 Found 4887 hits of ki data for polymerid = 2142,49000109
Found 4887 hits of ki data for polymerid = 2142,49000109 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044610
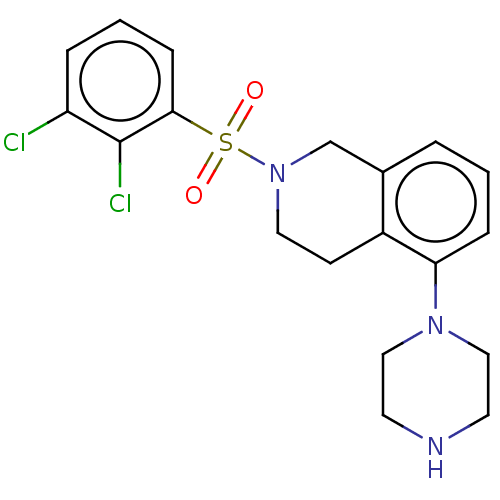
(CHEMBL3329438)Show SMILES Clc1cccc(c1Cl)S(=O)(=O)N1CCc2c(C1)cccc2N1CCNCC1 Show InChI InChI=1S/C19H21Cl2N3O2S/c20-16-4-2-6-18(19(16)21)27(25,26)24-10-7-15-14(13-24)3-1-5-17(15)23-11-8-22-9-12-23/h1-6,22H,7-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5HT6 receptor expressed in CHO cells |
J Med Chem 57: 7160-81 (2014)
Article DOI: 10.1021/jm5003952
BindingDB Entry DOI: 10.7270/Q2X92CZZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166333
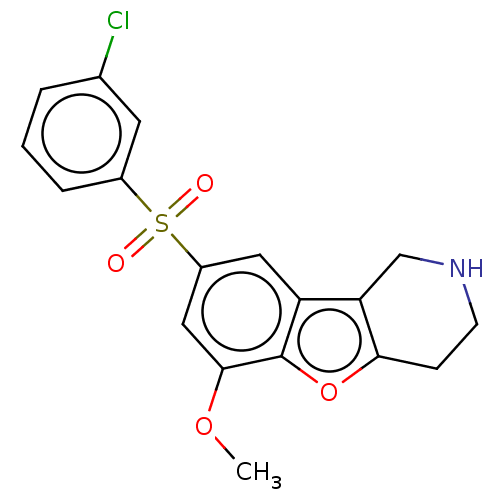
(US9067949, 190)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H16ClNO4S/c1-23-17-9-13(25(21,22)12-4-2-3-11(19)7-12)8-14-15-10-20-6-5-16(15)24-18(14)17/h2-4,7-9,20H,5-6,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415991

(CHEMBL1084794)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C19H20N2O2S/c20-11-14-5-3-4-13-10-15(8-9-16(13)14)24(22,23)19-12-21-18-7-2-1-6-17(18)19/h1-2,6-10,12,14,21H,3-5,11,20H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477491

(CHEMBL398034)Show SMILES Clc1cccc(Cl)c1S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H19Cl2N3O3S/c19-13-3-1-4-14(20)18(13)27(24,25)23-11-12-26-17-15(5-2-6-16(17)23)22-9-7-21-8-10-22/h1-6,21H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415975
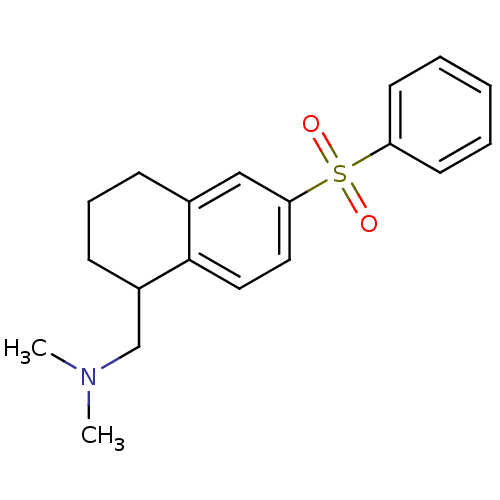
(CHEMBL1085462)Show InChI InChI=1S/C19H23NO2S/c1-20(2)14-16-8-6-7-15-13-18(11-12-19(15)16)23(21,22)17-9-4-3-5-10-17/h3-5,9-13,16H,6-8,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415981

(CHEMBL1086326)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C17H18FNO2S/c18-14-5-2-6-15(10-14)22(20,21)16-7-8-17-12(9-16)3-1-4-13(17)11-19/h2,5-10,13H,1,3-4,11,19H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166213

(US9067949, 70)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C20H18N2O4S/c1-25-19-10-14(9-15-16-11-21-6-5-18(16)26-20(15)19)27(23,24)13-2-3-17-12(8-13)4-7-22-17/h2-4,7-10,21-22H,5-6,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0830 | -57.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328391
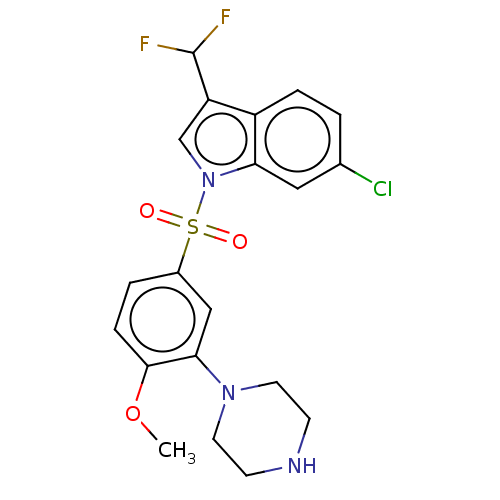
(6-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Cl)cc12 Show InChI InChI=1S/C20H20ClF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318625

