 Found 2489 hits of ki data for polymerid = 2154
Found 2489 hits of ki data for polymerid = 2154 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359960
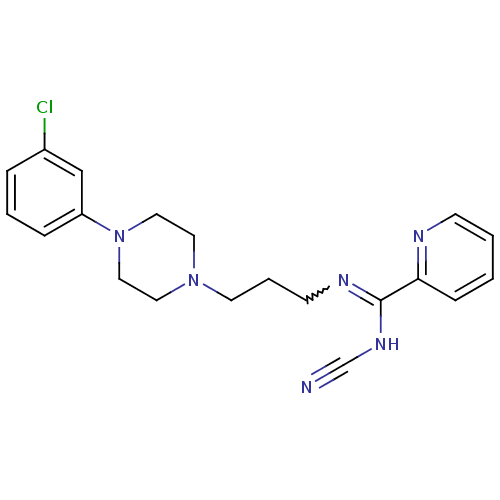
(CHEMBL1927094)Show SMILES Clc1cccc(c1)N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:14.14| Show InChI InChI=1S/C20H23ClN6/c21-17-5-3-6-18(15-17)27-13-11-26(12-14-27)10-4-9-24-20(25-16-22)19-7-1-2-8-23-19/h1-3,5-8,15H,4,9-14H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359961
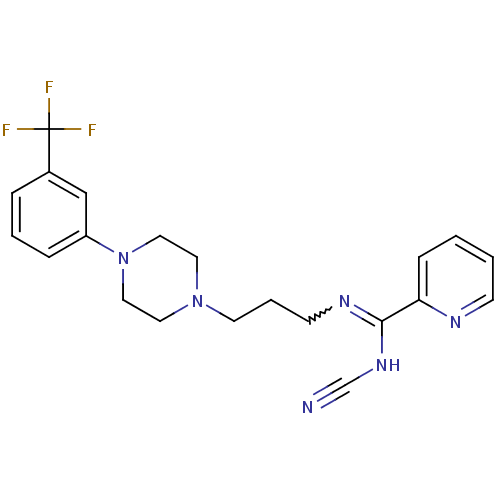
(CHEMBL1927095)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:17.17| Show InChI InChI=1S/C21H23F3N6/c22-21(23,24)17-5-3-6-18(15-17)30-13-11-29(12-14-30)10-4-9-27-20(28-16-25)19-7-1-2-8-26-19/h1-3,5-8,15H,4,9-14H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359954
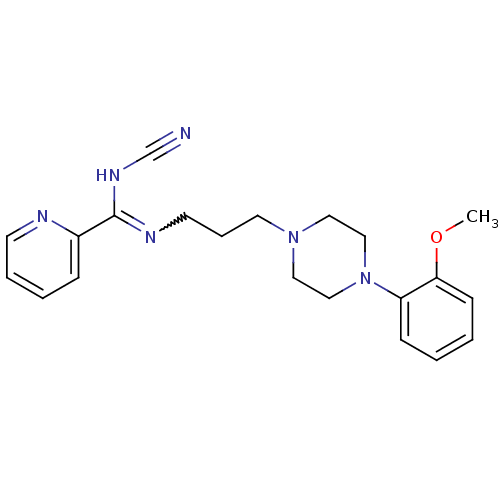
(CHEMBL1927088)Show SMILES COc1ccccc1N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:15.15| Show InChI InChI=1S/C21H26N6O/c1-28-20-9-3-2-8-19(20)27-15-13-26(14-16-27)12-6-11-24-21(25-17-22)18-7-4-5-10-23-18/h2-5,7-10H,6,11-16H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50145591
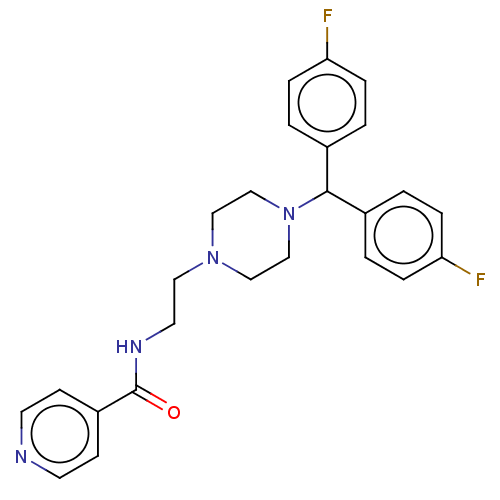
(CHEMBL3765471)Show SMILES Cl.Fc1ccc(cc1)C(N1CCN(CCNC(=O)c2ccncc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C25H26F2N4O.ClH/c26-22-5-1-19(2-6-22)24(20-3-7-23(27)8-4-20)31-17-15-30(16-18-31)14-13-29-25(32)21-9-11-28-12-10-21;/h1-12,24H,13-18H2,(H,29,32);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00956 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis |
Eur J Med Chem 110: 133-50 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.021
BindingDB Entry DOI: 10.7270/Q24X59N9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50145612
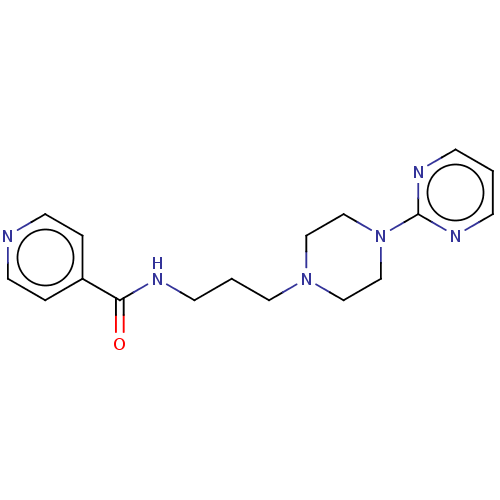
