 Found 187 hits of ki data for polymerid = 2161,50000035
Found 187 hits of ki data for polymerid = 2161,50000035 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | CHEMBL5272537
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
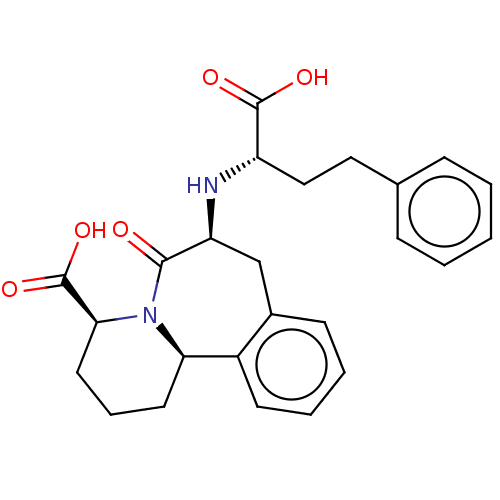
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044868
(7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...)Show SMILES OC(=O)[C@H](CCc1ccccc1)N[C@@H]1Cc2ccccc2C2CCC[C@@H](N2C1=O)C(O)=O Show InChI InChI=1S/C25H28N2O5/c28-23-20(26-19(24(29)30)14-13-16-7-2-1-3-8-16)15-17-9-4-5-10-18(17)21-11-6-12-22(25(31)32)27(21)23/h1-5,7-10,19-22,26H,6,11-15H2,(H,29,30)(H,31,32)/t19-,20+,21?,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120
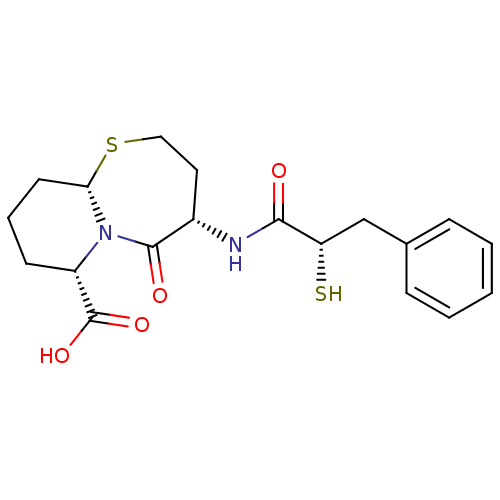
((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human fully glycosylated ACE N-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... |
J Med Chem 61: 10141-10154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01309
BindingDB Entry DOI: 10.7270/Q2862K4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human fully glycosylated ACE C-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... |
J Med Chem 61: 10141-10154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01309
BindingDB Entry DOI: 10.7270/Q2862K4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044866
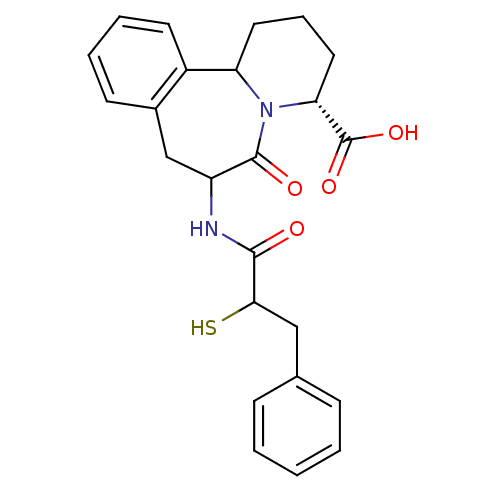
(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044866

(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50369460
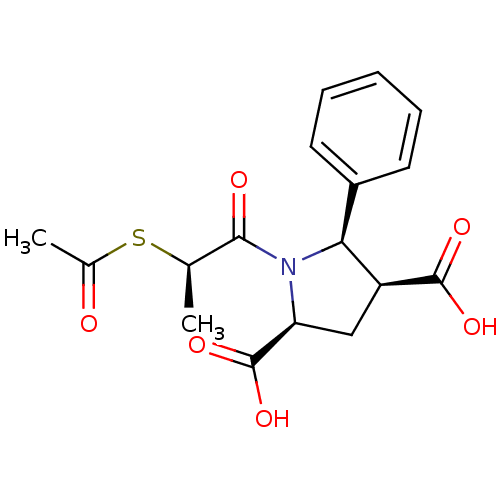
(CHEMBL1788109)Show SMILES C[C@@H](SC(C)=O)C(=O)N1[C@@H](C[C@@H]([C@@H]1c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H19NO6S/c1-9(25-10(2)19)15(20)18-13(17(23)24)8-12(16(21)22)14(18)11-6-4-3-5-7-11/h3-7,9,12-14H,8H2,1-2H3,(H,21,22)(H,23,24)/t9-,12+,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Enzyme inhibitory activity towards Angiotensin I converting enzyme |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50018850