(CHEMBL1083654 | N-Methyl-(3-phenylsulfonyl)-7,8-di...)Show InChI InChI=1S/C16H16N4O2S/c1-17-15-14(23(21,22)12-7-3-2-4-8-12)16-18-10-11-6-5-9-13(11)20(16)19-15/h2-4,7-8,10H,5-6,9H2,1H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]lysergic acid diethylamide from human recombinant 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 5186-96 (2010)
Article DOI: 10.1021/jm100350r
BindingDB Entry DOI: 10.7270/Q26T0NMP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579331
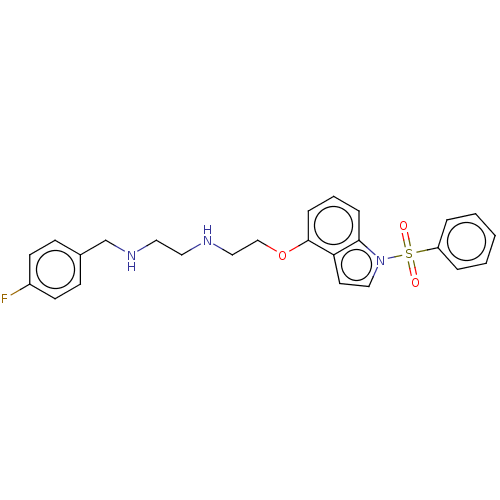
(CHEMBL4852099)Show SMILES Fc1ccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415993
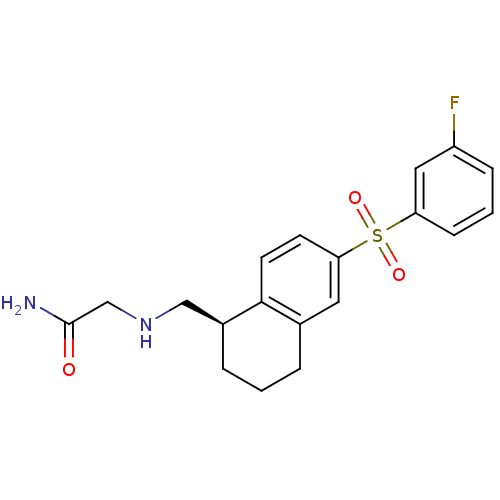
(CHEMBL1086252)Show SMILES NC(=O)CNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H21FN2O3S/c20-15-5-2-6-16(10-15)26(24,25)17-7-8-18-13(9-17)3-1-4-14(18)11-22-12-19(21)23/h2,5-10,14,22H,1,3-4,11-12H2,(H2,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416007

(CHEMBL1085120)Show SMILES OCC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H20FNO4S/c20-15-5-2-6-16(10-15)26(24,25)17-7-8-18-13(9-17)3-1-4-14(18)11-21-19(23)12-22/h2,5-10,14,22H,1,3-4,11-12H2,(H,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166328
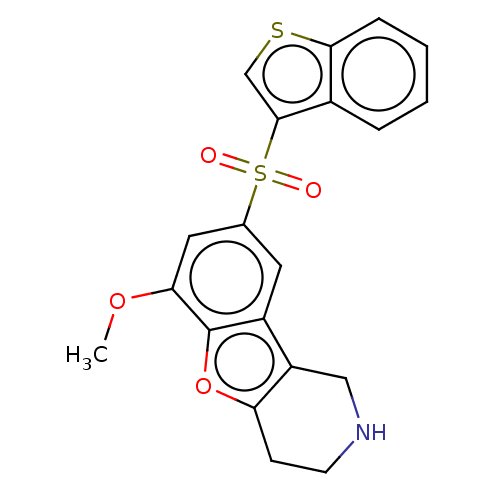
(US9067949, 185)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1csc2ccccc12 Show InChI InChI=1S/C20H17NO4S2/c1-24-17-9-12(8-14-15-10-21-7-6-16(15)25-20(14)17)27(22,23)19-11-26-18-5-3-2-4-13(18)19/h2-5,8-9,11,21H,6-7,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416028
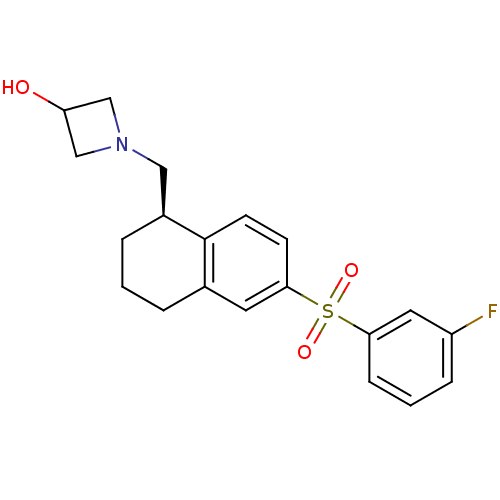
(CHEMBL1085658)Show SMILES OC1CN(C[C@@H]2CCCc3cc(ccc23)S(=O)(=O)c2cccc(F)c2)C1 |r| Show InChI InChI=1S/C20H22FNO3S/c21-16-5-2-6-18(10-16)26(24,25)19-7-8-20-14(9-19)3-1-4-15(20)11-22-12-17(23)13-22/h2,5-10,15,17,23H,1,3-4,11-13H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50163035
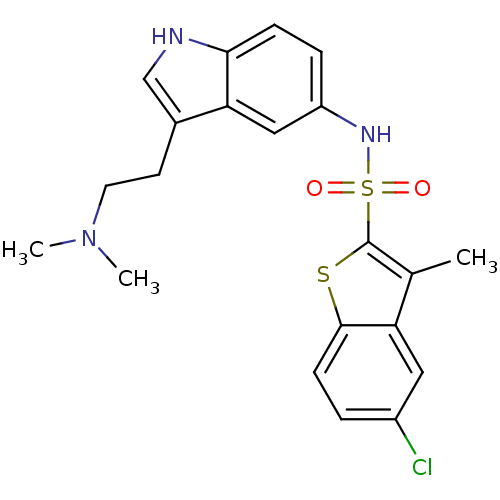
(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic aci...)Show SMILES CN(C)CCc1c[nH]c2ccc(NS(=O)(=O)c3sc4ccc(Cl)cc4c3C)cc12 Show InChI InChI=1S/C21H22ClN3O2S2/c1-13-17-10-15(22)4-7-20(17)28-21(13)29(26,27)24-16-5-6-19-18(11-16)14(12-23-19)8-9-25(2)3/h4-7,10-12,23-24H,8-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve S.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LSD binding to human 5-hydroxytryptamine 6 receptor expressed in HEK293 cells |
J Med Chem 48: 1781-95 (2005)
Article DOI: 10.1021/jm049615n
BindingDB Entry DOI: 10.7270/Q2ZP45N9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416015