(CHEMBL3764177)Show InChI InChI=1S/C17H22N6O/c24-16(15-3-8-18-9-4-15)19-7-2-10-22-11-13-23(14-12-22)17-20-5-1-6-21-17/h1,3-6,8-9H,2,7,10-14H2,(H,19,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00985 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis |
Eur J Med Chem 110: 133-50 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.021
BindingDB Entry DOI: 10.7270/Q24X59N9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM84928
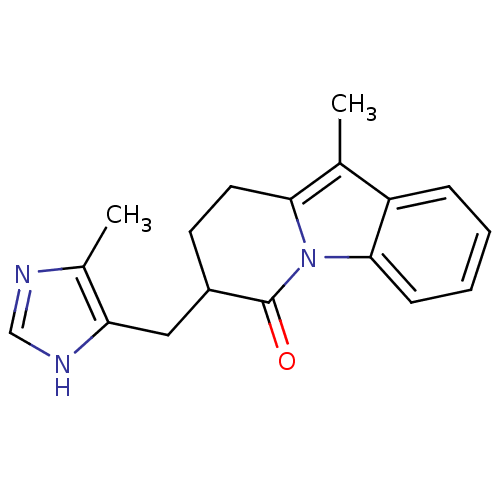
(CAS_125368 | FK 1052 | NSC_125368)Show InChI InChI=1S/C18H19N3O/c1-11-14-5-3-4-6-17(14)21-16(11)8-7-13(18(21)22)9-15-12(2)19-10-20-15/h3-6,10,13H,7-9H2,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 752-8 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4G73 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50145627
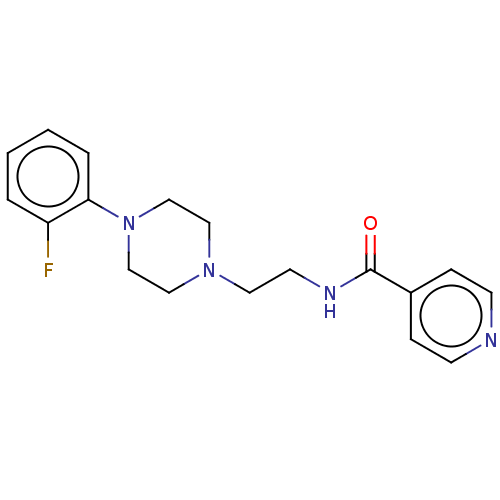
(CHEMBL3763812)Show InChI InChI=1S/C18H21FN4O/c19-16-3-1-2-4-17(16)23-13-11-22(12-14-23)10-9-21-18(24)15-5-7-20-8-6-15/h1-8H,9-14H2,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis |
Eur J Med Chem 110: 133-50 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.021
BindingDB Entry DOI: 10.7270/Q24X59N9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Clin Psychiatry 5-12 (1994)
BindingDB Entry DOI: 10.7270/Q2CR5RW8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359962
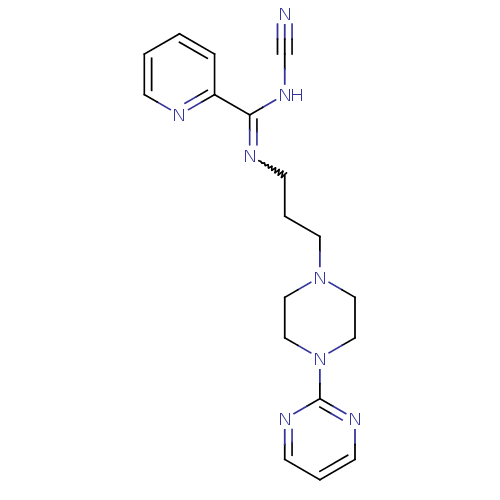
(CHEMBL1927096)Show SMILES N#CNC(=NCCCN1CCN(CC1)c1ncccn1)c1ccccn1 |w:4.4| Show InChI InChI=1S/C18H22N8/c19-15-24-17(16-5-1-2-6-20-16)21-9-4-10-25-11-13-26(14-12-25)18-22-7-3-8-23-18/h1-3,5-8H,4,9-14H2,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50451055
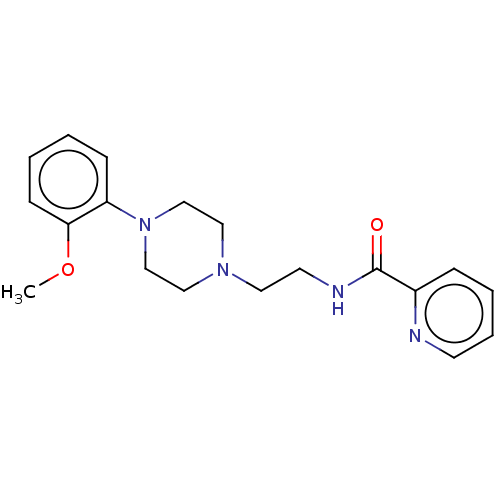
(CHEMBL4205290)Show InChI InChI=1S/C19H24N4O2/c1-25-18-8-3-2-7-17(18)23-14-12-22(13-15-23)11-10-21-19(24)16-6-4-5-9-20-16/h2-9H,10-15H2,1H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli "Federico II" Via D. Montesano
