC(=O)O[C@H]1CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r,t:12| Show InChI InChI=1S/C22H32O3/c1-4-20(24)25-19-8-7-17-16-6-5-14-13-15(23)9-11-21(14,2)18(16)10-12-22(17,19)3/h13,16-19H,4-12H2,1-3H3/t16-,17-,18-,19-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 17: 4487-90 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.007
BindingDB Entry DOI: 10.7270/Q2NV9HZ9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | -50.7 | n/a | n/a | 1 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSC-Scientific Computing Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostate |
J Med Chem 48: 917-25 (2005)
Article DOI: 10.1021/jm0495879
BindingDB Entry DOI: 10.7270/Q2SJ1PBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM29320

(BMS-665139 | oxazolidin-2-imine, 6c)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 0.200 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM29323
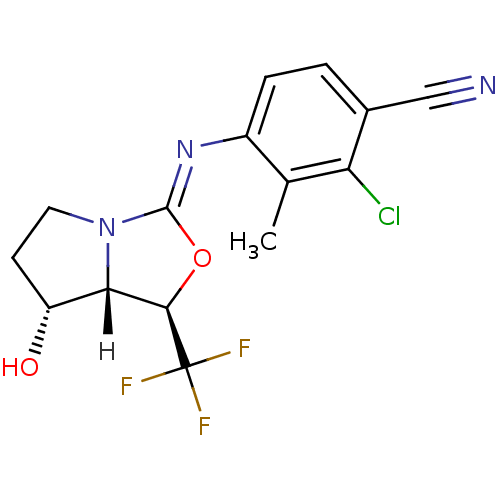
(oxazolidin-2-imine, 6f)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12+,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 1.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50099679
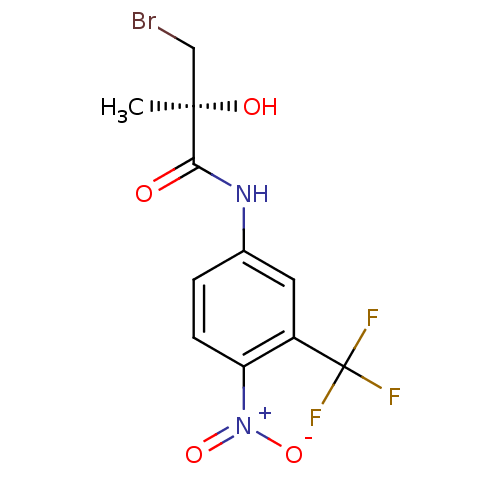
(3-Bromo-2-hydroxy-2-methyl-N-(4-nitro-3-trifluorom...)Show SMILES C[C@@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114119
BindingDB Entry DOI: 10.7270/Q2P55SHP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29320

(BMS-665139 | oxazolidin-2-imine, 6c)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00716
BindingDB Entry DOI: 10.7270/Q2J1076H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -56.5 | n/a | n/a | 5.70 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 2486-96 (2007)
Article DOI: 10.1021/jm061329j
BindingDB Entry DOI: 10.7270/Q20R9MNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699