(CHEMBL1086113)Show SMILES Oc1c[nH]c(NC[C@@H]2CCCc3cc(ccc23)S(=O)(=O)c2cccc(F)c2)n1 |r| Show InChI InChI=1S/C20H20FN3O3S/c21-15-5-2-6-16(10-15)28(26,27)17-7-8-18-13(9-17)3-1-4-14(18)11-22-20-23-12-19(25)24-20/h2,5-10,12,14,25H,1,3-4,11H2,(H2,22,23,24)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50001524
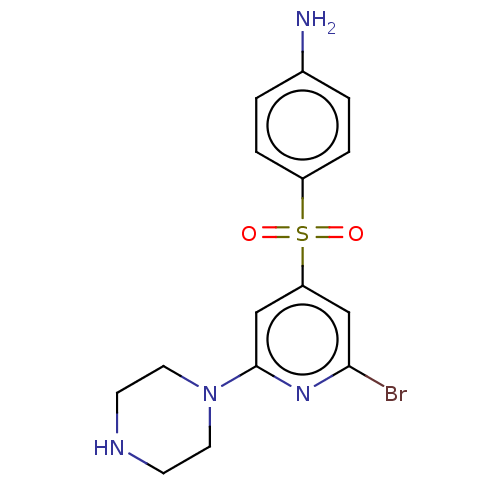
(CHEMBL24474)Show InChI InChI=1S/C15H17BrN4O2S/c16-14-9-13(10-15(19-14)20-7-5-18-6-8-20)23(21,22)12-3-1-11(17)2-4-12/h1-4,9-10,18H,5-8,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche
Curated by ChEMBL
| Assay Description
Binding constant towards serotonin receptor subtype 5-hydroxytryptamine 6 receptor expressed in HeLa cells using [3H]LSD as radioligand |
J Med Chem 46: 1273-6 (2003)
Checked by Author
Article DOI: 10.1021/jm021085c
BindingDB Entry DOI: 10.7270/Q20G3MCZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166358
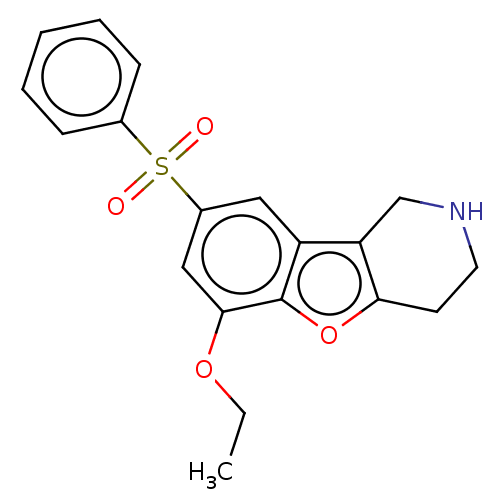
(US9067949, 215)Show InChI InChI=1S/C19H19NO4S/c1-2-23-18-11-14(25(21,22)13-6-4-3-5-7-13)10-15-16-12-20-9-8-17(16)24-19(15)18/h3-7,10-11,20H,2,8-9,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166331