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from serotonin 5-HT2A receptor in Sprague-Dawley rat brain cortex homogenates incubated for 15 mins by liquid scintill... |
Bioorg Med Chem 25: 5820-5837 (2017)
Article DOI: 10.1016/j.bmc.2017.09.018
BindingDB Entry DOI: 10.7270/Q2VD721F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50145609
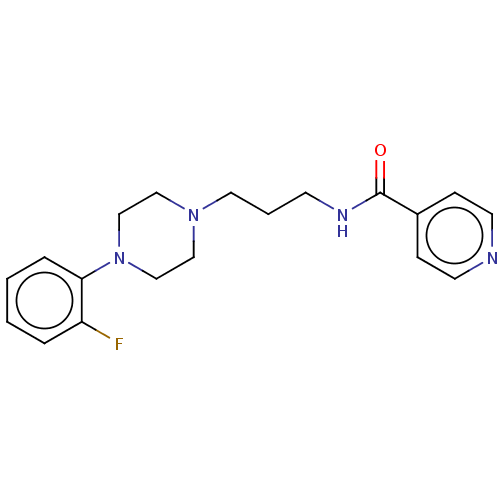
(CHEMBL3763474)Show InChI InChI=1S/C19H23FN4O/c20-17-4-1-2-5-18(17)24-14-12-23(13-15-24)11-3-8-22-19(25)16-6-9-21-10-7-16/h1-2,4-7,9-10H,3,8,11-15H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis |
Eur J Med Chem 110: 133-50 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.021
BindingDB Entry DOI: 10.7270/Q24X59N9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50165203
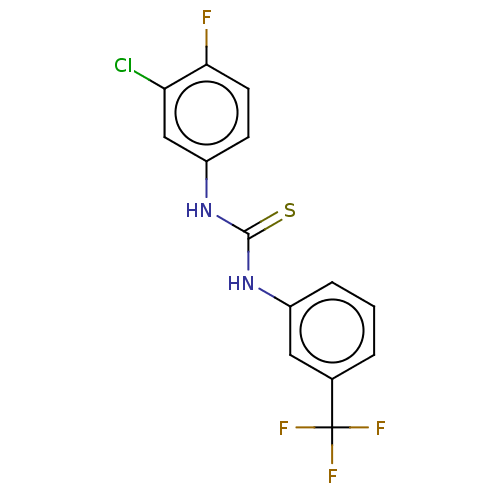
(CHEMBL3617113)Show InChI InChI=1S/C14H9ClF4N2S/c15-11-7-10(4-5-12(11)16)21-13(22)20-9-3-1-2-8(6-9)14(17,18)19/h1-7H,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins |
Eur J Med Chem 116: 173-186 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.073
BindingDB Entry DOI: 10.7270/Q22B90X6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50145628

(CHEMBL3765231)Show InChI InChI=1S/C18H21FN4O/c19-16-1-3-17(4-2-16)23-13-11-22(12-14-23)10-9-21-18(24)15-5-7-20-8-6-15/h1-8H,9-14H2,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis |
Eur J Med Chem 110: 133-50 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.021
BindingDB Entry DOI: 10.7270/Q24X59N9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50010044
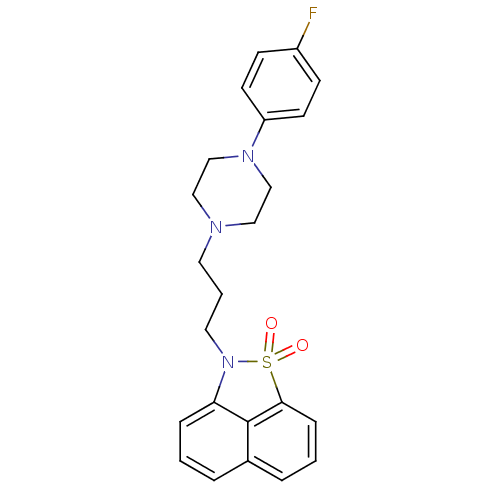
(2-{3-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-propyl}-...)Show SMILES Fc1ccc(cc1)N1CCN(CCCN2c3cccc4cccc(c34)S2(=O)=O)CC1 Show InChI InChI=1S/C23H24FN3O2S/c24-19-8-10-20(11-9-19)26-16-14-25(15-17-26)12-3-13-27-21-6-1-4-18-5-2-7-22(23(18)21)30(27,28)29/h1-2,4-11H,3,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cracow University of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in rat cortical membranes incubated for 15 mins by liquid scintillation spectrometry |
