 Found 41 hits of ki data for polymerid = 2448,50001791
Found 41 hits of ki data for polymerid = 2448,50001791 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538
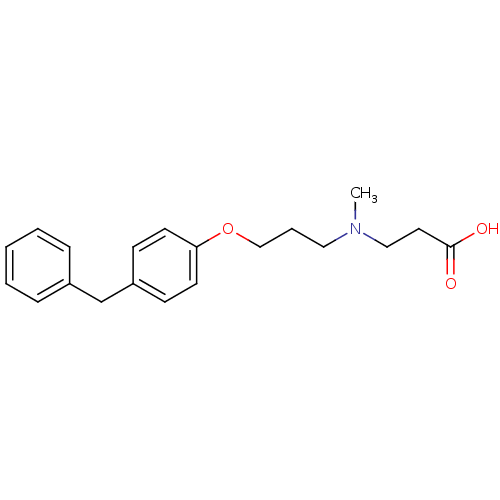
(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50505156
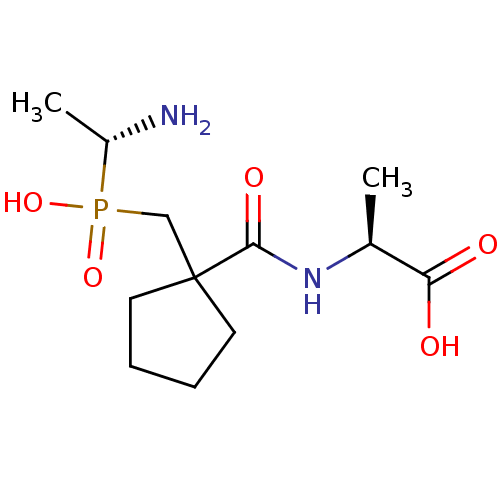
(CHEMBL4559677)Show SMILES C[C@H](N)P(O)(=O)CC1(CCCC1)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C12H23N2O5P/c1-8(10(15)16)14-11(17)12(5-3-4-6-12)7-20(18,19)9(2)13/h8-9H,3-7,13H2,1-2H3,(H,14,17)(H,15,16)(H,18,19)/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4H by L-(4-benzoyl)phenylalanyl-beta-naphthylamide fluorigenic substrate by fluorescence based assay |
Bioorg Med Chem Lett 29: 1031-1042 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DR2ZSK |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50505153
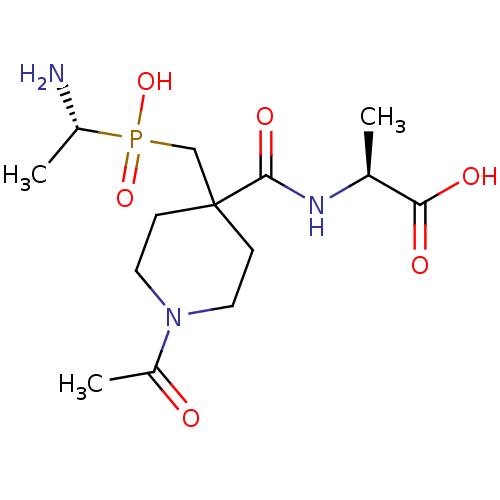
(CHEMBL4543986)Show SMILES C[C@H](N)P(O)(=O)CC1(CCN(CC1)C(C)=O)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C14H26N3O6P/c1-9(12(19)20)16-13(21)14(8-24(22,23)10(2)15)4-6-17(7-5-14)11(3)18/h9-10H,4-8,15H2,1-3H3,(H,16,21)(H,19,20)(H,22,23)/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4H by L-(4-benzoyl)phenylalanyl-beta-naphthylamide fluorigenic substrate by fluorescence based assay |
Bioorg Med Chem Lett 29: 1031-1042 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DR2ZSK |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264
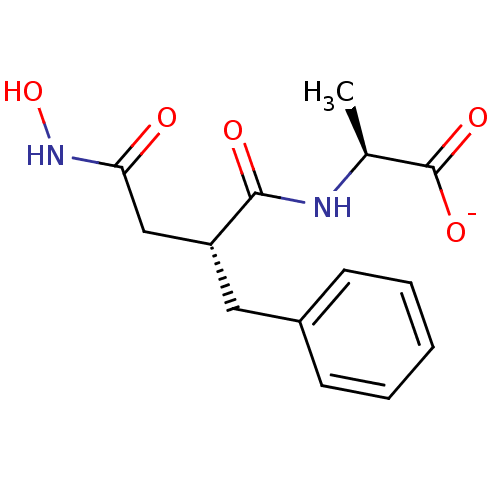
(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against LTA 4 hydrolase in aminopeptidase assay |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264