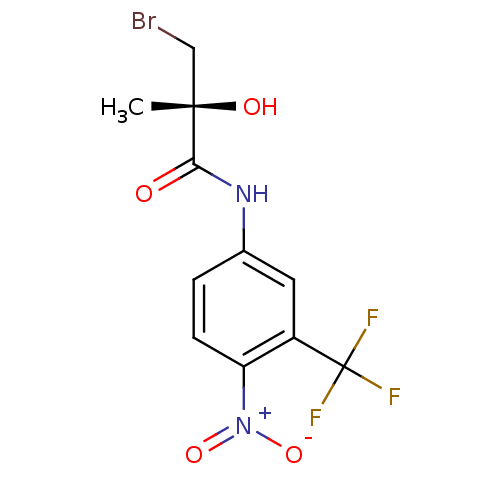
((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM29319
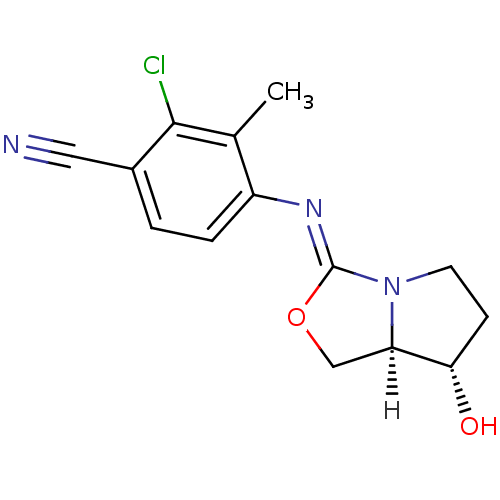
(oxazolidin-2-imine, 6b)Show SMILES [H][C@]12CO\C(=N/c3ccc(C#N)c(Cl)c3C)N1CC[C@@H]2O |r| Show InChI InChI=1S/C14H14ClN3O2/c1-8-10(3-2-9(6-16)13(8)15)17-14-18-5-4-12(19)11(18)7-20-14/h2-3,11-12,19H,4-5,7H2,1H3/b17-14-/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 14 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 2.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18699

((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | -50.5 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699

((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18699

((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSC-Scientific Computing Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostate |
J Med Chem 48: 917-25 (2005)
Article DOI: 10.1021/jm0495879
BindingDB Entry DOI: 10.7270/Q2SJ1PBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916
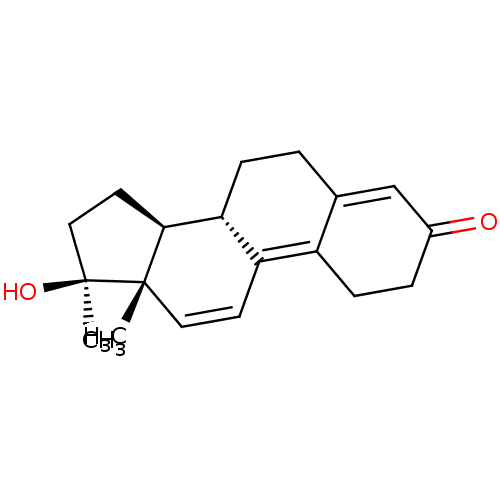
(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18605
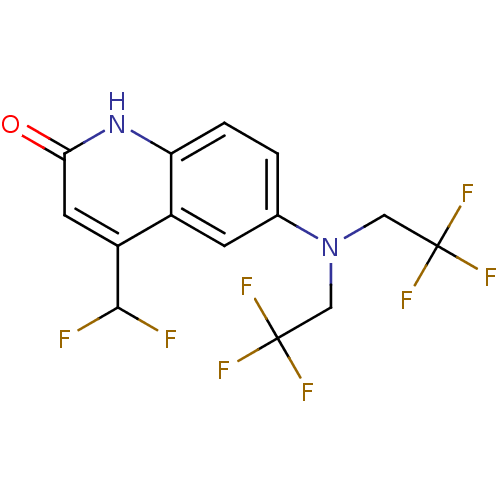
(6-[bis(2,2,2-trifluoroethyl)amino]-4-(difluorometh...)Show SMILES FC(F)c1cc(=O)[nH]c2ccc(cc12)N(CC(F)(F)F)CC(F)(F)F Show InChI InChI=1S/C14H10F8N2O/c15-12(16)9-4-11(25)23-10-2-1-7(3-8(9)10)24(5-13(17,18)19)6-14(20,21)22/h1-4,12H,5-6H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
Bioorg Med Chem Lett 17: 1527-31 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.001
BindingDB Entry DOI: 10.7270/Q2XS5SP9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215713
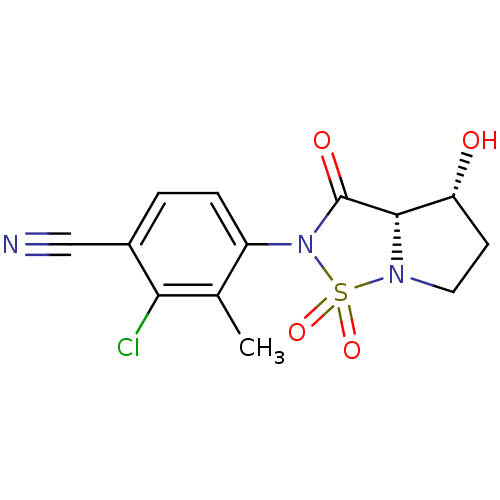