Bioorg Med Chem 27: 3396-3407 (2019)
Article DOI: 10.1016/j.bmc.2019.06.028
BindingDB Entry DOI: 10.7270/Q20K2D06 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007692
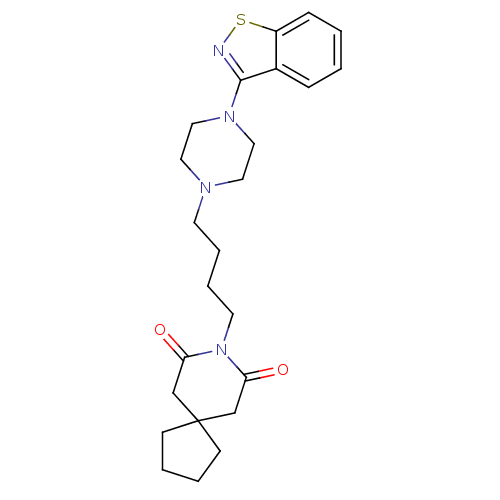
(8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C24H32N4O2S/c29-21-17-24(9-3-4-10-24)18-22(30)28(21)12-6-5-11-26-13-15-27(16-14-26)23-19-7-1-2-8-20(19)31-25-23/h1-2,7-8H,3-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 120: 365-8 (1995)
Article DOI: 10.1007/bf02311185
BindingDB Entry DOI: 10.7270/Q2VT1QKB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007692

(8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C24H32N4O2S/c29-21-17-24(9-3-4-10-24)18-22(30)28(21)12-6-5-11-26-13-15-27(16-14-26)23-19-7-1-2-8-20(19)31-25-23/h1-2,7-8H,3-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 1403-10 (1994)
BindingDB Entry DOI: 10.7270/Q2154FJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007692

(8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C24H32N4O2S/c29-21-17-24(9-3-4-10-24)18-22(30)28(21)12-6-5-11-26-13-15-27(16-14-26)23-19-7-1-2-8-20(19)31-25-23/h1-2,7-8H,3-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 1361-5 (1992)
BindingDB Entry DOI: 10.7270/Q2513WPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133231
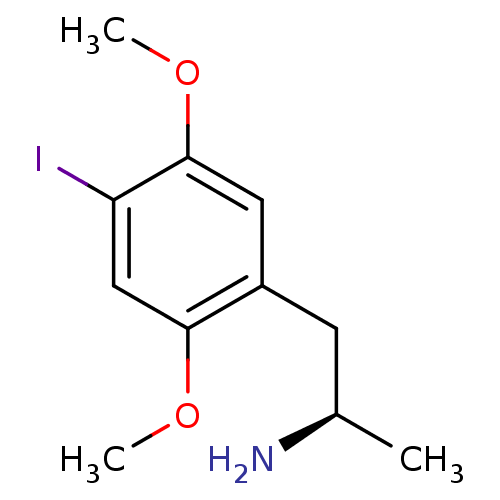
((R)-1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine |...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against [125I]DOI binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex |
J Med Chem 46: 4188-95 (2003)
Article DOI: 10.1021/jm030205t
BindingDB Entry DOI: 10.7270/Q2Z320C1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50095027
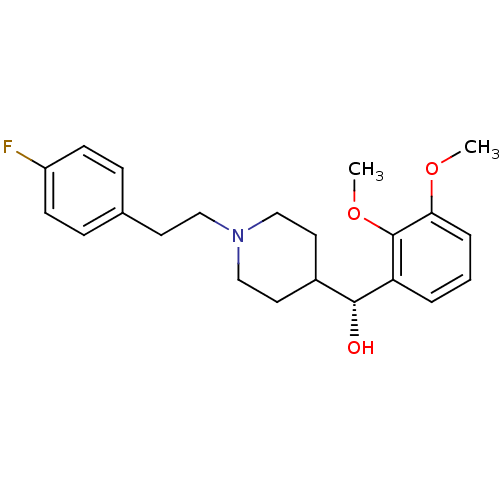