(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against LTA 4 hydrolase in epoxide hydrolase assay |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50046316
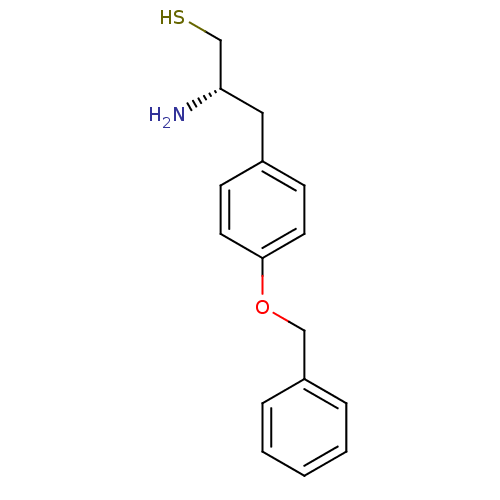
(2-Amino-3-(4-benzyloxy-phenyl)-propane-1-thiol | C...)Show InChI InChI=1S/C16H19NOS/c17-15(12-19)10-13-6-8-16(9-7-13)18-11-14-4-2-1-3-5-14/h1-9,15,19H,10-12,17H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes |
J Med Chem 36: 211-20 (1993)
BindingDB Entry DOI: 10.7270/Q2T152P3 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50046314
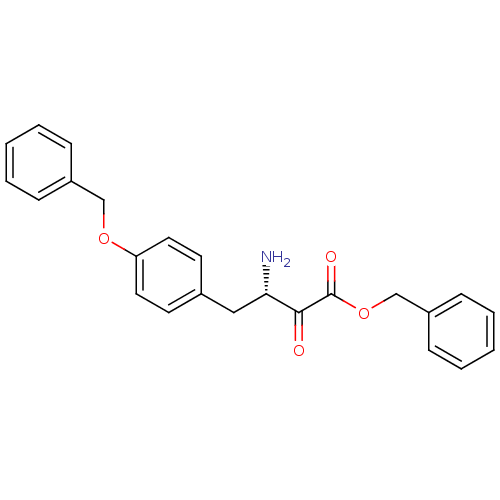
(3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...)Show SMILES N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)C(=O)OCc1ccccc1 Show InChI InChI=1S/C24H23NO4/c25-22(23(26)24(27)29-17-20-9-5-2-6-10-20)15-18-11-13-21(14-12-18)28-16-19-7-3-1-4-8-19/h1-14,22H,15-17,25H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes |
J Med Chem 36: 211-20 (1993)
BindingDB Entry DOI: 10.7270/Q2T152P3 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441909

(CHEMBL2437292)Show SMILES Clc1ccc(cc1)-c1csc(=N)n1CC(=O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C21H21ClN4OS/c22-17-8-6-16(7-9-17)19-15-28-21(23)26(19)14-20(27)25-12-10-24(11-13-25)18-4-2-1-3-5-18/h1-9,15,23H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197085
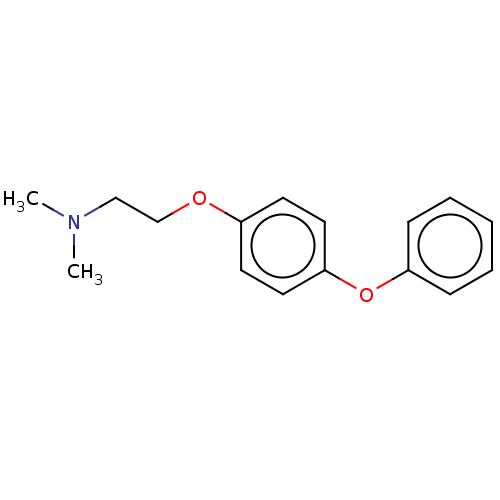
(CHEMBL3921982)Show InChI InChI=1S/C16H19NO2/c1-17(2)12-13-18-14-8-10-16(11-9-14)19-15-6-4-3-5-7-15/h3-11H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441915
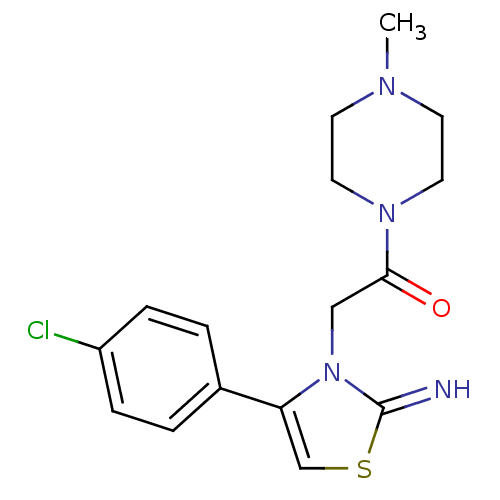