(US9067949, 188)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cc(F)cc(Cl)c1 Show InChI InChI=1S/C18H15ClFNO4S/c1-24-17-8-13(26(22,23)12-5-10(19)4-11(20)6-12)7-14-15-9-21-3-2-16(15)25-18(14)17/h4-8,21H,2-3,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166303
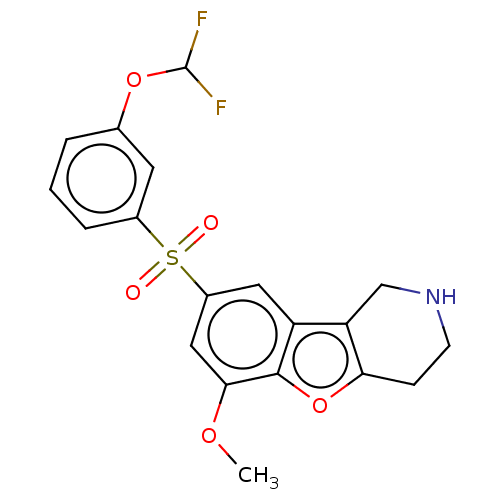
(US9067949, 160)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cccc(OC(F)F)c1 Show InChI InChI=1S/C19H17F2NO5S/c1-25-17-9-13(8-14-15-10-22-6-5-16(15)27-18(14)17)28(23,24)12-4-2-3-11(7-12)26-19(20)21/h2-4,7-9,19,22H,5-6,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318626
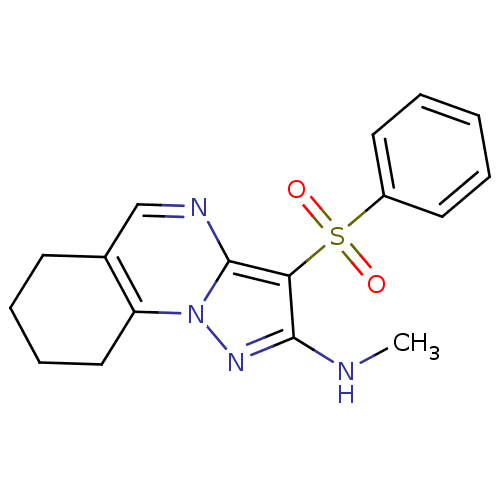
(CHEMBL1082763 | N-Methyl-(3-phenylsulfonyl)-6,7,8,...)Show InChI InChI=1S/C17H18N4O2S/c1-18-16-15(24(22,23)13-8-3-2-4-9-13)17-19-11-12-7-5-6-10-14(12)21(17)20-16/h2-4,8-9,11H,5-7,10H2,1H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]lysergic acid diethylamide from human recombinant 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 5186-96 (2010)
Article DOI: 10.1021/jm100350r
BindingDB Entry DOI: 10.7270/Q26T0NMP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166197
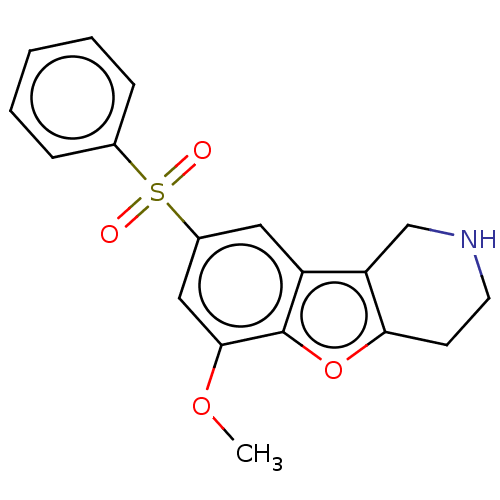
(US9067949, 54)Show InChI InChI=1S/C18H17NO4S/c1-22-17-10-13(24(20,21)12-5-3-2-4-6-12)9-14-15-11-19-8-7-16(15)23-18(14)17/h2-6,9-10,19H,7-8,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416012
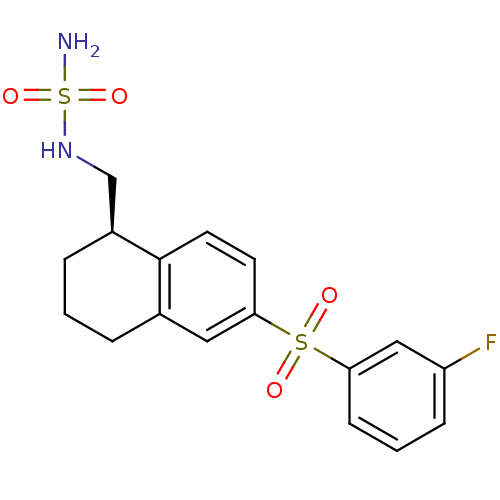
(CHEMBL1084711)Show SMILES NS(=O)(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C17H19FN2O4S2/c18-14-5-2-6-15(10-14)25(21,22)16-7-8-17-12(9-16)3-1-4-13(17)11-20-26(19,23)24/h2,5-10,13,20H,1,3-4,11H2,(H2,19,23,24)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415996

(CHEMBL1085037)Show SMILES OCCNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H22FNO3S/c20-16-5-2-6-17(12-16)25(23,24)18-7-8-19-14(11-18)3-1-4-15(19)13-21-9-10-22/h2,5-8,11-12,15,21-22H,1,3-4,9-10,13H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416006
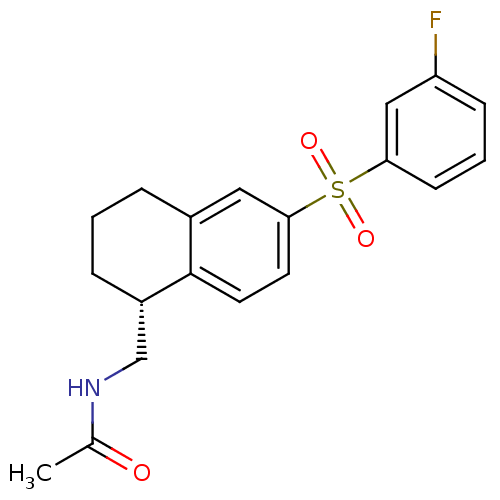
(CHEMBL1083886)Show SMILES CC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H20FNO3S/c1-13(22)21-12-15-5-2-4-14-10-18(8-9-19(14)15)25(23,24)17-7-3-6-16(20)11-17/h3,6-11,15H,2,4-5,12H2,1H3,(H,21,22)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50001827