((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50005257
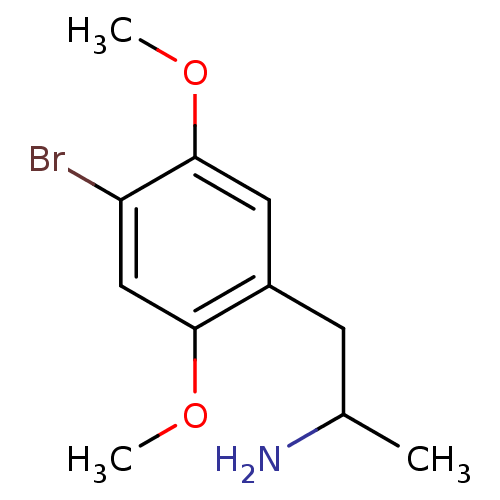
((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for 5-HT2A serotonin receptor |
J Med Chem 47: 6034-41 (2004)
Article DOI: 10.1021/jm040082s
BindingDB Entry DOI: 10.7270/Q2R78DQ8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM423299
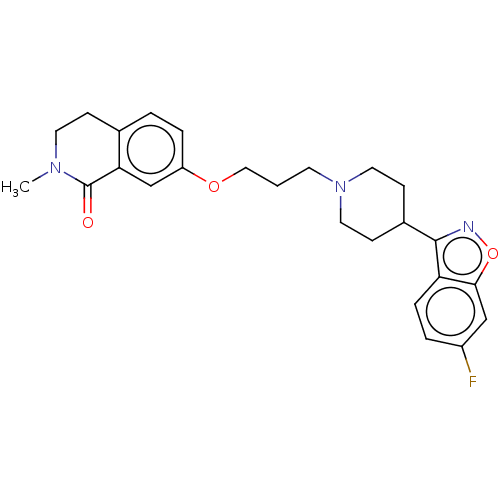
(US10501452, Compound 5)Show SMILES CN1CCc2ccc(OCCCN3CCC(CC3)c3noc4cc(F)ccc34)cc2C1=O Show InChI InChI=1S/C25H28FN3O3/c1-28-11-7-17-3-5-20(16-22(17)25(28)30)31-14-2-10-29-12-8-18(9-13-29)24-21-6-4-19(26)15-23(21)32-27-24/h3-6,15-16,18H,2,7-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION
US Patent
| Assay Description
5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... |
US Patent US10501452 (2019)
BindingDB Entry DOI: 10.7270/Q2BK1FR6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50236715
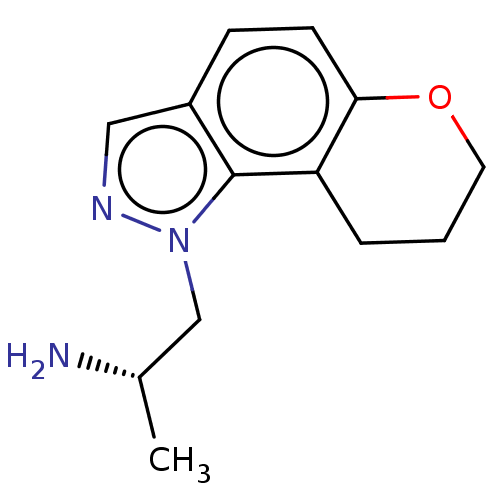
(CHEMBL4067210)Show InChI InChI=1S/C13H17N3O/c1-9(14)8-16-13-10(7-15-16)4-5-12-11(13)3-2-6-17-12/h4-5,7,9H,2-3,6,8,14H2,1H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 |
J Med Chem 60: 2605-2628 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00085
BindingDB Entry DOI: 10.7270/Q2DJ5HWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM84994
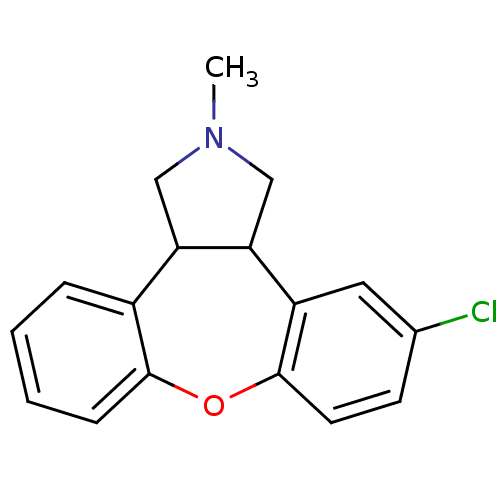
(CAS_163091 | NSC_163091 | ORG-5222)Show InChI InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17)15(14)10-19/h2-8,14-15H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50113332
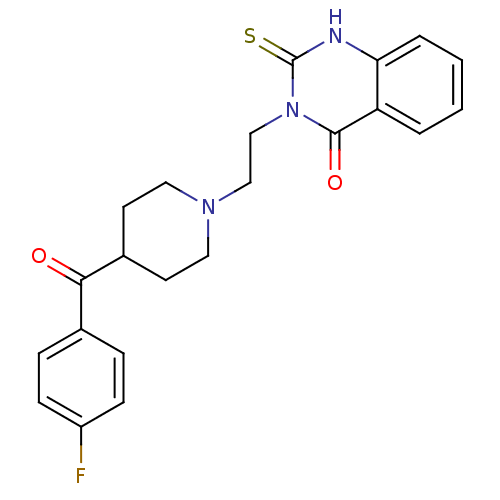
(3-(2-(4-(4-fluorobenzoyl)piperidin-1-yl)ethyl)-2-t...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=S)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O2S/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
| Assay Description
Displacement of [3H]altanserine from rat cortical membrane 5HT2A receptor |