(2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...)Show SMILES Cc1c(Cl)c(ccc1N1C(=O)[C@@H]2[C@H](O)CCN2S1(=O)=O)C#N |r| Show InChI InChI=1S/C13H12ClN3O4S/c1-7-9(3-2-8(6-15)11(7)14)17-13(19)12-10(18)4-5-16(12)22(17,20)21/h2-3,10,12,18H,4-5H2,1H3/t10-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00716
BindingDB Entry DOI: 10.7270/Q2J1076H |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215713

(2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...)Show SMILES Cc1c(Cl)c(ccc1N1C(=O)[C@@H]2[C@H](O)CCN2S1(=O)=O)C#N |r| Show InChI InChI=1S/C13H12ClN3O4S/c1-7-9(3-2-8(6-15)11(7)14)17-13(19)12-10(18)4-5-16(12)22(17,20)21/h2-3,10,12,18H,4-5H2,1H3/t10-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215713

(2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...)Show SMILES Cc1c(Cl)c(ccc1N1C(=O)[C@@H]2[C@H](O)CCN2S1(=O)=O)C#N |r| Show InChI InChI=1S/C13H12ClN3O4S/c1-7-9(3-2-8(6-15)11(7)14)17-13(19)12-10(18)4-5-16(12)22(17,20)21/h2-3,10,12,18H,4-5H2,1H3/t10-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 17: 4487-90 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.007
BindingDB Entry DOI: 10.7270/Q2NV9HZ9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18607
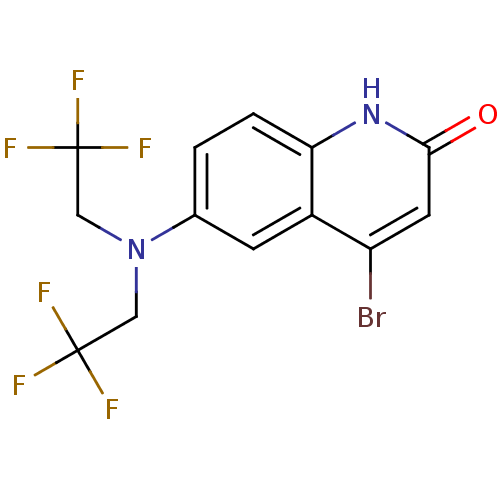
(6-[bis(2,2,2-trifluoroethyl)amino]-4-bromo-1,2-dih...)Show SMILES FC(F)(F)CN(CC(F)(F)F)c1ccc2[nH]c(=O)cc(Br)c2c1 Show InChI InChI=1S/C13H9BrF6N2O/c14-9-4-11(23)21-10-2-1-7(3-8(9)10)22(5-12(15,16)17)6-13(18,19)20/h1-4H,5-6H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
Bioorg Med Chem Lett 17: 1527-31 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.001
BindingDB Entry DOI: 10.7270/Q2XS5SP9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18606
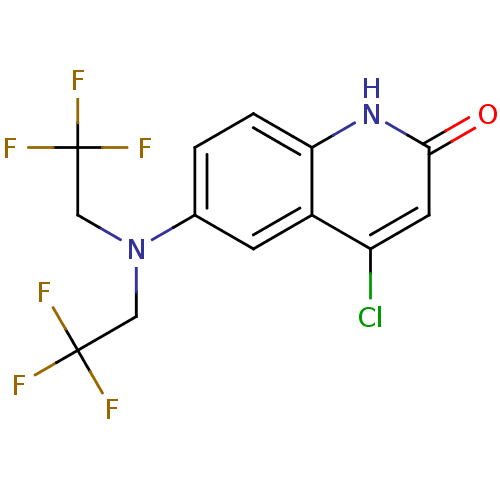
(6-[bis(2,2,2-trifluoroethyl)amino]-4-chloro-1,2-di...)Show SMILES FC(F)(F)CN(CC(F)(F)F)c1ccc2[nH]c(=O)cc(Cl)c2c1 Show InChI InChI=1S/C13H9ClF6N2O/c14-9-4-11(23)21-10-2-1-7(3-8(9)10)22(5-12(15,16)17)6-13(18,19)20/h1-4H,5-6H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