(CHEMBL363792)Show SMILES CON1CCN(CC1)c1ccc(cc1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C21H22N2O3S/c1-26-23-15-13-22(14-16-23)18-9-11-19(12-10-18)27(24,25)21-8-4-6-17-5-2-3-7-20(17)21/h2-12H,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 6 receptor |
J Med Chem 48: 4216-9 (2005)
Checked by Author
Article DOI: 10.1021/jm050247c
BindingDB Entry DOI: 10.7270/Q2736SD0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50001832
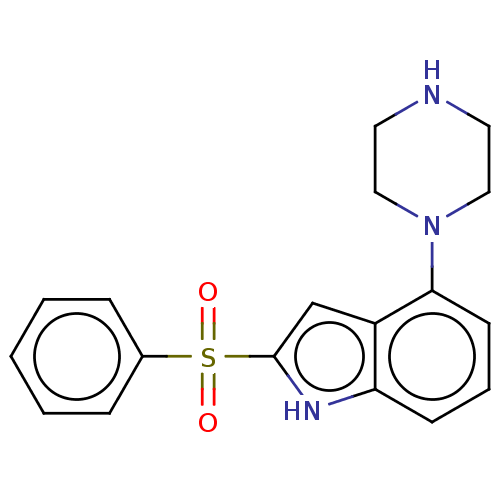
(CHEMBL365569)Show SMILES O=S(=O)(c1cc2c(cccc2[nH]1)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C18H19N3O2S/c22-24(23,14-5-2-1-3-6-14)18-13-15-16(20-18)7-4-8-17(15)21-11-9-19-10-12-21/h1-8,13,19-20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 6 receptor |
J Med Chem 48: 4216-9 (2005)
Checked by Author
Article DOI: 10.1021/jm050247c
BindingDB Entry DOI: 10.7270/Q2736SD0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166310

(US9067949, 167)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H16F3NO4S/c1-26-17-9-13(8-14-15-10-23-6-5-16(15)27-18(14)17)28(24,25)12-4-2-3-11(7-12)19(20,21)22/h2-4,7-9,23H,5-6,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
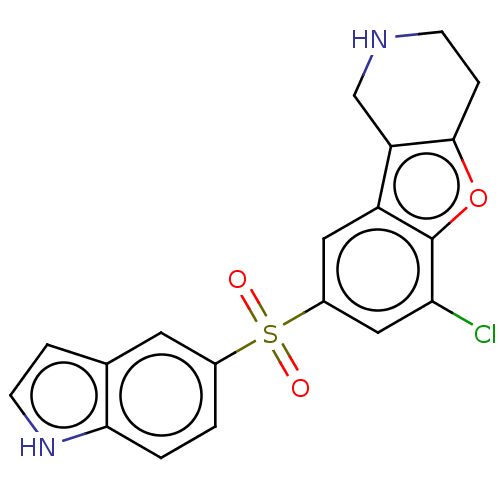
(Homo sapiens (Human)) | BDBM166244
(US9067949, 101 | US9067949, 143)Show SMILES Clc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C19H15ClN2O3S/c20-16-9-13(8-14-15-10-21-5-4-18(15)25-19(14)16)26(23,24)12-1-2-17-11(7-12)3-6-22-17/h1-3,6-9,21-22H,4-5,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166244

(US9067949, 101 | US9067949, 143)Show SMILES Clc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C19H15ClN2O3S/c20-16-9-13(8-14-15-10-21-5-4-18(15)25-19(14)16)26(23,24)12-1-2-17-11(7-12)3-6-22-17/h1-3,6-9,21-22H,4-5,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50358778