Bioorg Med Chem 17: 2989-3002 (2009)
Article DOI: 10.1016/j.bmc.2009.03.021
BindingDB Entry DOI: 10.7270/Q21V5DW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50113332

(3-(2-(4-(4-fluorobenzoyl)piperidin-1-yl)ethyl)-2-t...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=S)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O2S/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007568
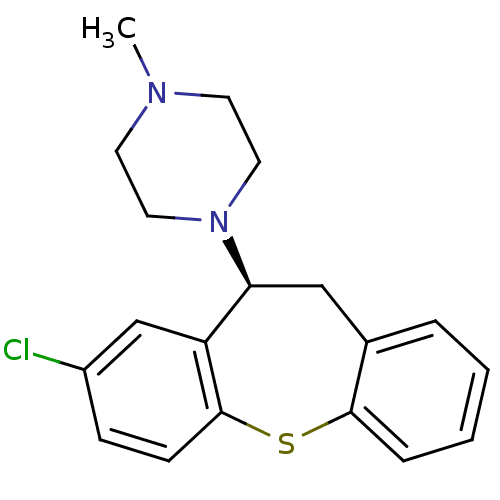
(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 2A receptor in rat tissue homogenate using [3H]ketanserin as radioligand |
J Med Chem 47: 143-57 (2003)
Article DOI: 10.1021/jm0309811
BindingDB Entry DOI: 10.7270/Q2VM4CRH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007568

(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex homogenate |
J Med Chem 45: 344-59 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3G26 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry |
J Med Chem 52: 151-69 (2009)
Article DOI: 10.1021/jm800689g
BindingDB Entry DOI: 10.7270/Q2J67GSD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. |
J Med Chem 38: 708-14 (1995)
BindingDB Entry DOI: 10.7270/Q2PG1SCG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 18: 63-101 (1998)
Article DOI: 10.1016/S0893-133X(97)00112-7
BindingDB Entry DOI: 10.7270/Q2SF2TQB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in rat brain |
Bioorg Med Chem 15: 7361-7 (2007)
Article DOI: 10.1016/j.bmc.2007.07.018
BindingDB Entry DOI: 10.7270/Q251420V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from 5-HT2A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting ana... |
Eur J Med Chem 124: 713-728 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.008
BindingDB Entry DOI: 10.7270/Q2SQ92C5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserine from serotonin 5-HT2A receptor in rat brain cortex homogenates incubated for 30 mins by liquid scintillation countin... |