Bioorg Med Chem Lett 17: 1527-31 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.001
BindingDB Entry DOI: 10.7270/Q2XS5SP9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18578
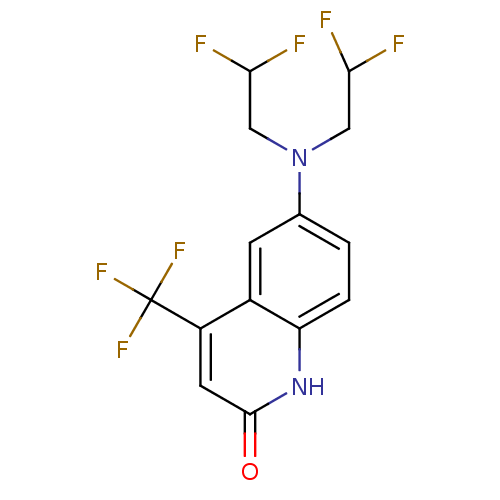
(6-[bis(2,2-difluoroethyl)amino]-4-(trifluoromethyl...)Show SMILES FC(F)CN(CC(F)F)c1ccc2[nH]c(=O)cc(c2c1)C(F)(F)F Show InChI InChI=1S/C14H11F7N2O/c15-11(16)5-23(6-12(17)18)7-1-2-10-8(3-7)9(14(19,20)21)4-13(24)22-10/h1-4,11-12H,5-6H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | -55.2 | n/a | n/a | 0.400 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
Bioorg Med Chem Lett 17: 1527-31 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.001
BindingDB Entry DOI: 10.7270/Q2XS5SP9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50157826
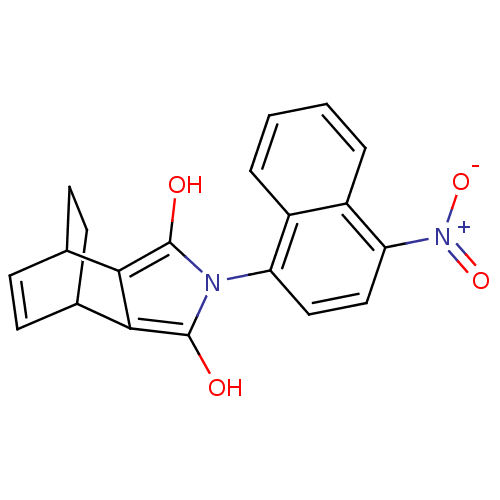
((2S,6R)-4-(4-Nitro-naphthalen-1-yl)-4-aza-tricyclo...)Show SMILES Oc1c2C3CCC(C=C3)c2c(O)n1-c1ccc([N+]([O-])=O)c2ccccc12 |c:7,THB:10:9:7.8:4.5,(.45,2.28,;-.79,1.39,;-2.24,1.86,;-3.71,2.32,;-4.12,3.9,;-4.98,2.62,;-4.64,1.11,;-6.17,.83,;-5.2,2.02,;-3.15,.6,;-2.24,-.63,;-2.72,-2.1,;-.79,-.16,;.54,-.93,;.54,-2.47,;1.86,-3.23,;3.19,-2.47,;4.53,-3.23,;4.53,-4.76,;5.83,-2.48,;3.19,-.93,;4.53,-.16,;4.53,1.39,;3.17,2.14,;1.86,1.39,;1.86,-.16,)| Show InChI InChI=1S/C20H16N2O4/c23-19-17-11-5-6-12(8-7-11)18(17)20(24)21(19)15-9-10-16(22(25)26)14-4-2-1-3-13(14)15/h1-6,9-12,23-24H,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for mutant T877A Androgen receptor in human LNCaP cells |
Bioorg Med Chem Lett 15: 271-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.085
BindingDB Entry DOI: 10.7270/Q2N58KVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18188
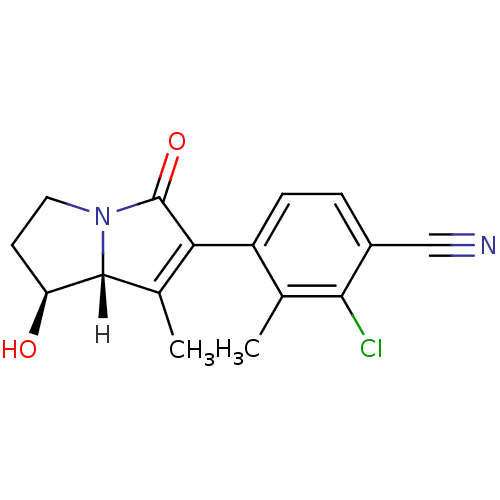