(CHEMBL1922616)Show InChI InChI=1S/C14H14ClN5O2S/c1-8-10(15)12(16)20-14(18-8)11(13(17-2)19-20)23(21,22)9-6-4-3-5-7-9/h3-7H,16H2,1-2H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-HT6 receptor expressed in human HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation |
J Med Chem 54: 8161-73 (2011)
Article DOI: 10.1021/jm201079g
BindingDB Entry DOI: 10.7270/Q2X067F0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166225
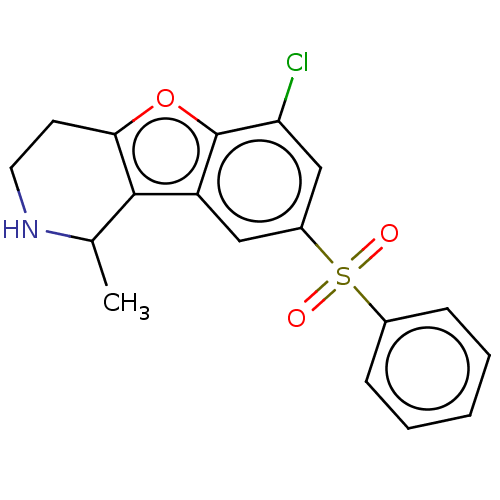
(US9067949, 81a | US9067949, 82a | US9067949, 82b)Show SMILES CC1NCCc2oc3c(Cl)cc(cc3c12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H16ClNO3S/c1-11-17-14-9-13(24(21,22)12-5-3-2-4-6-12)10-15(19)18(14)23-16(17)7-8-20-11/h2-6,9-11,20H,7-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.150 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415987
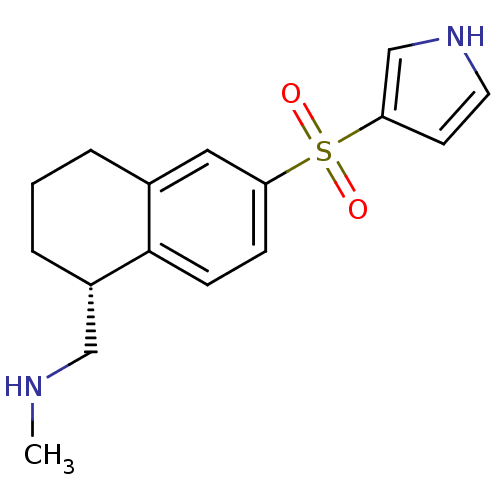
(CHEMBL1085617)Show SMILES CNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cc[nH]c1 |r| Show InChI InChI=1S/C16H20N2O2S/c1-17-10-13-4-2-3-12-9-14(5-6-16(12)13)21(19,20)15-7-8-18-11-15/h5-9,11,13,17-18H,2-4,10H2,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415978
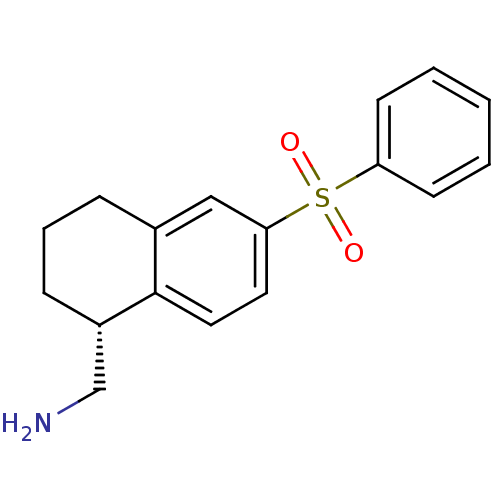
(CHEMBL1086323)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C17H19NO2S/c18-12-14-6-4-5-13-11-16(9-10-17(13)14)21(19,20)15-7-2-1-3-8-15/h1-3,7-11,14H,4-6,12,18H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416000
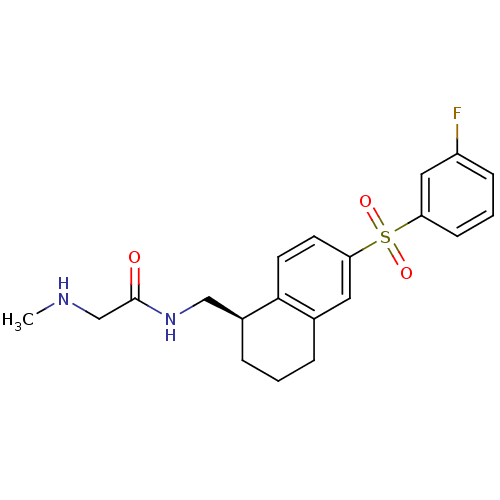
(CHEMBL1085585)Show SMILES CNCC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C20H23FN2O3S/c1-22-13-20(24)23-12-15-5-2-4-14-10-18(8-9-19(14)15)27(25,26)17-7-3-6-16(21)11-17/h3,6-11,15,22H,2,4-5,12-13H2,1H3,(H,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416019
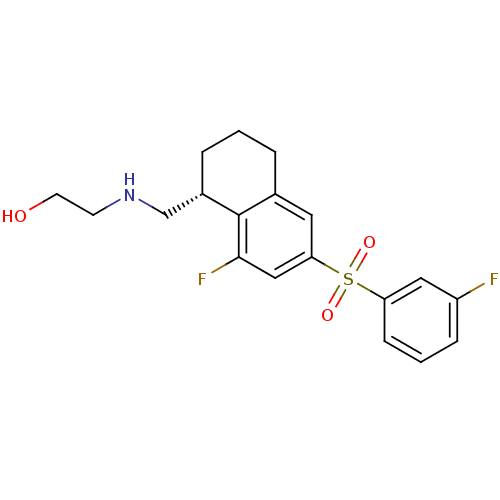
(CHEMBL1084336)Show SMILES OCCNC[C@@H]1CCCc2cc(cc(F)c12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H21F2NO3S/c20-15-5-2-6-16(10-15)26(24,25)17-9-13-3-1-4-14(12-22-7-8-23)19(13)18(21)11-17/h2,5-6,9-11,14,22-23H,1,3-4,7-8,12H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415972