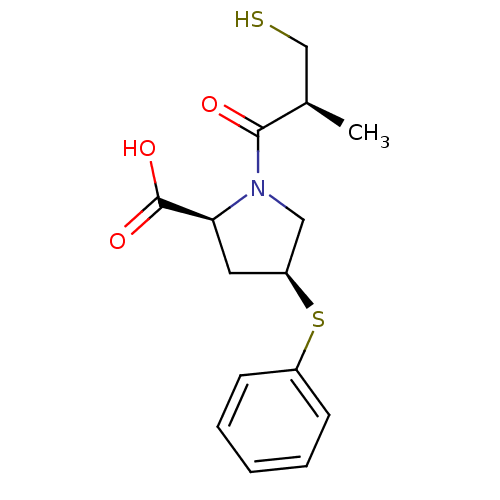
(1-(3-Mercapto-2-methyl-propionyl)-4-phenylsulfanyl...)Show SMILES C[C@H](CS)C(=O)N1C[C@H](C[C@H]1C(O)=O)Sc1ccccc1 Show InChI InChI=1S/C15H19NO3S2/c1-10(9-20)14(17)16-8-12(7-13(16)15(18)19)21-11-5-3-2-4-6-11/h2-6,10,12-13,20H,7-9H2,1H3,(H,18,19)/t10-,12+,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit lung Angiotensin I converting enzyme |
J Med Chem 31: 1148-60 (1988)
BindingDB Entry DOI: 10.7270/Q2V125DX |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303321
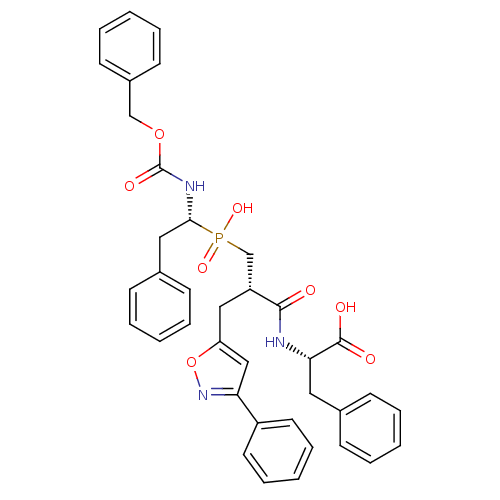
((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O8P/c42-36(39-34(37(43)44)21-27-13-5-1-6-14-27)31(23-32-24-33(41-49-32)30-19-11-4-12-20-30)26-50(46,47)35(22-28-15-7-2-8-16-28)40-38(45)48-25-29-17-9-3-10-18-29/h1-20,24,31,34-35H,21-23,25-26H2,(H,39,42)(H,40,45)(H,43,44)(H,46,47)/t31-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303319
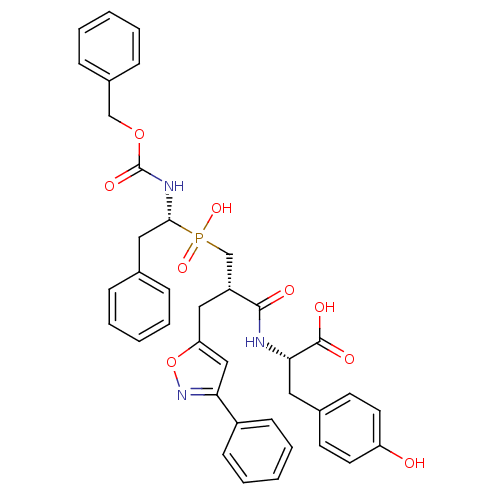
((S)-2-[(S)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367249

(CHEMBL309601)Show SMILES C[C@H](NP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H23N2O5P/c1-12(15(19)18-10-5-8-14(18)16(20)21)17-24(22,23)11-9-13-6-3-2-4-7-13/h2-4,6-7,12,14H,5,8-11H2,1H3,(H,20,21)(H2,17,22,23)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021119
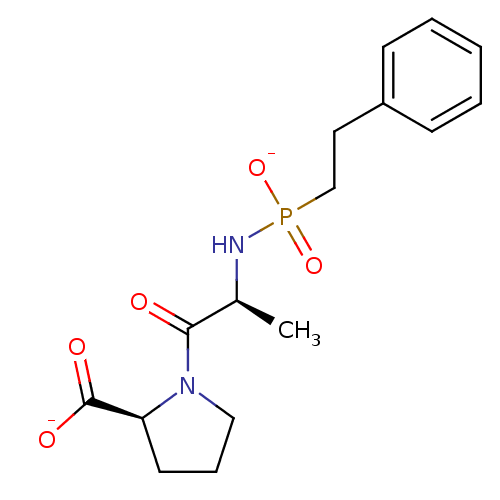
(CHEMBL34389 | disodium (2S)-1-((2S)-2-{[(2-phenyle...)Show SMILES C[C@H](NP([O-])(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C16H23N2O5P/c1-12(15(19)18-10-5-8-14(18)16(20)21)17-24(22,23)11-9-13-6-3-2-4-7-13/h2-4,6-7,12,14H,5,8-11H2,1H3,(H,20,21)(H2,17,22,23)/p-2/t12-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of buffer |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021118
