 Found 763 hits of ki data for polymerid = 350,5758
Found 763 hits of ki data for polymerid = 350,5758 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931
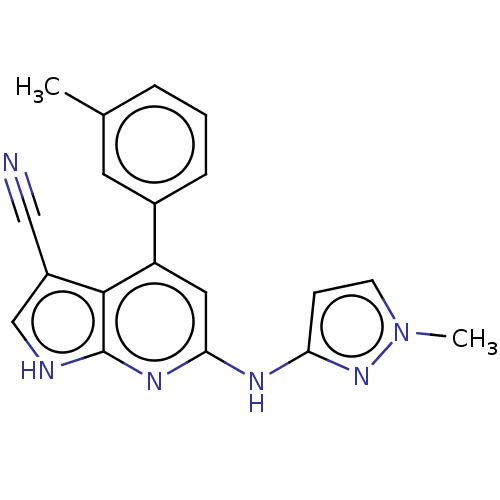
(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582714

(CHEMBL5075978)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cnn(C)c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931

(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582715

(CHEMBL5091909)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cn[nH]c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM182716
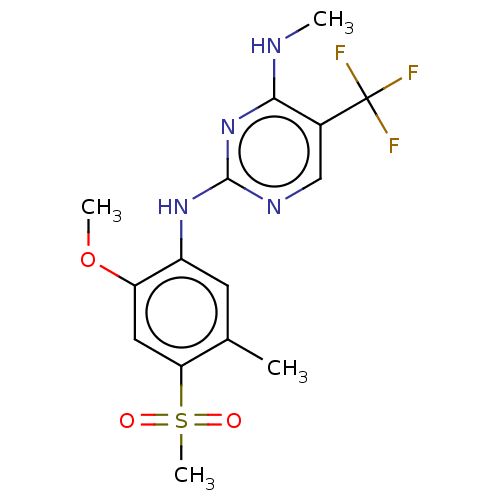
(US9145402, 23 | US9145402, 36)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C15H17F3N4O3S/c1-8-5-10(11(25-3)6-12(8)26(4,23)24)21-14-20-7-9(15(16,17)18)13(19-2)22-14/h5-7H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US9145402 (2015)
BindingDB Entry DOI: 10.7270/Q2J67FQ3 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50393038

(CHEMBL2152707)Show SMILES Cn1cc(C=Cc2[nH]nc3ccnc(OC4CCCCC4)c23)cn1 |w:5.5| Show InChI InChI=1S/C18H21N5O/c1-23-12-13(11-20-23)7-8-15-17-16(22-21-15)9-10-19-18(17)24-14-5-3-2-4-6-14/h7-12,14H,2-6H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to LRRK2 |
ACS Med Chem Lett 3: 701-702 (2012)
Article DOI: 10.1021/ml300200p
BindingDB Entry DOI: 10.7270/Q2514092 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM41571
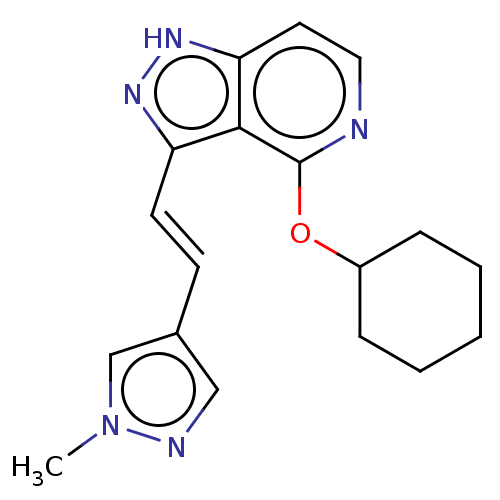
(US8569281, 52)Show InChI InChI=1S/C15H21N5O3S/c1-10-8-11(19-23-10)14(21)16-5-4-13-18-12(9-24-13)15(22)17-6-7-20(2)3/h8-9H,4-7H2,1-3H3,(H,16,21)(H,17,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. |
US Patent US8569281 (2013)
BindingDB Entry DOI: 10.7270/Q2DN43PM |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582714