(CHEMBL1086079)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-1-[#6]-[#6]-[#6]-c2cc(ccc-12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H21N3O2S/c19-18(20)21-12-14-6-4-5-13-11-16(9-10-17(13)14)24(22,23)15-7-2-1-3-8-15/h1-3,7-11,14H,4-6,12H2,(H4,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477488
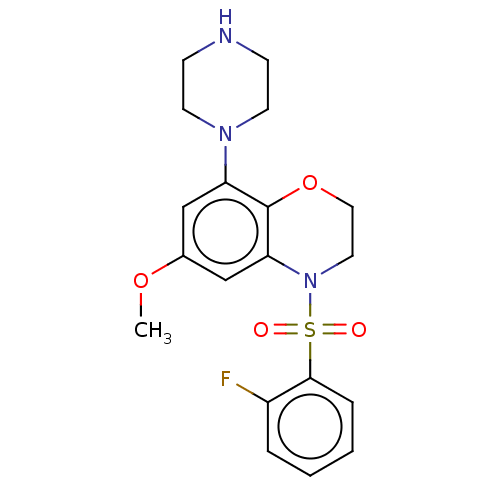
(CHEMBL394690)Show SMILES COc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H22FN3O4S/c1-26-14-12-16(22-8-6-21-7-9-22)19-17(13-14)23(10-11-27-19)28(24,25)18-5-3-2-4-15(18)20/h2-5,12-13,21H,6-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50492797
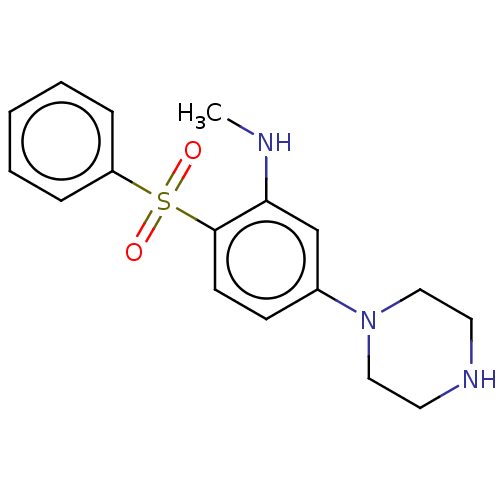
(CHEMBL2413990)Show InChI InChI=1S/C17H21N3O2S/c1-18-16-13-14(20-11-9-19-10-12-20)7-8-17(16)23(21,22)15-5-3-2-4-6-15/h2-8,13,18-19H,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute (CDRI)
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human 5-HT6 receptor expressed in HEK293 cells assessed as 5-HT-induced intracellular cAMP production after 30 min... |
Bioorg Med Chem 21: 4614-27 (2013)
Article DOI: 10.1016/j.bmc.2013.05.040
BindingDB Entry DOI: 10.7270/Q2DJ5JK0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166327
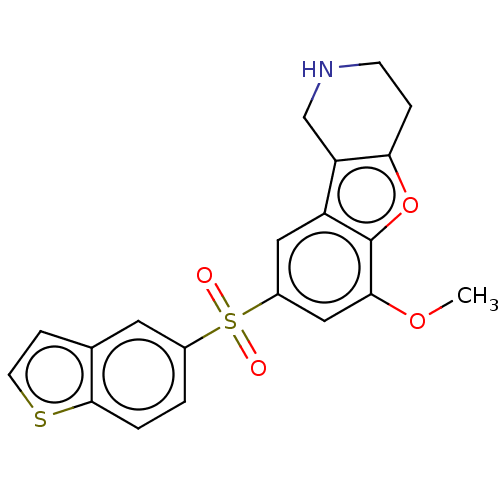
(US9067949, 184)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1ccc2sccc2c1 Show InChI InChI=1S/C20H17NO4S2/c1-24-18-10-14(9-15-16-11-21-6-4-17(16)25-20(15)18)27(22,23)13-2-3-19-12(8-13)5-7-26-19/h2-3,5,7-10,21H,4,6,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166326
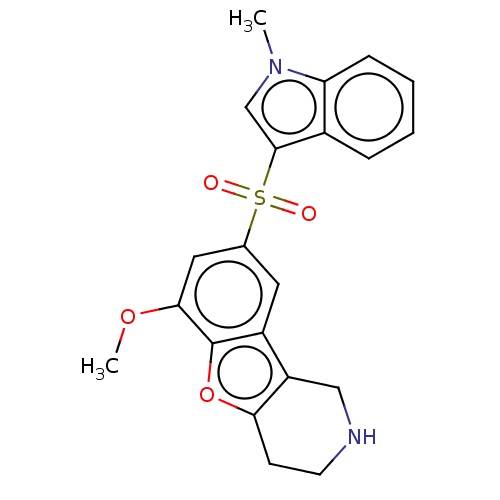
(US9067949, 183)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cn(C)c2ccccc12 Show InChI InChI=1S/C21H20N2O4S/c1-23-12-20(14-5-3-4-6-17(14)23)28(24,25)13-9-15-16-11-22-8-7-18(16)27-21(15)19(10-13)26-2/h3-6,9-10,12,22H,7-8,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166335
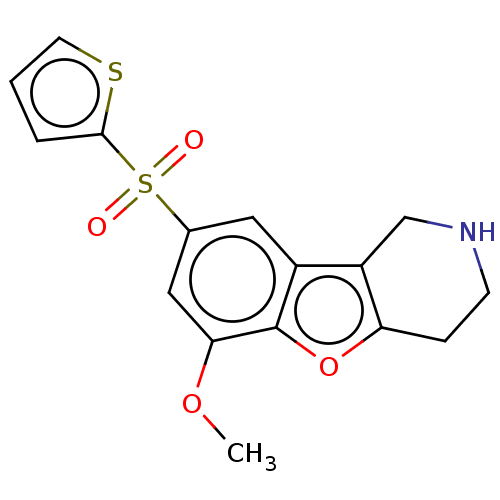
(US9067949, 192)Show InChI InChI=1S/C16H15NO4S2/c1-20-14-8-10(23(18,19)15-3-2-6-22-15)7-11-12-9-17-5-4-13(12)21-16(11)14/h2-3,6-8,17H,4-5,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166193
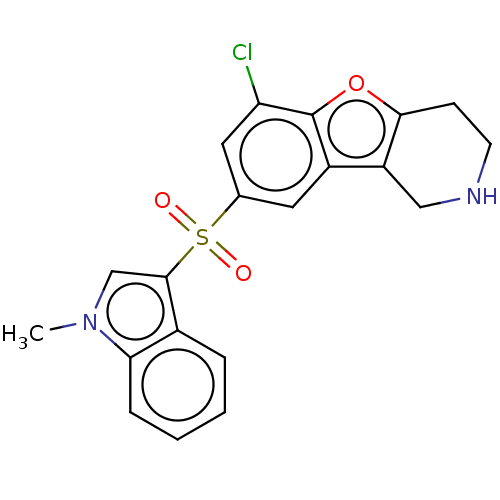
(US9067949, 50)Show SMILES Cn1cc(c2ccccc12)S(=O)(=O)c1cc(Cl)c2oc3CCNCc3c2c1 Show InChI InChI=1S/C20H17ClN2O3S/c1-23-11-19(13-4-2-3-5-17(13)23)27(24,25)12-8-14-15-10-22-7-6-18(15)26-20(14)16(21)9-12/h2-5,8-9,11,22H,6-7,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT6 receptor expressed in human HeLa cells after 1 hr by scintillation spectroscopic analysis |
J Med Chem 57: 5823-8 (2014)
Article DOI: 10.1021/jm5003759
BindingDB Entry DOI: 10.7270/Q2D79D04 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592784
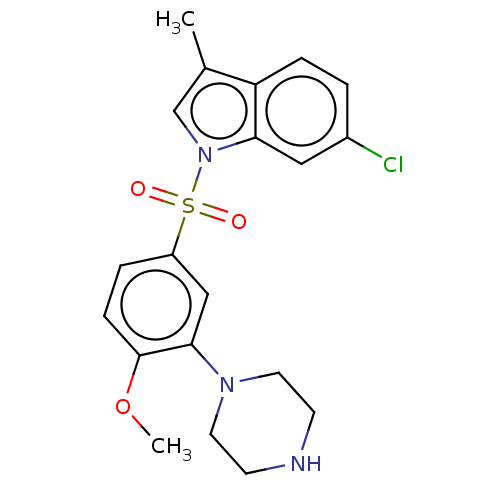
(CHEMBL5177311)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Cl)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50417934