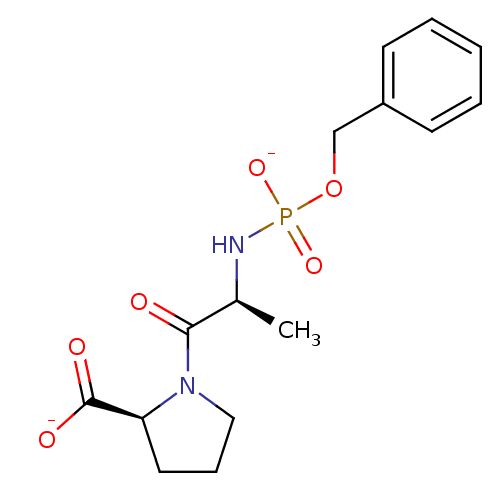
(CHEMBL30836 | dipotassium (2S)-1-((2S)-2-{[(benzyl...)Show SMILES C[C@H](NP([O-])(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C15H21N2O6P/c1-11(14(18)17-9-5-8-13(17)15(19)20)16-24(21,22)23-10-12-6-3-2-4-7-12/h2-4,6-7,11,13H,5,8-10H2,1H3,(H,19,20)(H2,16,21,22)/p-2/t11-,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
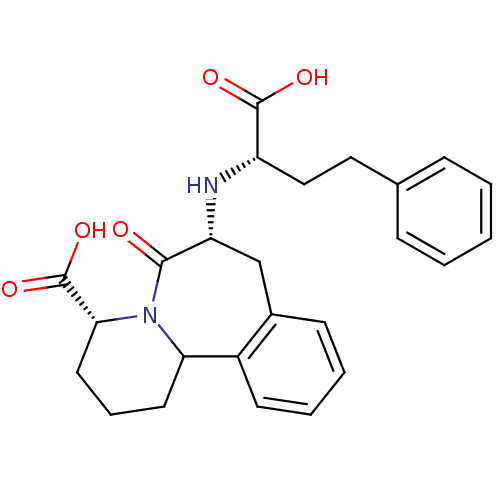
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50011270

(2-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...)Show SMILES OC(=O)C(CCc1ccccc1)NC1Cc2ccccc2[C@H]2CCC[C@H](N2C1=O)C(O)=O Show InChI InChI=1S/C25H28N2O5/c28-23-20(26-19(24(29)30)14-13-16-7-2-1-3-8-16)15-17-9-4-5-10-18(17)21-11-6-12-22(25(31)32)27(21)23/h1-5,7-10,19-22,26H,6,11-15H2,(H,29,30)(H,31,32)/t19?,20?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calgary
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory potency against angiotensin I converting enzyme. |
J Med Chem 34: 511-7 (1991)
BindingDB Entry DOI: 10.7270/Q2JS9R1G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021123
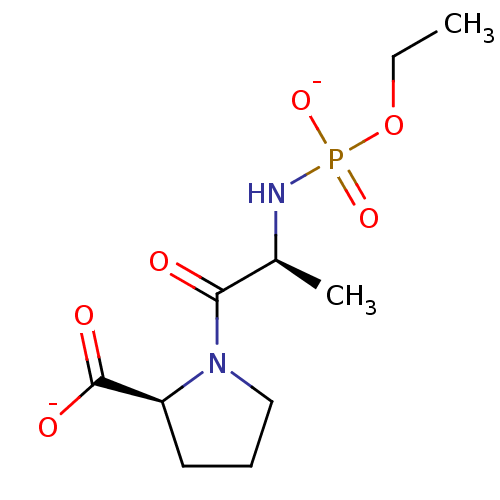
(CHEMBL30505 | dipotassium (2S)-1-{(2S)-2-[(ethoxyp...)Show SMILES CCOP([O-])(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C10H19N2O6P/c1-3-18-19(16,17)11-7(2)9(13)12-6-4-5-8(12)10(14)15/h7-8H,3-6H2,1-2H3,(H,14,15)(H2,11,16,17)/p-2/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50115848
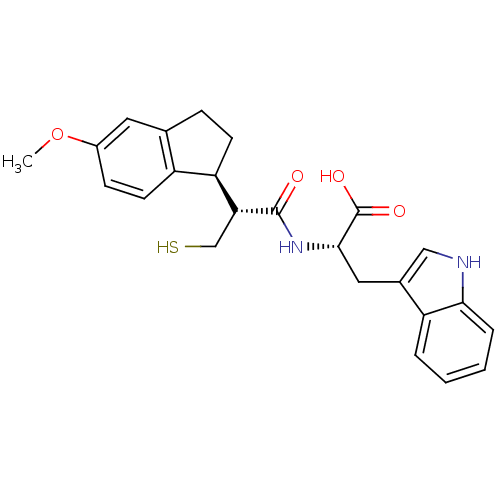
((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...)Show SMILES COc1ccc2[C@@H](CCc2c1)[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-30-16-7-9-17-14(10-16)6-8-19(17)20(13-31)23(27)26-22(24(28)29)11-15-12-25-21-5-3-2-4-18(15)21/h2-5,7,9-10,12,19-20,22,25,31H,6,8,11,13H2,1H3,(H,26,27)(H,28,29)/t19-,20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against angiotensin I converting enzyme |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50020843