(CHEMBL5075978)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cnn(C)c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human GST tagged truncated LRRK2 G2019S mutant incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196826

(US9212186, 23)Show InChI InChI=1S/C15H19F3N6O/c1-4-19-12-9(15(16,17)18)5-20-13(23-12)22-10-6-21-24-8-14(2,3)25-7-11(10)24/h5-6H,4,7-8H2,1-3H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212186 (2015)
BindingDB Entry DOI: 10.7270/Q2NS0SPX |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196757

(US9212173, 35)Show SMILES CCNc1nc(Nc2cn(nc2C)C(C)(C)c2ccnn2C)ncc1C(F)(F)F Show InChI InChI=1S/C18H23F3N8/c1-6-22-15-12(18(19,20)21)9-23-16(26-15)25-13-10-29(27-11(13)2)17(3,4)14-7-8-24-28(14)5/h7-10H,6H2,1-5H3,(H2,22,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582707
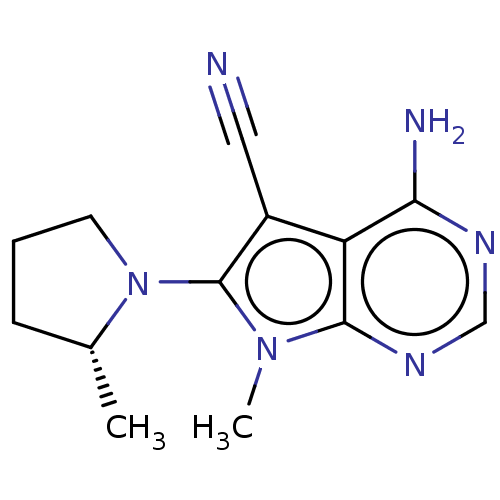
(CHEMBL5086513) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582715

(CHEMBL5091909)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cn[nH]c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human GST tagged truncated LRRK2 G2019S mutant incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196754
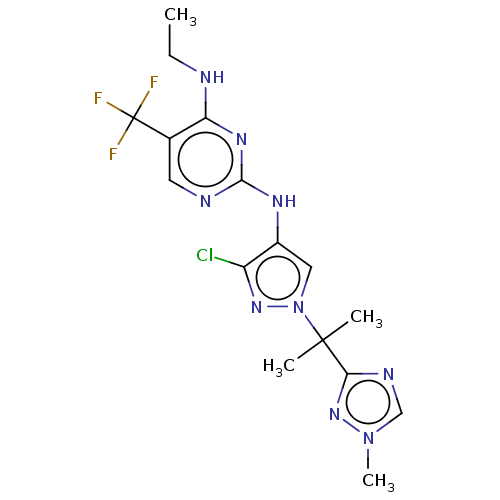
(US9212173, 32)Show SMILES CCNc1nc(Nc2cn(nc2Cl)C(C)(C)c2ncn(C)n2)ncc1C(F)(F)F Show InChI InChI=1S/C16H19ClF3N9/c1-5-21-12-9(16(18,19)20)6-22-14(25-12)24-10-7-29(26-11(10)17)15(2,3)13-23-8-28(4)27-13/h6-8H,5H2,1-4H3,(H2,21,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254929
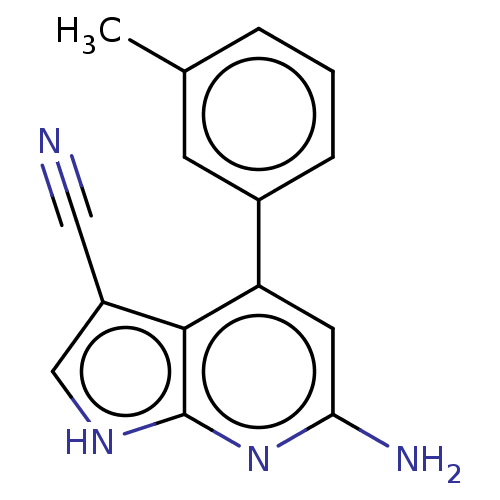
(US9499542, 12 | US9675594, 12)Show InChI InChI=1S/C15H12N4/c1-9-3-2-4-10(5-9)12-6-13(17)19-15-14(12)11(7-16)8-18-15/h2-6,8H,1H3,(H3,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50507669