(CHEMBL1668590 | US8829009, 1.1(7))Show InChI InChI=1S/C15H15ClN4O2S/c1-9-7-10(2)20-15(18-9)13(14(17-3)19-20)23(21,22)12-6-4-5-11(16)8-12/h4-8H,1-3H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation after ... |
Bioorg Med Chem 19: 1482-91 (2011)
Article DOI: 10.1016/j.bmc.2010.12.055
BindingDB Entry DOI: 10.7270/Q21G0NJS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166311
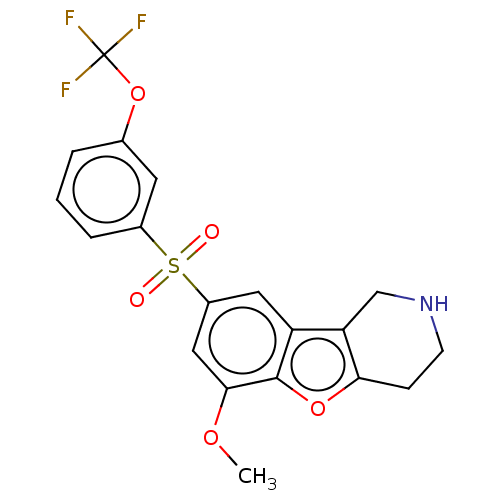
(US9067949, 168)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C19H16F3NO5S/c1-26-17-9-13(8-14-15-10-23-6-5-16(15)27-18(14)17)29(24,25)12-4-2-3-11(7-12)28-19(20,21)22/h2-4,7-9,23H,5-6,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166306

(US9067949, 163)Show InChI InChI=1S/C18H16FNO4S/c1-23-16-9-11(25(21,22)17-5-3-2-4-14(17)19)8-12-13-10-20-7-6-15(13)24-18(12)16/h2-5,8-9,20H,6-7,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50291284
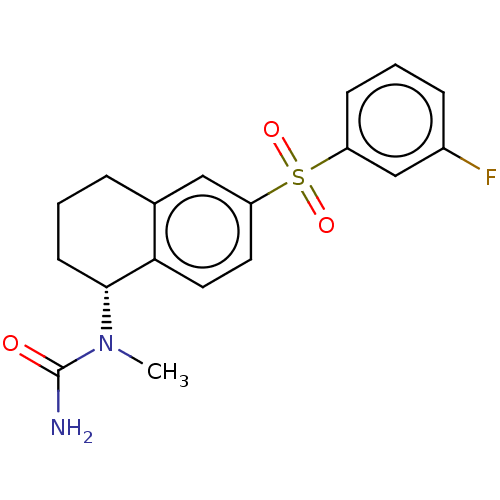
(CHEMBL4170220)Show SMILES CN([C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1)C(N)=O |r| Show InChI InChI=1S/C18H19FN2O3S/c1-21(18(20)22)17-7-2-4-12-10-15(8-9-16(12)17)25(23,24)14-6-3-5-13(19)11-14/h3,5-6,8-11,17H,2,4,7H2,1H3,(H2,20,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM166309
(US9067949, 166)Show SMILES COc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C18H16FNO4S/c1-23-17-9-13(25(21,22)12-4-2-3-11(19)7-12)8-14-15-10-20-6-5-16(15)24-18(14)17/h2-4,7-9,20H,5-6,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc.
US Patent
| Assay Description
For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... |
US Patent US9067949 (2015)
BindingDB Entry DOI: 10.7270/Q23777FB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data