(2-[(2S)-2-carboxypyrrolidin-1-yl]-1-methyl-2-oxoet...)Show SMILES C[C@@H](NP([O-])([O-])=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C8H15N2O6P/c1-5(9-17(14,15)16)7(11)10-4-2-3-6(10)8(12)13/h5-6H,2-4H2,1H3,(H,12,13)(H3,9,14,15,16)/p-2/t5?,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-1 converting enzyme |
J Med Chem 31: 1148-60 (1988)
BindingDB Entry DOI: 10.7270/Q2V125DX |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021121
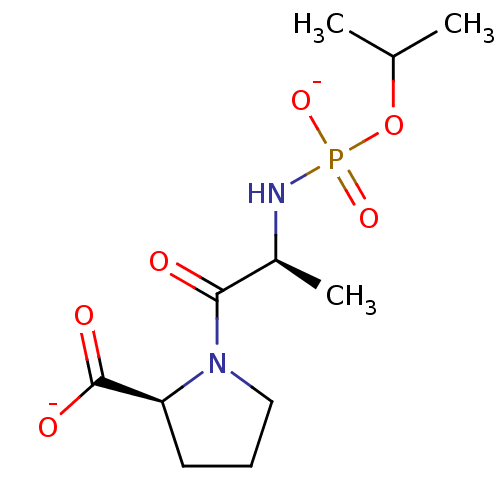
(CHEMBL286181 | dipotassium (2S)-1-{(2S)-2-[(isopro...)Show SMILES CC(C)OP([O-])(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C11H21N2O6P/c1-7(2)19-20(17,18)12-8(3)10(14)13-6-4-5-9(13)11(15)16/h7-9H,4-6H2,1-3H3,(H,15,16)(H2,12,17,18)/p-2/t8-,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021114

(CHEMBL282987 | tripotassium (2S)-1-[(2S)-2-(phosph...)Show SMILES C[C@H](NP([O-])([O-])=O)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C8H15N2O6P/c1-5(9-17(14,15)16)7(11)10-4-2-3-6(10)8(12)13/h5-6H,2-4H2,1H3,(H,12,13)(H3,9,14,15,16)/p-3/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303322
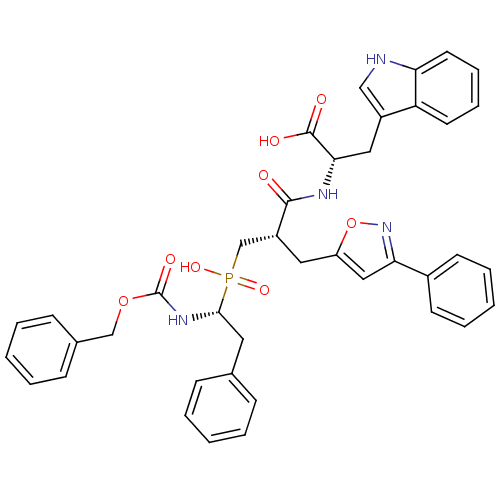
((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C40H39N4O8P/c45-38(42-36(39(46)47)22-30-24-41-34-19-11-10-18-33(30)34)31(21-32-23-35(44-52-32)29-16-8-3-9-17-29)26-53(49,50)37(20-27-12-4-1-5-13-27)43-40(48)51-25-28-14-6-2-7-15-28/h1-19,23-24,31,36-37,41H,20-22,25-26H2,(H,42,45)(H,43,48)(H,46,47)(H,49,50)/t31-,36+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50018849