(4-[(1S,7aR)-1-hydroxy-7-methyl-5-oxo-2,3,5,7a-tetr...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)C(=C2C)c1ccc(C#N)c(Cl)c1C |r,c:10| Show InChI InChI=1S/C16H15ClN2O2/c1-8-11(4-3-10(7-18)14(8)17)13-9(2)15-12(20)5-6-19(15)16(13)21/h3-4,12,15,20H,5-6H2,1-2H3/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | 2 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50158075
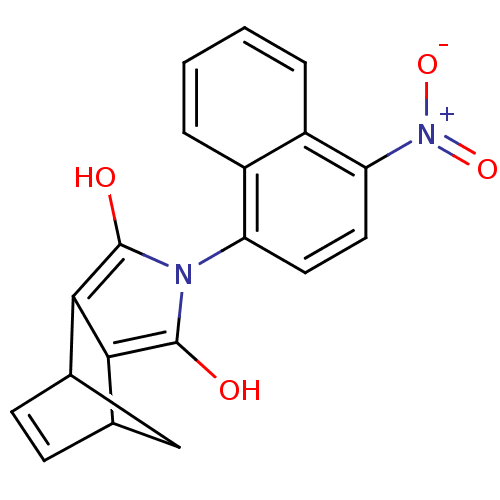
((2S,6R)-4-(4-Nitro-naphthalen-1-yl)-4-aza-tricyclo...)Show SMILES Oc1c2C3CC(C=C3)c2c(O)n1-c1ccc([N+]([O-])=O)c2ccccc12 |c:6,TLB:1:2:7.6:4,THB:9:8:7.6:4,(.02,2.61,;-1.14,1.58,;-2.64,1.89,;-4.27,2.42,;-4.54,3.83,;-4.67,1.18,;-5.99,.53,;-5.37,1.65,;-3.41,.55,;-2.38,-.59,;-2.7,-2.09,;-.97,.04,;.36,-.73,;.36,-2.28,;1.69,-3.05,;3.02,-2.28,;4.36,-3.05,;4.36,-4.59,;5.71,-2.28,;3.02,-.73,;4.36,.04,;4.36,1.58,;3.02,2.33,;1.69,1.57,;1.69,.04,)| Show InChI InChI=1S/C19H14N2O4/c22-18-16-10-5-6-11(9-10)17(16)19(23)20(18)14-7-8-15(21(24)25)13-4-2-1-3-12(13)14/h1-8,10-11,22-23H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DHT binding to T877A androgen receptor of LNCaP cells |
Bioorg Med Chem Lett 15: 389-93 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.051
BindingDB Entry DOI: 10.7270/Q2QZ29GJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50594916
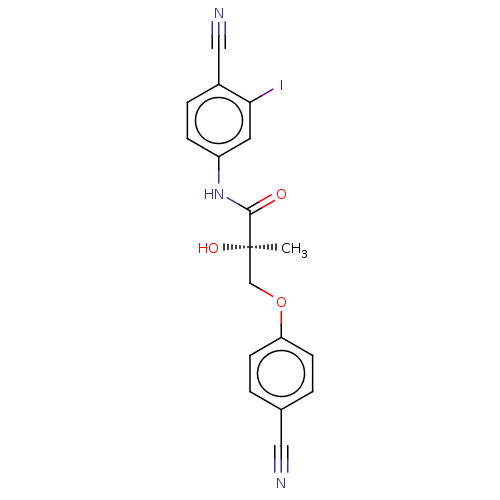
(CHEMBL469759)Show SMILES C[C@@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(I)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114119
BindingDB Entry DOI: 10.7270/Q2P55SHP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26260
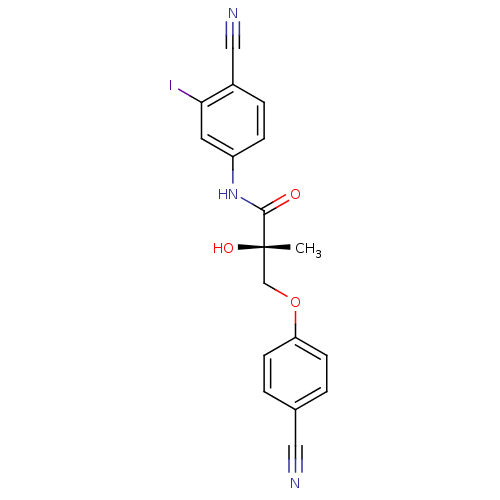