(CHEMBL4539170)Show InChI InChI=1S/C17H22N6O/c1-3-12-10-14(22-23(12)2)16-15-13(20-21-16)4-7-18-17(15)19-11-5-8-24-9-6-11/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,18,19)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... |
Bioorg Med Chem Lett 29: 674-680 (2019)
Article DOI: 10.1016/j.bmcl.2018.10.017
BindingDB Entry DOI: 10.7270/Q2HH6PCX |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196827
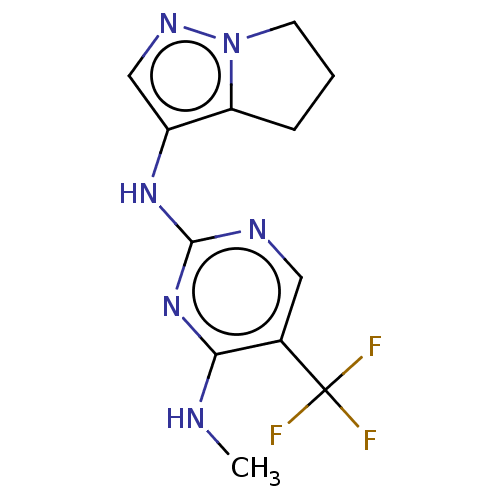
(US9212186, 24)Show InChI InChI=1S/C12H13F3N6/c1-16-10-7(12(13,14)15)5-17-11(20-10)19-8-6-18-21-4-2-3-9(8)21/h5-6H,2-4H2,1H3,(H2,16,17,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212186 (2015)
BindingDB Entry DOI: 10.7270/Q2NS0SPX |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196762