(4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...)Show SMILES OC(=O)[C@@H]1C[C@H](CN1C(=O)CP(O)(=O)CCCCc1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H34NO5P/c25-22(17-30(28,29)14-8-7-11-18-9-3-1-4-10-18)24-16-20(15-21(24)23(26)27)19-12-5-2-6-13-19/h1,3-4,9-10,19-21H,2,5-8,11-17H2,(H,26,27)(H,28,29)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50018849

(4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...)Show SMILES OC(=O)[C@@H]1C[C@H](CN1C(=O)CP(O)(=O)CCCCc1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H34NO5P/c25-22(17-30(28,29)14-8-7-11-18-9-3-1-4-10-18)24-16-20(15-21(24)23(26)27)19-12-5-2-6-13-19/h1,3-4,9-10,19-21H,2,5-8,11-17H2,(H,26,27)(H,28,29)/t20-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-1 converting enzyme |
J Med Chem 31: 1148-60 (1988)
BindingDB Entry DOI: 10.7270/Q2V125DX |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50281632
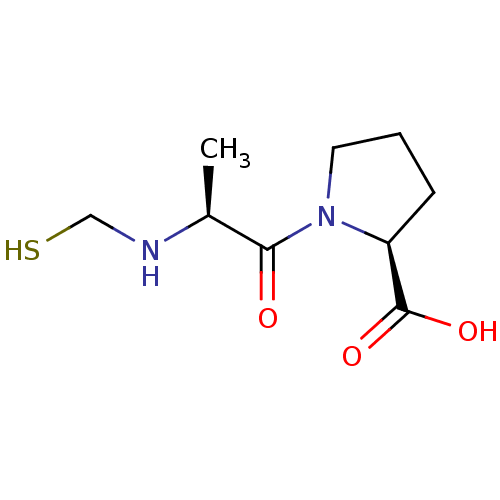
((2S)-1-{(2S)-2-[(lambda~4~-sulfanylmethyl)amino]pr...)Show InChI InChI=1S/C9H16N2O3S/c1-6(10-5-15)8(12)11-4-2-3-7(11)9(13)14/h6-7,10,15H,2-5H2,1H3,(H,13,14)/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Angiotensin I converting enzyme |
Bioorg Med Chem Lett 3: 799-802 (1993)
Article DOI: 10.1016/S0960-894X(00)80669-1
BindingDB Entry DOI: 10.7270/Q29S1QZZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-1 converting enzyme |
J Med Chem 31: 1148-60 (1988)
BindingDB Entry DOI: 10.7270/Q2V125DX |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044869
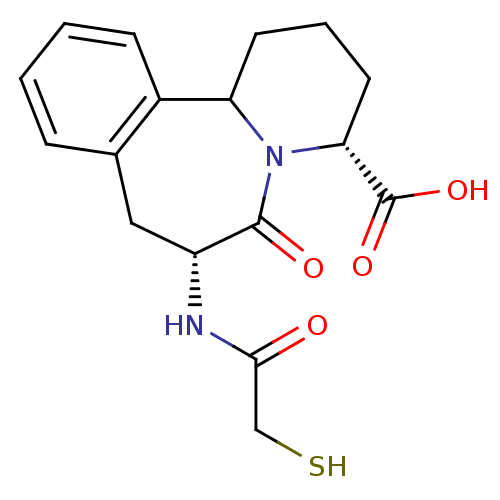
(7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6,7,8,12b...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@@H](Cc1ccccc21)NC(=O)CS Show InChI InChI=1S/C17H20N2O4S/c20-15(9-24)18-12-8-10-4-1-2-5-11(10)13-6-3-7-14(17(22)23)19(13)16(12)21/h1-2,4-5,12-14,24H,3,6-9H2,(H,18,20)(H,22,23)/t12-,13?,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044867
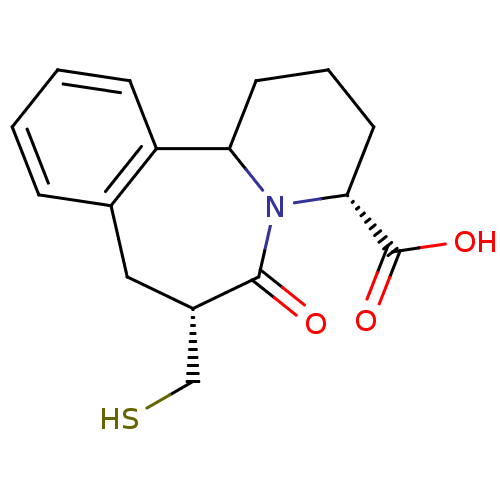