J Med Chem 61: 10017-10039 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01096
BindingDB Entry DOI: 10.7270/Q2NS0XK7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM30704
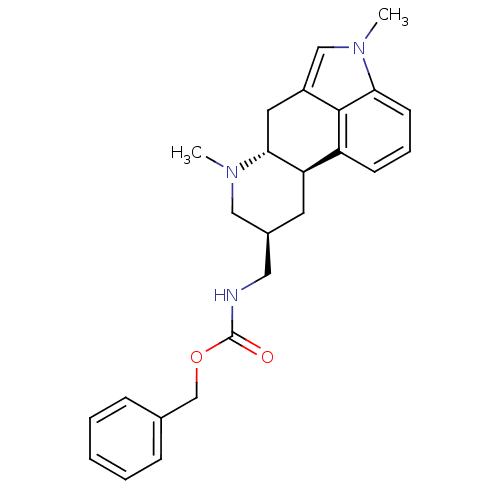
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690
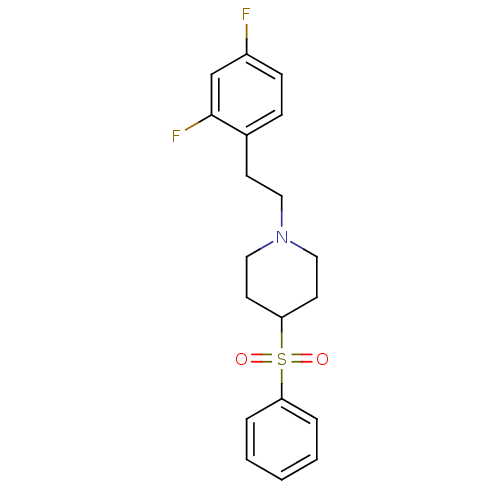
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690

(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]ketanserin binding from 5-hydroxytryptamine 2A receptor in rat striatal membrane. |
J Med Chem 42: 2774-97 (1999)
Article DOI: 10.1021/jm981094e
BindingDB Entry DOI: 10.7270/Q2BG2RR8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133233
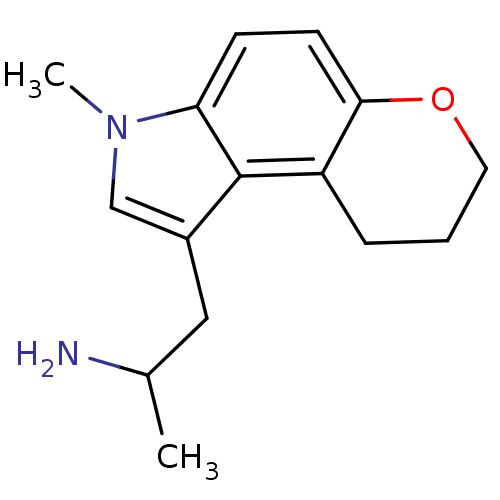
(1-Methyl-2-(3-methyl-3,7,8,9-tetrahydro-pyrano[3,2...)Show InChI InChI=1S/C15H20N2O/c1-10(16)8-11-9-17(2)13-5-6-14-12(15(11)13)4-3-7-18-14/h5-6,9-10H,3-4,7-8,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against [125I]DOI binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex |
J Med Chem 46: 4188-95 (2003)
Article DOI: 10.1021/jm030205t
BindingDB Entry DOI: 10.7270/Q2Z320C1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133232
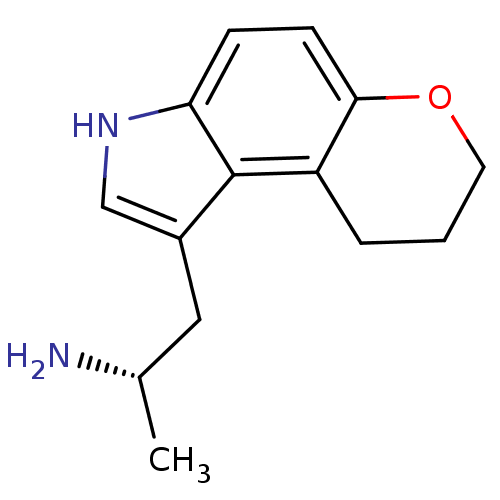
((S)-1-Methyl-2-(3,7,8,9-tetrahydro-pyrano[3,2-e]in...)Show InChI InChI=1S/C14H18N2O/c1-9(15)7-10-8-16-12-4-5-13-11(14(10)12)3-2-6-17-13/h4-5,8-9,16H,2-3,6-7,15H2,1H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory constant against [125I]DOI binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex |
J Med Chem 46: 4188-95 (2003)
Article DOI: 10.1021/jm030205t
BindingDB Entry DOI: 10.7270/Q2Z320C1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin (0.5 nM) from rat cerebral cortex 5-hydroxytryptamine 2A receptors |
J Med Chem 46: 265-83 (2003)
Article DOI: 10.1021/jm020938y