(US9212173, 40)Show SMILES CNc1nc(Nc2cnn(C3CCOC[C@@H]3F)c2C)ncc1C(F)(F)F |r| Show InChI InChI=1S/C15H18F4N6O/c1-8-11(6-22-25(8)12-3-4-26-7-10(12)16)23-14-21-5-9(15(17,18)19)13(20-2)24-14/h5-6,10,12H,3-4,7H2,1-2H3,(H2,20,21,23,24)/t10-,12?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196759
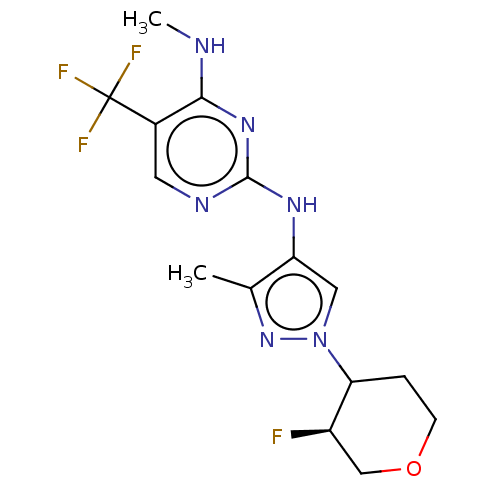
(US9212173, 37)Show SMILES CNc1nc(Nc2cn(nc2C)C2CCOC[C@H]2F)ncc1C(F)(F)F |r| Show InChI InChI=1S/C15H18F4N6O/c1-8-11(6-25(24-8)12-3-4-26-7-10(12)16)22-14-21-5-9(15(17,18)19)13(20-2)23-14/h5-6,10,12H,3-4,7H2,1-2H3,(H2,20,21,22,23)/t10-,12?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196752
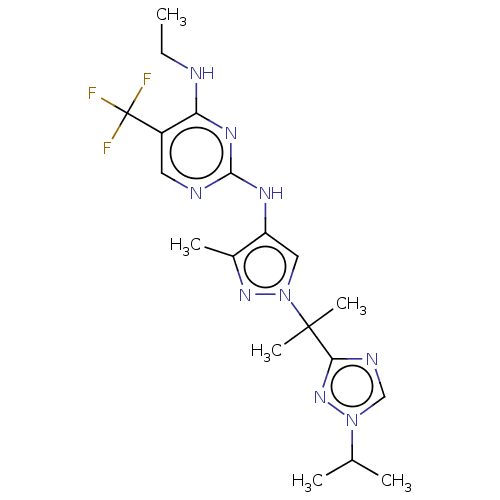
(US9212173, 30)Show SMILES CCNc1nc(Nc2cn(nc2C)C(C)(C)c2ncn(n2)C(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C19H26F3N9/c1-7-23-15-13(19(20,21)22)8-24-17(27-15)26-14-9-31(28-12(14)4)18(5,6)16-25-10-30(29-16)11(2)3/h8-11H,7H2,1-6H3,(H2,23,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448118
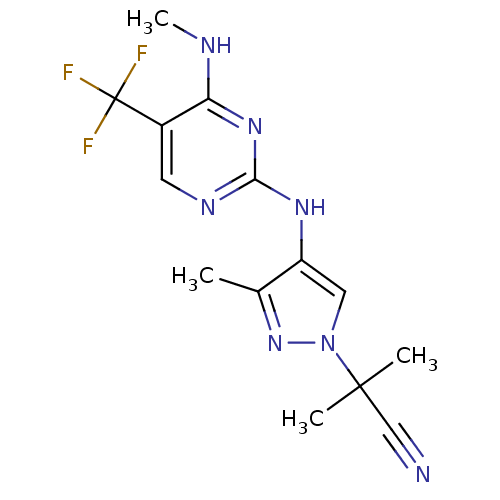
(CHEMBL3122113 | US10590114, No. 80 | US11111235, N...)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-24(23-8)13(2,3)7-18)21-12-20-5-9(14(15,16)17)11(19-4)22-12/h5-6H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LRRK2 G2019S mutant (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129179
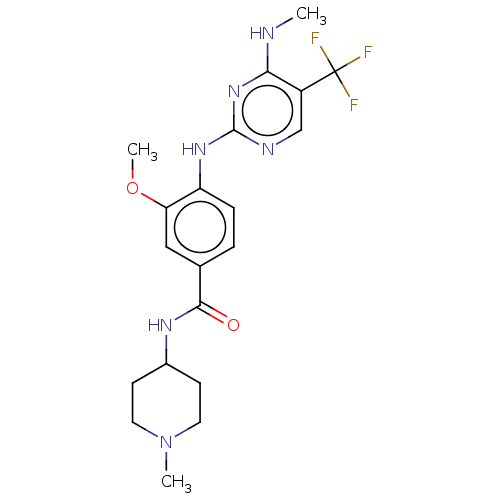
(US8802674, 282)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)NC2CCN(C)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-24-17-14(20(21,22)23)11-25-19(28-17)27-15-5-4-12(10-16(15)31-3)18(30)26-13-6-8-29(2)9-7-13/h4-5,10-11,13H,6-9H2,1-3H3,(H,26,30)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129199
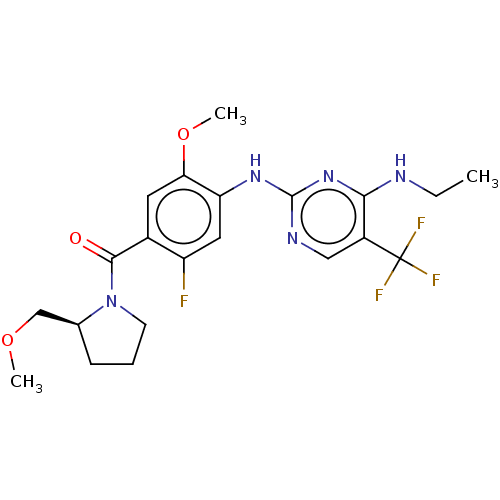
(US8802674, 306)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCC[C@H]2COC)ncc1C(F)(F)F |r| Show InChI InChI=1S/C21H25F4N5O3/c1-4-26-18-14(21(23,24)25)10-27-20(29-18)28-16-9-15(22)13(8-17(16)33-3)19(31)30-7-5-6-12(30)11-32-2/h8-10,12H,4-7,11H2,1-3H3,(H2,26,27,28,29)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448127
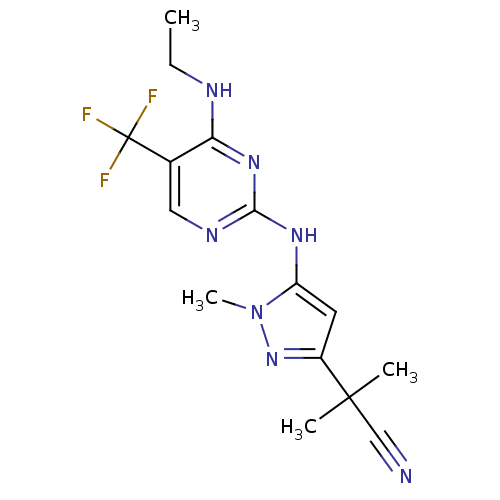
(CHEMBL3122119 | US9212173, 44)Show InChI InChI=1S/C15H18F3N7/c1-5-20-12-9(15(16,17)18)7-21-13(23-12)22-11-6-10(24-25(11)4)14(2,3)8-19/h6-7H,5H2,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448118