(CHEMBL2437298)Show InChI InChI=1S/C16H19ClN4OS/c1-19-6-8-20(9-7-19)15(22)10-21-14(11-23-16(21)18)12-2-4-13(17)5-3-12/h2-5,11,18H,6-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197084
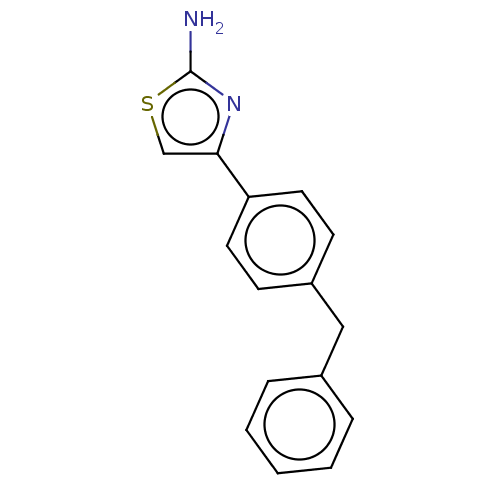
(CHEMBL3883608)Show InChI InChI=1S/C16H14N2S/c17-16-18-15(11-19-16)14-8-6-13(7-9-14)10-12-4-2-1-3-5-12/h1-9,11H,10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441914
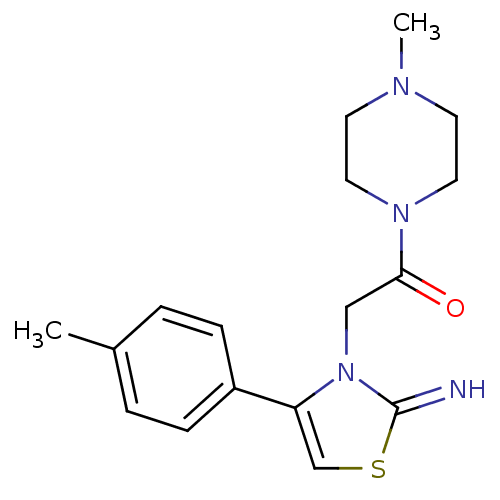
(CHEMBL2437296)Show InChI InChI=1S/C17H22N4OS/c1-13-3-5-14(6-4-13)15-12-23-17(18)21(15)11-16(22)20-9-7-19(2)8-10-20/h3-6,12,18H,7-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441912
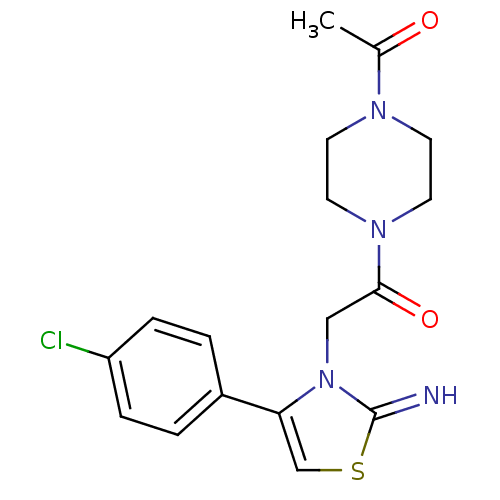
(CHEMBL2437295)Show SMILES CC(=O)N1CCN(CC1)C(=O)Cn1c(csc1=N)-c1ccc(Cl)cc1 Show InChI InChI=1S/C17H19ClN4O2S/c1-12(23)20-6-8-21(9-7-20)16(24)10-22-15(11-25-17(22)19)13-2-4-14(18)5-3-13/h2-5,11,19H,6-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 607 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441916
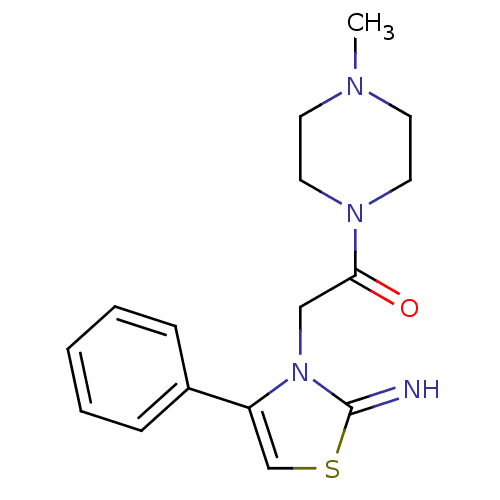
(CHEMBL2437300)Show InChI InChI=1S/C16H20N4OS/c1-18-7-9-19(10-8-18)15(21)11-20-14(12-22-16(20)17)13-5-3-2-4-6-13/h2-6,12,17H,7-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441910
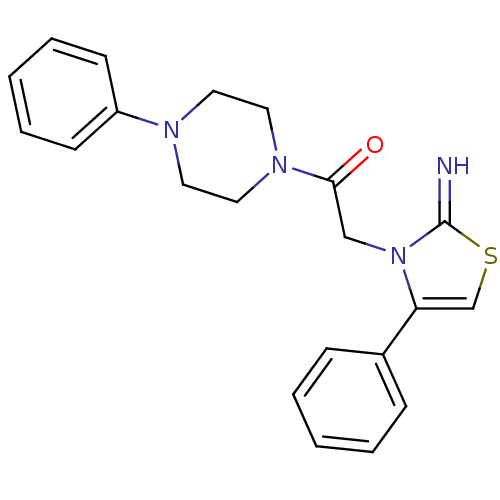
(CHEMBL2437293)Show SMILES N=c1scc(-c2ccccc2)n1CC(=O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C21H22N4OS/c22-21-25(19(16-27-21)17-7-3-1-4-8-17)15-20(26)24-13-11-23(12-14-24)18-9-5-2-6-10-18/h1-10,16,22H,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 643 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441918