(7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@H](CS)Cc1ccccc21 Show InChI InChI=1S/C16H19NO3S/c18-15-11(9-21)8-10-4-1-2-5-12(10)13-6-3-7-14(16(19)20)17(13)15/h1-2,4-5,11,13-14,21H,3,6-9H2,(H,19,20)/t11-,13?,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E£tv£s Lor£nd University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) assessed as 3-Hydroxybutyril-glycil-glycil-glycine conversion to 3-hydroxybutyric acid after 60 mins by WST assay |
J Med Chem 56: 8377-88 (2013)
Article DOI: 10.1021/jm400813y
BindingDB Entry DOI: 10.7270/Q2MG7SGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | CHEMBL5281720
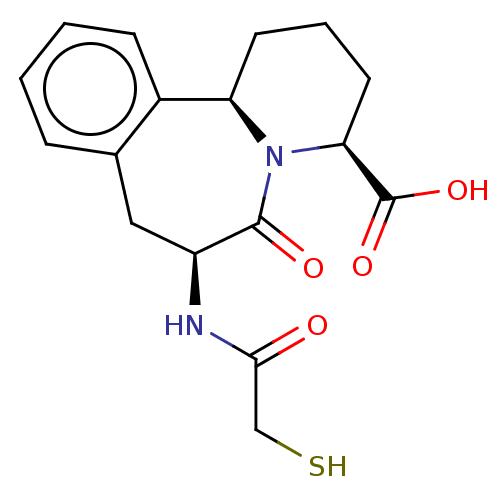
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406931
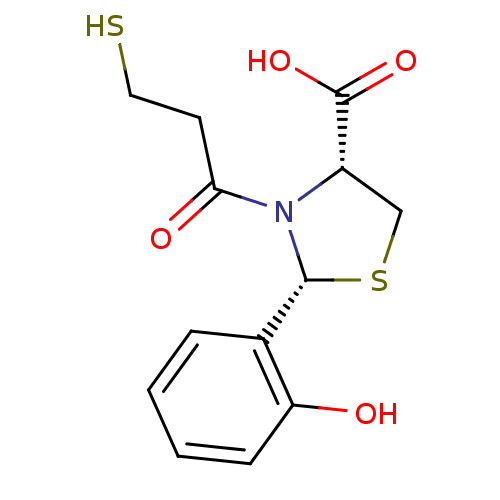
(RENTIAPRIL)Show InChI InChI=1S/C13H15NO4S2/c15-10-4-2-1-3-8(10)12-14(11(16)5-6-19)9(7-20-12)13(17)18/h1-4,9,12,15,19H,5-7H2,(H,17,18)/t9-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021124
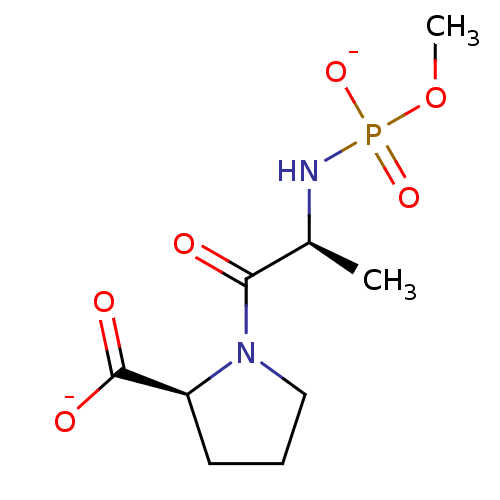
(CHEMBL34402 | dipotassium (2S)-1-{(2S)-2-[(methoxy...)Show SMILES COP([O-])(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C9H17N2O6P/c1-6(10-18(15,16)17-2)8(12)11-5-3-4-7(11)9(13)14/h6-7H,3-5H2,1-2H3,(H,13,14)(H2,10,15,16)/p-2/t6-,7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303327
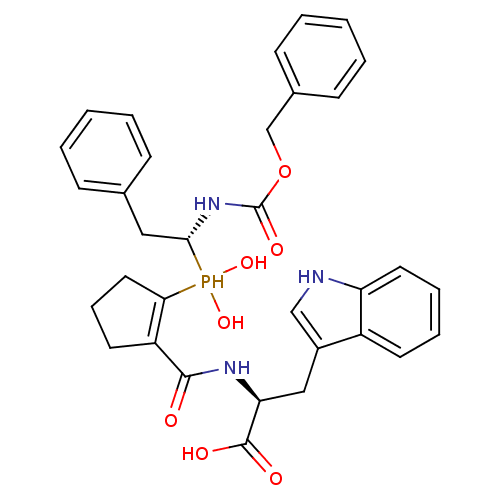
((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1=C(CCC1)P(O)(O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,t:19| Show InChI InChI=1S/C33H36N3O7P/c37-31(35-28(32(38)39)19-24-20-34-27-16-8-7-14-25(24)27)26-15-9-17-29(26)44(41,42)30(18-22-10-3-1-4-11-22)36-33(40)43-21-23-12-5-2-6-13-23/h1-8,10-14,16,20,28,30,34,41-42,44H,9,15,17-19,21H2,(H,35,37)(H,36,40)(H,38,39)/t28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303327