((2S)-N-(4-cyano-3-iodophenyl)-3-(4-cyanophenoxy)-2...)Show SMILES C[C@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(I)c1 |r| Show InChI InChI=1S/C18H14IN3O3/c1-18(24,11-25-15-6-2-12(9-20)3-7-15)17(23)22-14-5-4-13(10-21)16(19)8-14/h2-8,24H,11H2,1H3,(H,22,23)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.540 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529668

(Enobosarm | Gtx-024 | MK-2866 | Ostarine | US10806...)Show SMILES C[C@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-18(27,11-28-15-6-2-12(9-23)3-7-15)17(26)25-14-5-4-13(10-24)16(8-14)19(20,21)22/h2-8,27H,11H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00716
BindingDB Entry DOI: 10.7270/Q2J1076H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18681

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18681

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50158078
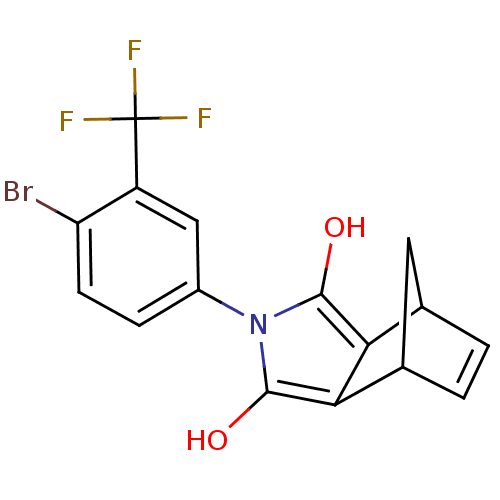
((2S,6R)-4-(4-Bromo-3-trifluoromethyl-phenyl)-4-aza...)Show SMILES Oc1c2C3CC(C=C3)c2c(O)n1-c1ccc(Br)c(c1)C(F)(F)F |c:6,THB:9:8:6.7:4| Show InChI InChI=1S/C16H11BrF3NO2/c17-11-4-3-9(6-10(11)16(18,19)20)21-14(22)12-7-1-2-8(5-7)13(12)15(21)23/h1-4,6-8,22-23H,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DHT binding to T877A androgen receptor of LNCaP cells |
Bioorg Med Chem Lett 15: 389-93 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.051
BindingDB Entry DOI: 10.7270/Q2QZ29GJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM86689
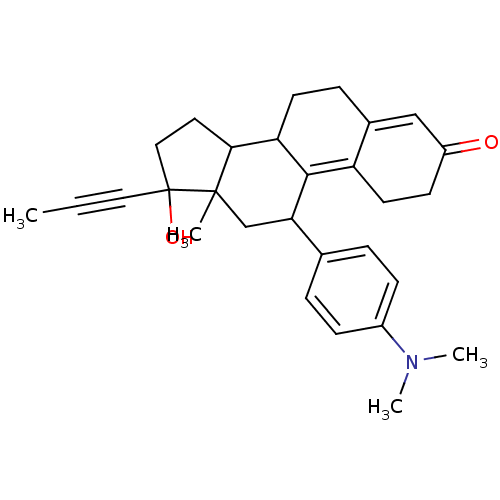
(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627
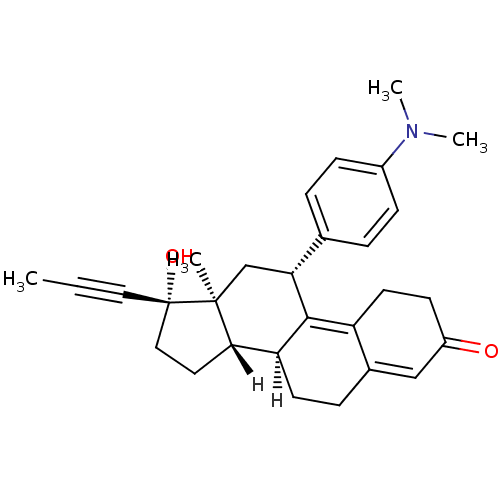
((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human androgen receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50145862
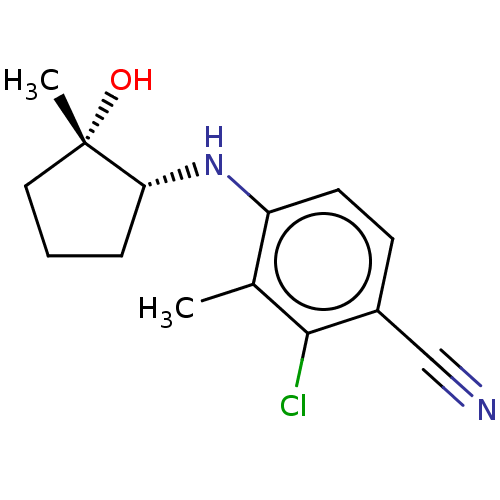
(CHEMBL3765171)Show InChI InChI=1S/C14H17ClN2O/c1-9-11(6-5-10(8-16)13(9)15)17-12-4-3-7-14(12,2)18/h5-6,12,17-18H,3-4,7H2,1-2H3/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against rat prostate cytosol androgen receptor using [3H]mibolerone |