(CHEMBL2437302)Show InChI InChI=1S/C15H16ClN3O2S/c16-12-3-1-11(2-4-12)13-10-22-15(17)19(13)9-14(20)18-5-7-21-8-6-18/h1-4,10,17H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441911
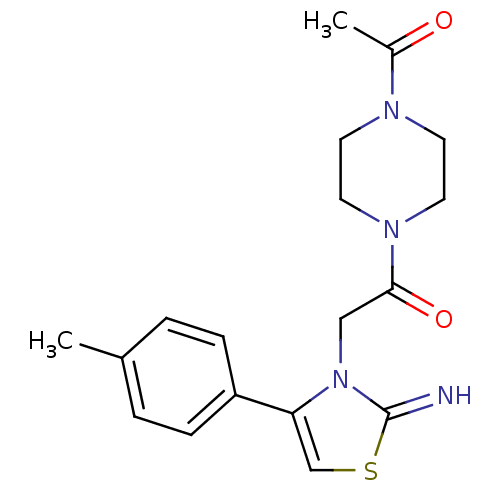
(CHEMBL2437294)Show SMILES CC(=O)N1CCN(CC1)C(=O)Cn1c(csc1=N)-c1ccc(C)cc1 Show InChI InChI=1S/C18H22N4O2S/c1-13-3-5-15(6-4-13)16-12-25-18(19)22(16)11-17(24)21-9-7-20(8-10-21)14(2)23/h3-6,12,19H,7-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441913
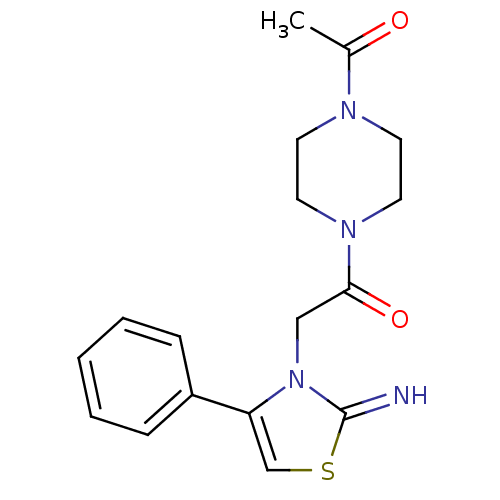
(CHEMBL2437297)Show InChI InChI=1S/C17H20N4O2S/c1-13(22)19-7-9-20(10-8-19)16(23)11-21-15(12-24-17(21)18)14-5-3-2-4-6-14/h2-6,12,18H,7-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441917
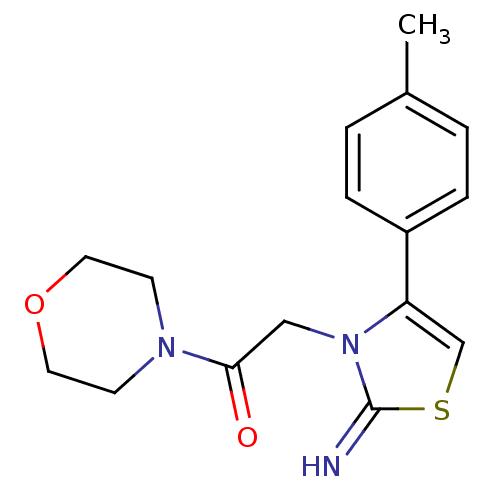
(CHEMBL2437299)Show InChI InChI=1S/C16H19N3O2S/c1-12-2-4-13(5-3-12)14-11-22-16(17)19(14)10-15(20)18-6-8-21-9-7-18/h2-5,11,17H,6-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441908
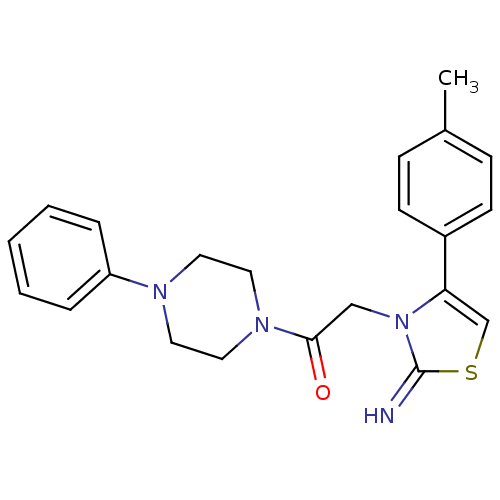
(CHEMBL2437291)Show SMILES Cc1ccc(cc1)-c1csc(=N)n1CC(=O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C22H24N4OS/c1-17-7-9-18(10-8-17)20-16-28-22(23)26(20)15-21(27)25-13-11-24(12-14-25)19-5-3-2-4-6-19/h2-10,16,23H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23978
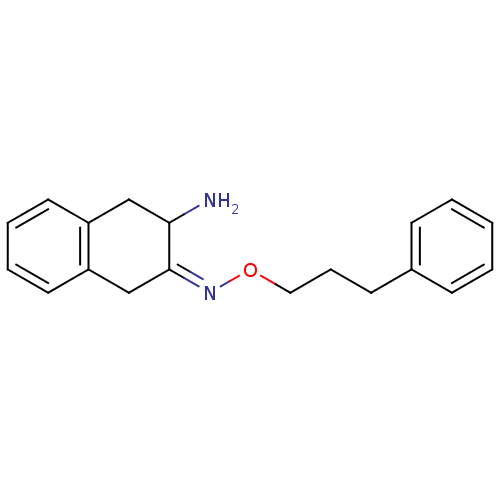
((3Z)-3-[(3-phenylpropoxy)imino]-1,2,3,4-tetrahydro...)Show InChI InChI=1S/C19H22N2O/c20-18-13-16-10-4-5-11-17(16)14-19(18)21-22-12-6-9-15-7-2-1-3-8-15/h1-5,7-8,10-11,18H,6,9,12-14,20H2/b21-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu
| Assay Description
Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... |
Bioorg Med Chem 14: 7241-57 (2006)