BindingDB Entry DOI: 10.7270/Q2TB17MV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 18: 63-101 (1998)
Article DOI: 10.1016/S0893-133X(97)00112-7
BindingDB Entry DOI: 10.7270/Q2SF2TQB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50590663
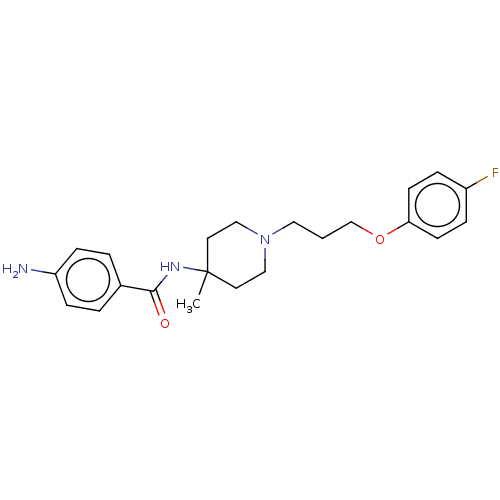
(CHEMBL5175726)Show SMILES CC1(CCN(CCCOc2ccc(F)cc2)CC1)NC(=O)c1ccc(N)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50064708
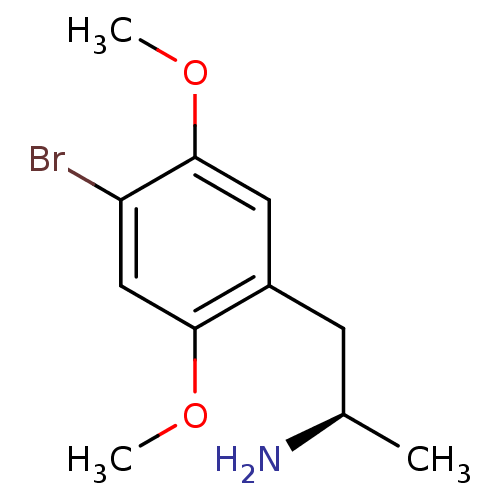
((R)-1-(4-bromo-2,5-dimethoxyphenyl)propan-2-amine ...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for 5-HT2A serotonin receptor |
J Med Chem 47: 6034-41 (2004)
Article DOI: 10.1021/jm040082s
BindingDB Entry DOI: 10.7270/Q2R78DQ8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 1361-5 (1992)
BindingDB Entry DOI: 10.7270/Q2513WPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50064708

((R)-1-(4-bromo-2,5-dimethoxyphenyl)propan-2-amine ...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
The compound was evaluated for binding affinity against Prothrombin |
J Med Chem 60: 2605-2628 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00085
BindingDB Entry DOI: 10.7270/Q2DJ5HWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50252513
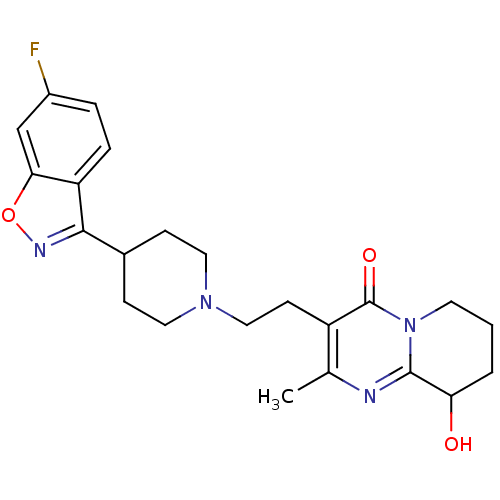
(3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1...)Show SMILES Cc1nc2C(O)CCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Clin Psychiatry 5-12 (1994)
BindingDB Entry DOI: 10.7270/Q2CR5RW8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50145610
(CHEMBL3764420)Show InChI InChI=1S/C19H23FN4O/c20-17-2-4-18(5-3-17)24-14-12-23(13-15-24)11-1-8-22-19(25)16-6-9-21-10-7-16/h2-7,9-10H,1,8,11-15H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis |
Eur J Med Chem 110: 133-50 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.021
BindingDB Entry DOI: 10.7270/Q24X59N9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data