((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1=C(CCC1)P(O)(O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,t:19| Show InChI InChI=1S/C33H36N3O7P/c37-31(35-28(32(38)39)19-24-20-34-27-16-8-7-14-25(24)27)26-15-9-17-29(26)44(41,42)30(18-22-10-3-1-4-11-22)36-33(40)43-21-23-12-5-2-6-13-23/h1-8,10-14,16,20,28,30,34,41-42,44H,9,15,17-19,21H2,(H,35,37)(H,36,40)(H,38,39)/t28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411736
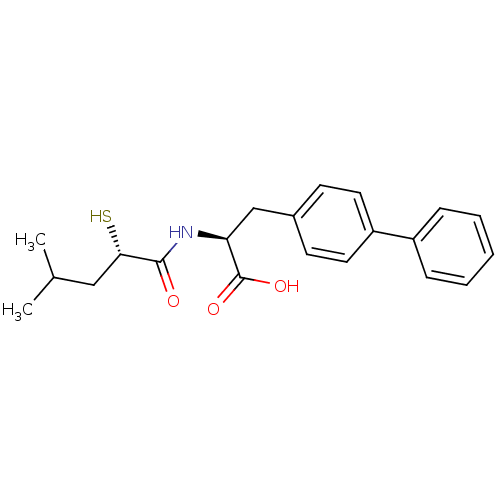
(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303323
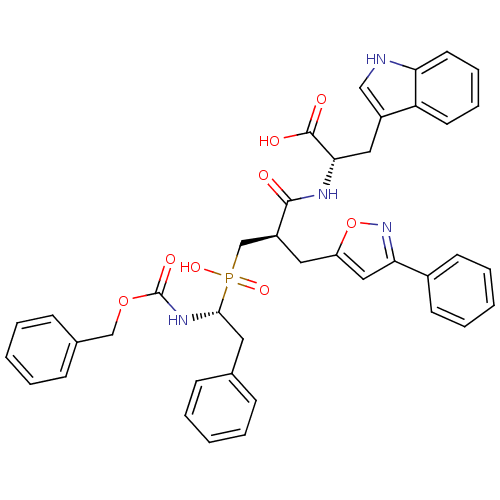
((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C40H39N4O8P/c45-38(42-36(39(46)47)22-30-24-41-34-19-11-10-18-33(30)34)31(21-32-23-35(44-52-32)29-16-8-3-9-17-29)26-53(49,50)37(20-27-12-4-1-5-13-27)43-40(48)51-25-28-14-6-2-7-15-28/h1-19,23-24,31,36-37,41H,20-22,25-26H2,(H,42,45)(H,43,48)(H,46,47)(H,49,50)/t31-,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303328

((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1{[(benzyloxy)c...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O8P/c42-36(39-34(37(43)44)21-27-13-5-1-6-14-27)31(23-32-24-33(41-49-32)30-19-11-4-12-20-30)26-50(46,47)35(22-28-15-7-2-8-16-28)40-38(45)48-25-29-17-9-3-10-18-29/h1-20,24,31,34-35H,21-23,25-26H2,(H,39,42)(H,40,45)(H,43,44)(H,46,47)/t31-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303328

((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1{[(benzyloxy)c...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O8P/c42-36(39-34(37(43)44)21-27-13-5-1-6-14-27)31(23-32-24-33(41-49-32)30-19-11-4-12-20-30)26-50(46,47)35(22-28-15-7-2-8-16-28)40-38(45)48-25-29-17-9-3-10-18-29/h1-20,24,31,34-35H,21-23,25-26H2,(H,39,42)(H,40,45)(H,43,44)(H,46,47)/t31-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of buffer |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of 1:100 diluted SHR rat plasma |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303325