Article DOI: 10.1016/j.bmc.2006.06.050
BindingDB Entry DOI: 10.7270/Q2N29V73 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23979
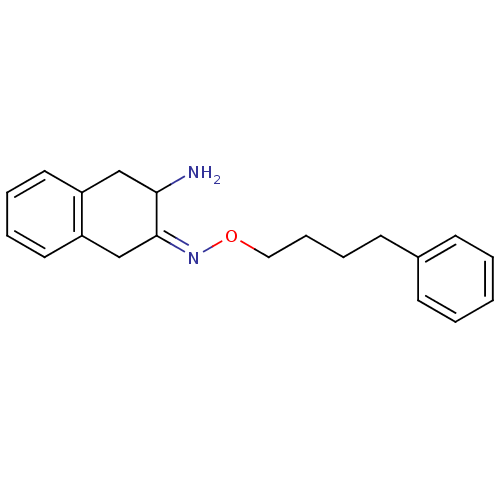
((3Z)-3-[(4-phenylbutoxy)imino]-1,2,3,4-tetrahydron...)Show InChI InChI=1S/C20H24N2O/c21-19-14-17-11-4-5-12-18(17)15-20(19)22-23-13-7-6-10-16-8-2-1-3-9-16/h1-5,8-9,11-12,19H,6-7,10,13-15,21H2/b22-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu
| Assay Description
Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... |
Bioorg Med Chem 14: 7241-57 (2006)
Article DOI: 10.1016/j.bmc.2006.06.050
BindingDB Entry DOI: 10.7270/Q2N29V73 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50441919
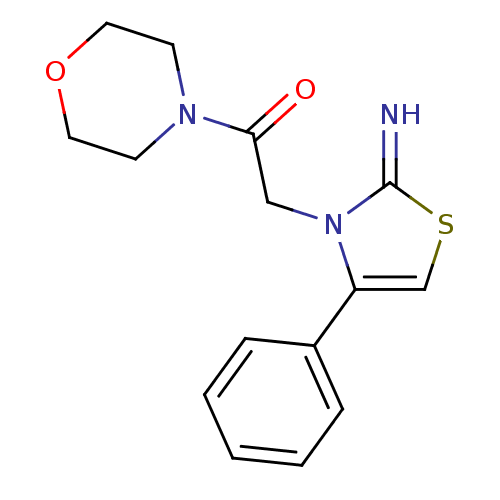
(CHEMBL2437301)Show InChI InChI=1S/C15H17N3O2S/c16-15-18(10-14(19)17-6-8-20-9-7-17)13(11-21-15)12-4-2-1-3-5-12/h1-5,11,16H,6-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mansoura
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A-4 hydrolase |
Eur J Med Chem 69: 908-19 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.021
BindingDB Entry DOI: 10.7270/Q2TM7CJN |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50046327
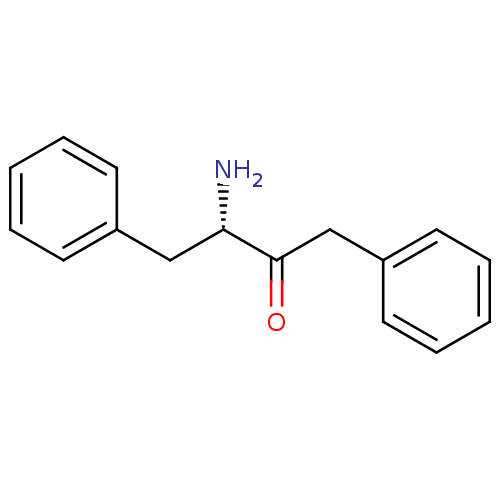
(3-Amino-1,4-diphenyl-butan-2-one | CHEMBL70658)Show InChI InChI=1S/C16H17NO/c17-15(11-13-7-3-1-4-8-13)16(18)12-14-9-5-2-6-10-14/h1-10,15H,11-12,17H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes |
J Med Chem 36: 211-20 (1993)
BindingDB Entry DOI: 10.7270/Q2T152P3 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50046331
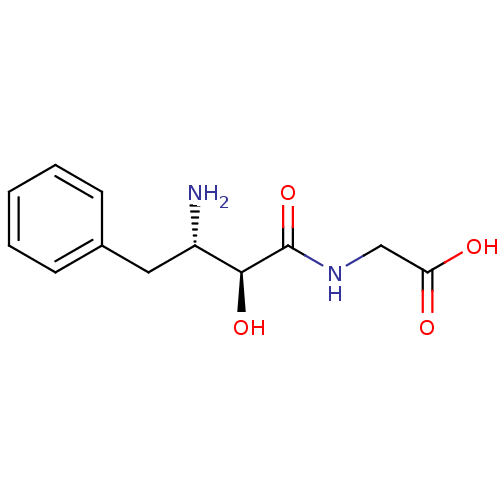