(CHEMBL3122113 | US10590114, No. 80 | US11111235, N...)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-24(23-8)13(2,3)7-18)21-12-20-5-9(14(15,16)17)11(19-4)22-12/h5-6H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448127

(CHEMBL3122119 | US9212173, 44)Show InChI InChI=1S/C15H18F3N7/c1-5-20-12-9(15(16,17)18)7-21-13(23-12)22-11-6-10(24-25(11)4)14(2,3)8-19/h6-7H,5H2,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582707

(CHEMBL5086513) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human GST tagged truncated LRRK2 G2019S mutant incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582712

(CHEMBL5090953) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129173
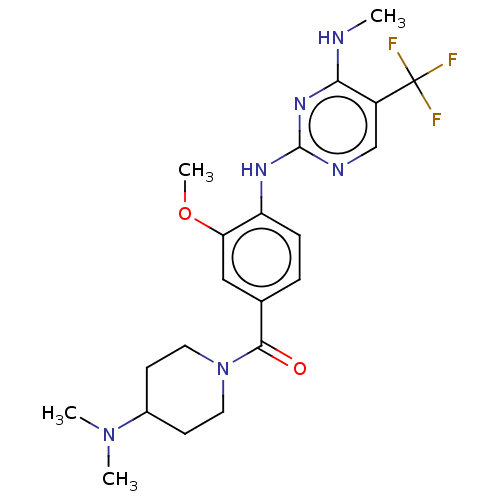
(US8802674, 276)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCC(CC2)N(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-25-18-15(21(22,23)24)12-26-20(28-18)27-16-6-5-13(11-17(16)32-4)19(31)30-9-7-14(8-10-30)29(2)3/h5-6,11-12,14H,7-10H2,1-4H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
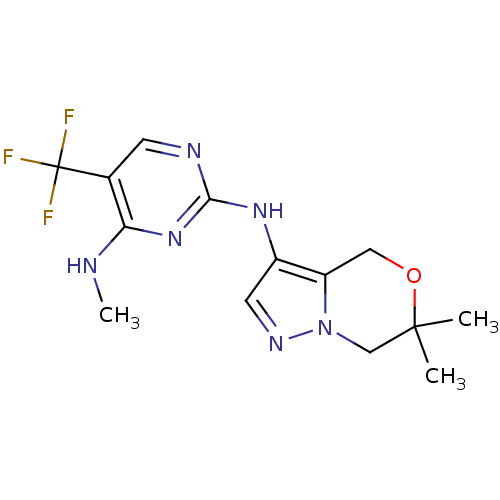
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448126
(CHEMBL3122105 | US9212186, 22)Show InChI InChI=1S/C14H17F3N6O/c1-13(2)7-23-10(6-24-13)9(5-20-23)21-12-19-4-8(14(15,16)17)11(18-3)22-12/h4-5H,6-7H2,1-3H3,(H2,18,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448126