J Med Chem 43: 3344-7 (2000)
BindingDB Entry DOI: 10.7270/Q2GQ6ZFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18183
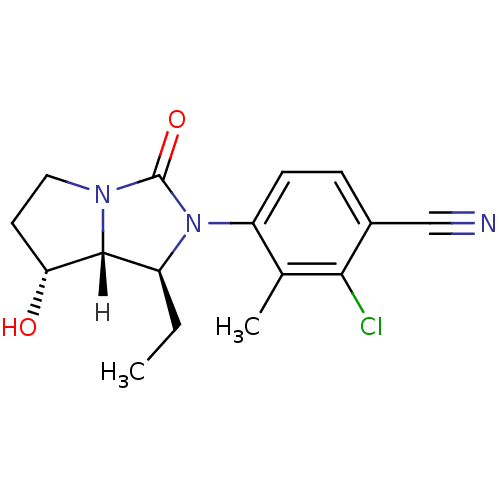
(4-[(1S,7R,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...)Show SMILES [H][C@@]12[C@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H18ClN3O2/c1-3-11-15-13(21)6-7-19(15)16(22)20(11)12-5-4-10(8-18)14(17)9(12)2/h4-5,11,13,15,21H,3,6-7H2,1-2H3/t11-,13+,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | 2.60 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29324
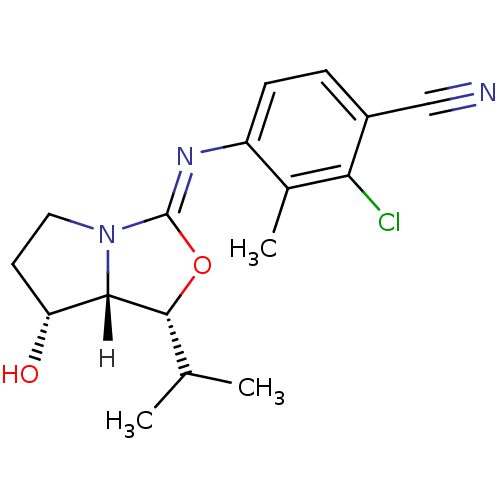
(oxazolidin-2-imine, 6g)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@@H]2C(C)C)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C17H20ClN3O2/c1-9(2)16-15-13(22)6-7-21(15)17(23-16)20-12-5-4-11(8-19)14(18)10(12)3/h4-5,9,13,15-16,22H,6-7H2,1-3H3/b20-17-/t13-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | 3.70 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205111
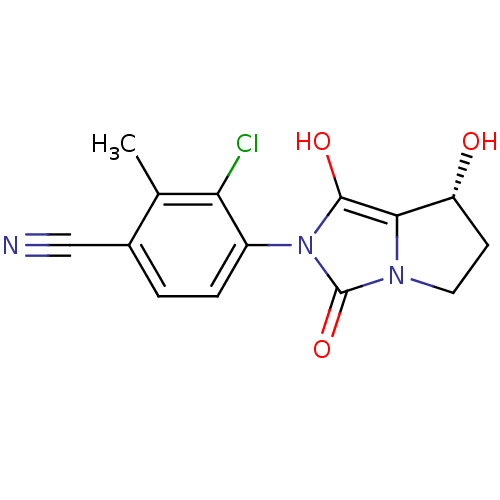
(3-chloro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydr...)Show SMILES Cc1c(Cl)c(ccc1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:14.15,(22.97,-37.01,;22.21,-35.67,;20.67,-35.65,;19.89,-36.98,;19.91,-34.31,;20.69,-32.99,;22.22,-32.99,;22.99,-34.34,;24.53,-34.34,;26.08,-34.34,;18.37,-34.31,;17.48,-33.06,;17.96,-31.6,;16.01,-33.53,;14.55,-33.04,;14.08,-31.57,;13.63,-34.28,;14.54,-35.53,;16,-35.06,;17.46,-35.55,;17.93,-37.02,)| Show InChI InChI=1S/C14H12ClN3O3/c1-7-8(6-16)2-3-9(11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50091401
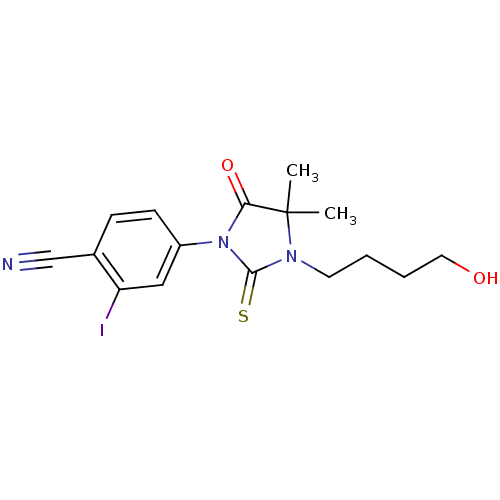