(((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyrylamino)-...)Show InChI InChI=1S/C12H16N2O4/c13-9(6-8-4-2-1-3-5-8)11(17)12(18)14-7-10(15)16/h1-5,9,11,17H,6-7,13H2,(H,14,18)(H,15,16)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes |
J Med Chem 36: 211-20 (1993)
BindingDB Entry DOI: 10.7270/Q2T152P3 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50046331

(((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyrylamino)-...)Show InChI InChI=1S/C12H16N2O4/c13-9(6-8-4-2-1-3-5-8)11(17)12(18)14-7-10(15)16/h1-5,9,11,17H,6-7,13H2,(H,14,18)(H,15,16)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Leukotriene A4 hydrolase from human leukocytes |
Bioorg Med Chem Lett 1: 551-556 (1991)
Article DOI: 10.1016/S0960-894X(01)80464-9
BindingDB Entry DOI: 10.7270/Q2M32W8B |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23980
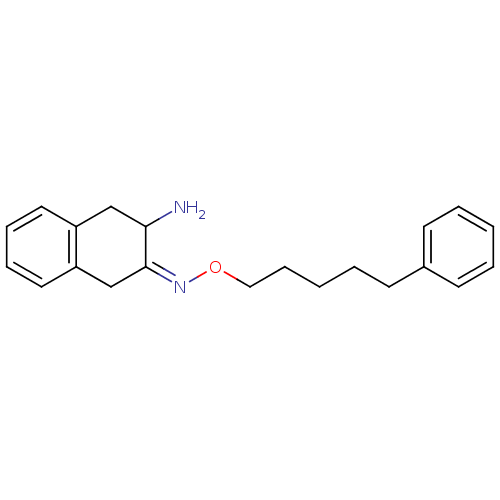
((3Z)-3-{[(5-phenylpentyl)oxy]imino}-1,2,3,4-tetrah...)Show InChI InChI=1S/C21H26N2O/c22-20-15-18-12-6-7-13-19(18)16-21(20)23-24-14-8-2-5-11-17-9-3-1-4-10-17/h1,3-4,6-7,9-10,12-13,20H,2,5,8,11,14-16,22H2/b23-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu
| Assay Description
Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... |
Bioorg Med Chem 14: 7241-57 (2006)
Article DOI: 10.1016/j.bmc.2006.06.050
BindingDB Entry DOI: 10.7270/Q2N29V73 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50279808
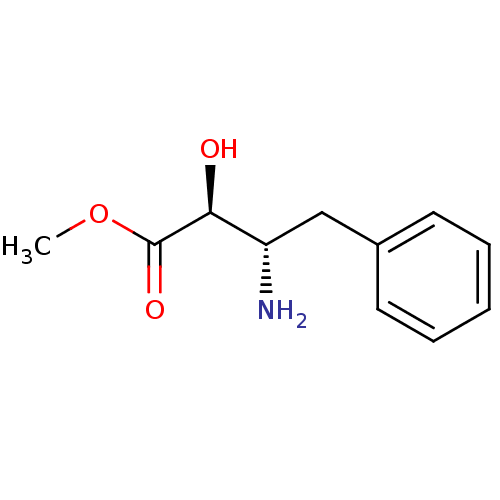
((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyric acid me...)Show InChI InChI=1S/C11H15NO3/c1-15-11(14)10(13)9(12)7-8-5-3-2-4-6-8/h2-6,9-10,13H,7,12H2,1H3/t9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Leukotriene A4 hydrolase from human leukocytes |