((2S)-2-{[3-(4',5'-Dihydro-3'-phenyl-5'-isoxazolyl)...)Show SMILES C[C@H](NC(=O)C(CC1CC(=NO1)c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(O)=O |r,c:9| Show InChI InChI=1S/C32H36N3O8P/c1-22(31(37)38)33-30(36)26(18-27-19-28(35-43-27)25-15-9-4-10-16-25)21-44(40,41)29(17-23-11-5-2-6-12-23)34-32(39)42-20-24-13-7-3-8-14-24/h2-16,22,26-27,29H,17-21H2,1H3,(H,33,36)(H,34,39)(H,37,38)(H,40,41)/t22-,26?,27?,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044867

(7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@H](CS)Cc1ccccc21 Show InChI InChI=1S/C16H19NO3S/c18-15-11(9-21)8-10-4-1-2-5-12(10)13-6-3-7-14(16(19)20)17(13)15/h1-2,4-5,11,13-14,21H,3,6-9H2,(H,19,20)/t11-,13?,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50393213
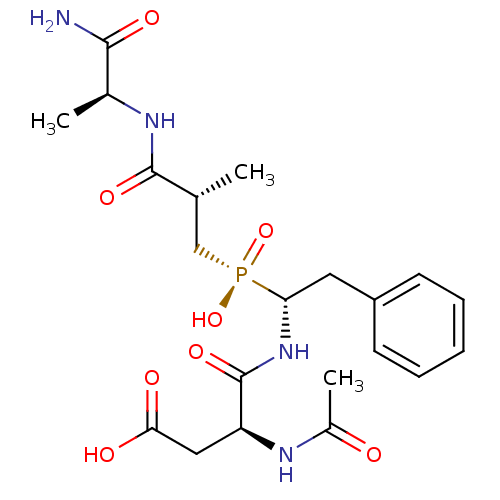
(CHEMBL1235767)Show SMILES C[C@H](C[P@](O)(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C21H31N4O8P/c1-12(20(30)23-13(2)19(22)29)11-34(32,33)17(9-15-7-5-4-6-8-15)25-21(31)16(10-18(27)28)24-14(3)26/h4-8,12-13,16-17H,9-11H2,1-3H3,(H2,22,29)(H,23,30)(H,24,26)(H,25,31)(H,27,28)(H,32,33)/t12-,13+,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of 1:50 diluted SHR rat plasma |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021116
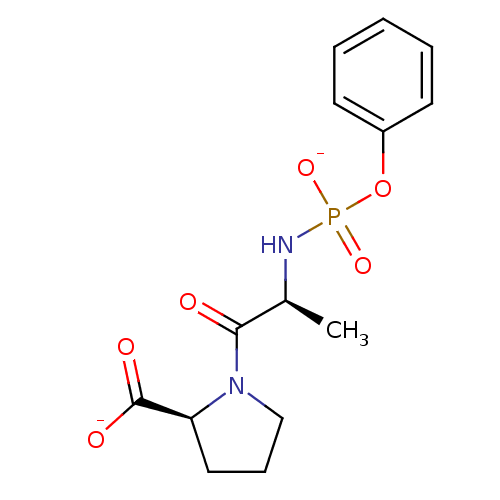
(CHEMBL33124 | dipotassium (2S)-1-{(2S)-2-[(phenoxy...)Show SMILES C[C@H](NP([O-])(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C([O-])=O Show InChI InChI=1S/C14H19N2O6P/c1-10(13(17)16-9-5-8-12(16)14(18)19)15-23(20,21)22-11-6-3-2-4-7-11/h2-4,6-7,10,12H,5,8-9H2,1H3,(H,18,19)(H2,15,20,21)/p-2/t10-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 |
J Med Chem 28: 1422-7 (1985)
BindingDB Entry DOI: 10.7270/Q2Z60N24 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411731
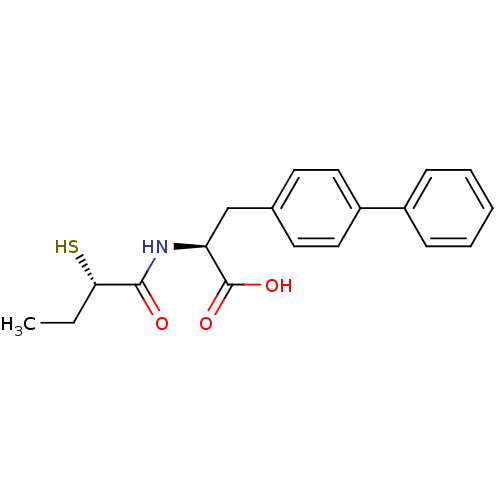
(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data