(4-[3-(4-Hydroxy-butyl)-4,4-dimethyl-5-oxo-2-thioxo...)Show InChI InChI=1S/C16H18IN3O2S/c1-16(2)14(22)20(15(23)19(16)7-3-4-8-21)12-6-5-11(10-18)13(17)9-12/h5-6,9,21H,3-4,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSC-Scientific Computing Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostate |
J Med Chem 48: 917-25 (2005)
Article DOI: 10.1021/jm0495879
BindingDB Entry DOI: 10.7270/Q2SJ1PBD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50091401

(4-[3-(4-Hydroxy-butyl)-4,4-dimethyl-5-oxo-2-thioxo...)Show InChI InChI=1S/C16H18IN3O2S/c1-16(2)14(22)20(15(23)19(16)7-3-4-8-21)12-6-5-11(10-18)13(17)9-12/h5-6,9,21H,3-4,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibition of rat prostate cytosolic androgen receptor |
Bioorg Med Chem Lett 14: 5285-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.031
BindingDB Entry DOI: 10.7270/Q29K4BZG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50091401

(4-[3-(4-Hydroxy-butyl)-4,4-dimethyl-5-oxo-2-thioxo...)Show InChI InChI=1S/C16H18IN3O2S/c1-16(2)14(22)20(15(23)19(16)7-3-4-8-21)12-6-5-11(10-18)13(17)9-12/h5-6,9,21H,3-4,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against rat prostate cytosol androgen receptor using [3H]mibolerone |
J Med Chem 43: 3344-7 (2000)
BindingDB Entry DOI: 10.7270/Q2GQ6ZFW |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26258
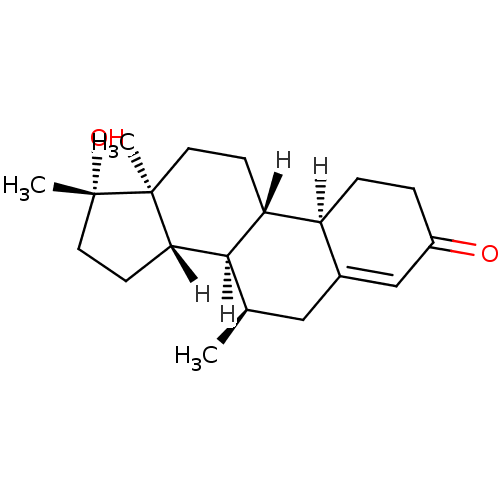
((1S,2R,9R,10R,11S,14S,15S)-14-hydroxy-9,14,15-trim...)Show SMILES [H][C@@]12CC[C@](C)(O)[C@@]1(C)CC[C@]1([H])[C@@]3([H])CCC(=O)C=C3C[C@@H](C)[C@@]21[H] |c:20| Show InChI InChI=1S/C20H30O2/c1-12-10-13-11-14(21)4-5-15(13)16-6-8-19(2)17(18(12)16)7-9-20(19,3)22/h11-12,15-18,22H,4-10H2,1-3H3/t12-,15+,16-,17+,18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against rat prostate cytosol androgen receptor using [3H]mibolerone |
J Med Chem 43: 3344-7 (2000)
BindingDB Entry DOI: 10.7270/Q2GQ6ZFW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data