Bioorg Med Chem Lett 1: 551-556 (1991)
Article DOI: 10.1016/S0960-894X(01)80464-9
BindingDB Entry DOI: 10.7270/Q2M32W8B |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50279808

((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyric acid me...)Show InChI InChI=1S/C11H15NO3/c1-15-11(14)10(13)9(12)7-8-5-3-2-4-6-8/h2-6,9-10,13H,7,12H2,1H3/t9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes |
J Med Chem 36: 211-20 (1993)
BindingDB Entry DOI: 10.7270/Q2T152P3 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397950
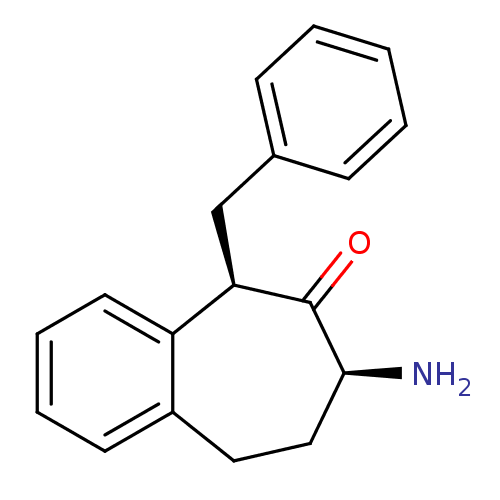
(CHEMBL2179972)Show InChI InChI=1S/C18H19NO/c19-17-11-10-14-8-4-5-9-15(14)16(18(17)20)12-13-6-2-1-3-7-13/h1-9,16-17H,10-12,19H2/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397951
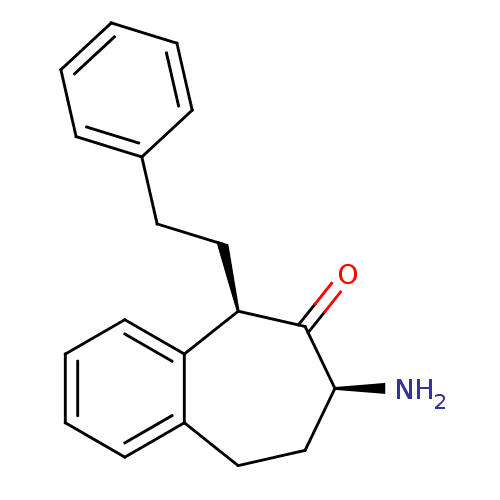
(CHEMBL2179981)Show SMILES N[C@H]1CCc2ccccc2[C@@H](CCc2ccccc2)C1=O |r| Show InChI InChI=1S/C19H21NO/c20-18-13-11-15-8-4-5-9-16(15)17(19(18)21)12-10-14-6-2-1-3-7-14/h1-9,17-18H,10-13,20H2/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397952
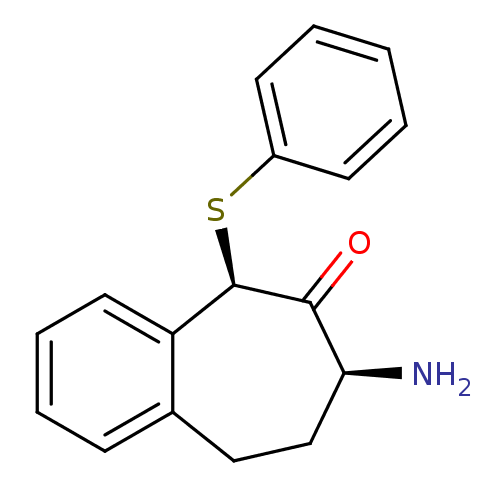
(CHEMBL2179979)Show InChI InChI=1S/C17H17NOS/c18-15-11-10-12-6-4-5-9-14(12)17(16(15)19)20-13-7-2-1-3-8-13/h1-9,15,17H,10-11,18H2/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397953
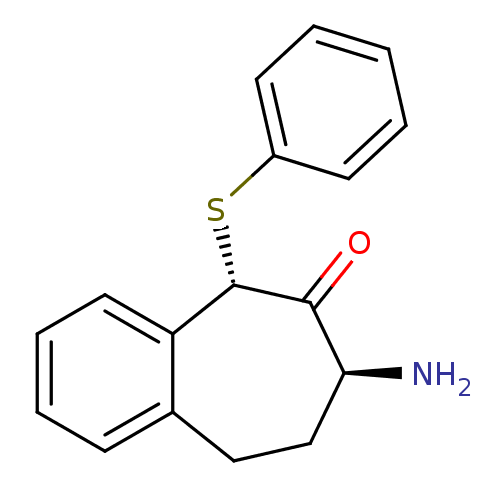
(CHEMBL2179978)Show InChI InChI=1S/C17H17NOS/c18-15-11-10-12-6-4-5-9-14(12)17(16(15)19)20-13-7-2-1-3-8-13/h1-9,15,17H,10-11,18H2/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397954
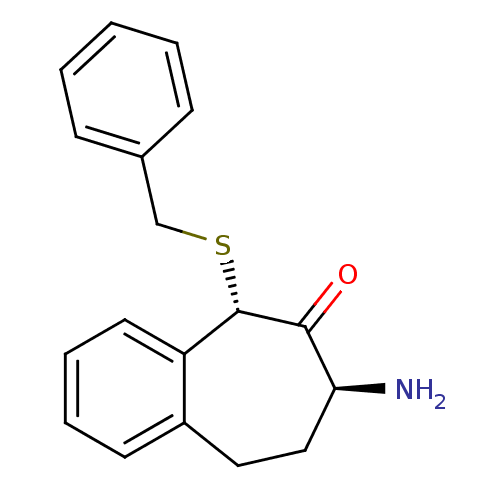
(CHEMBL2179977)Show InChI InChI=1S/C18H19NOS/c19-16-11-10-14-8-4-5-9-15(14)18(17(16)20)21-12-13-6-2-1-3-7-13/h1-9,16,18H,10-12,19H2/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397949
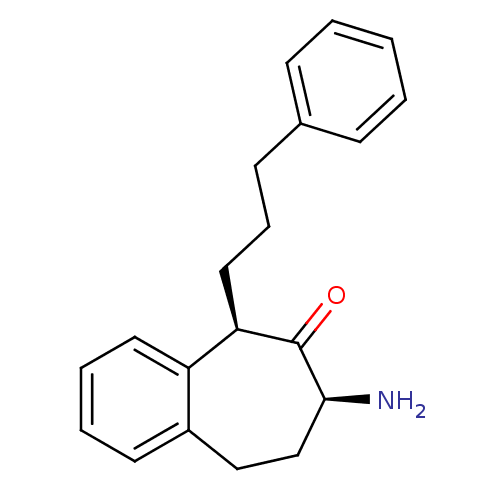
(CHEMBL2179973)Show SMILES N[C@H]1CCc2ccccc2[C@@H](CCCc2ccccc2)C1=O |r| Show InChI InChI=1S/C20H23NO/c21-19-14-13-16-10-4-5-11-17(16)18(20(19)22)12-6-9-15-7-2-1-3-8-15/h1-5,7-8,10-11,18-19H,6,9,12-14,21H2/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397956
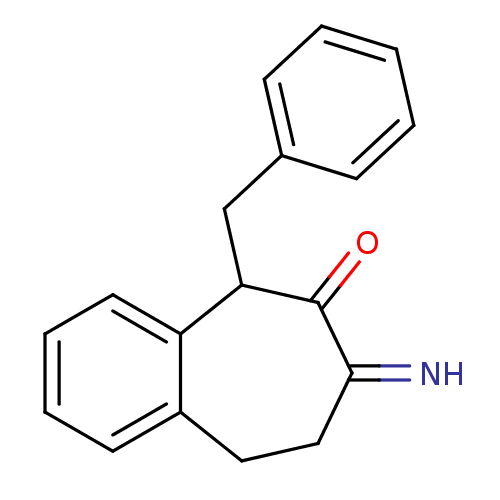
(CHEMBL2179974)Show InChI InChI=1S/C18H17NO/c19-17-11-10-14-8-4-5-9-15(14)16(18(17)20)12-13-6-2-1-3-7-13/h1-9,16,19H,10-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by Dixon-plot analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397957
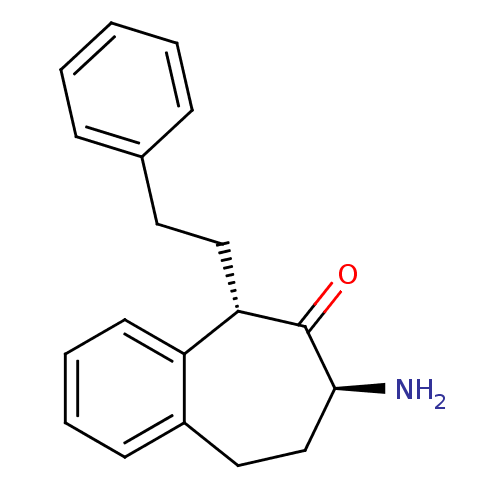
(CHEMBL2179980)Show InChI InChI=1S/C19H21NO/c20-18-13-11-15-8-4-5-9-16(15)17(19(18)21)12-10-14-6-2-1-3-7-14/h1-9,17-18H,10-13,20H2/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by Dixon-plot analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397955
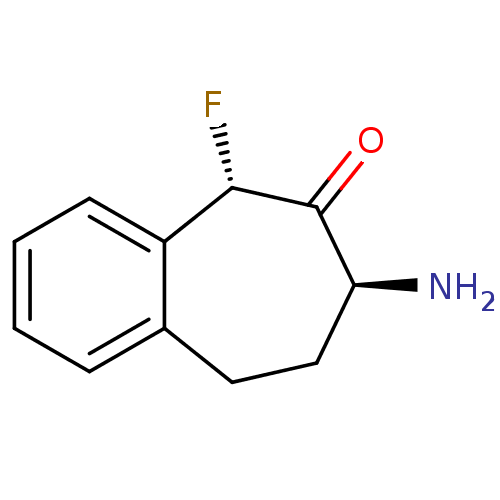
(CHEMBL2179976)Show InChI InChI=1S/C11H12FNO/c12-10-8-4-2-1-3-7(8)5-6-9(13)11(10)14/h1-4,9-10H,5-6,13H2/t9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50397948
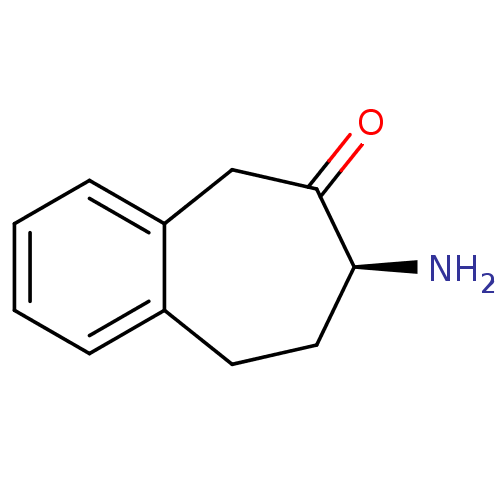
(CHEMBL2179975)Show InChI InChI=1S/C11H13NO/c12-10-6-5-8-3-1-2-4-9(8)7-11(10)13/h1-4,10H,5-7,12H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysis |
Bioorg Med Chem 20: 4942-53 (2012)
Article DOI: 10.1016/j.bmc.2012.06.041
BindingDB Entry DOI: 10.7270/Q2RB75Q1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data