(CHEMBL3122105 | US9212186, 22)Show InChI InChI=1S/C14H17F3N6O/c1-13(2)7-23-10(6-24-13)9(5-20-23)21-12-19-4-8(14(15,16)17)11(18-3)22-12/h4-5H,6-7H2,1-3H3,(H2,18,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212186 (2015)
BindingDB Entry DOI: 10.7270/Q2NS0SPX |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196742
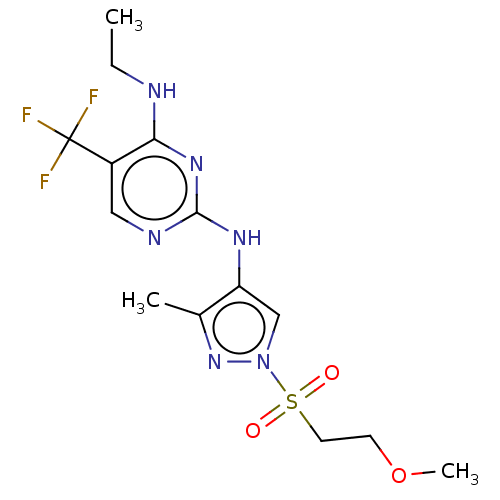
(US9212173, 20)Show SMILES CCNc1nc(Nc2cn(nc2C)S(=O)(=O)CCOC)ncc1C(F)(F)F Show InChI InChI=1S/C14H19F3N6O3S/c1-4-18-12-10(14(15,16)17)7-19-13(21-12)20-11-8-23(22-9(11)2)27(24,25)6-5-26-3/h7-8H,4-6H2,1-3H3,(H2,18,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196741
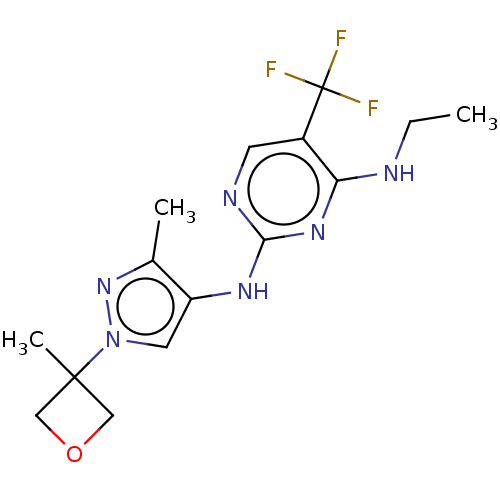
(US9212173, 19)Show InChI InChI=1S/C15H19F3N6O/c1-4-19-12-10(15(16,17)18)5-20-13(22-12)21-11-6-24(23-9(11)2)14(3)7-25-8-14/h5-6H,4,7-8H2,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.975 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127896
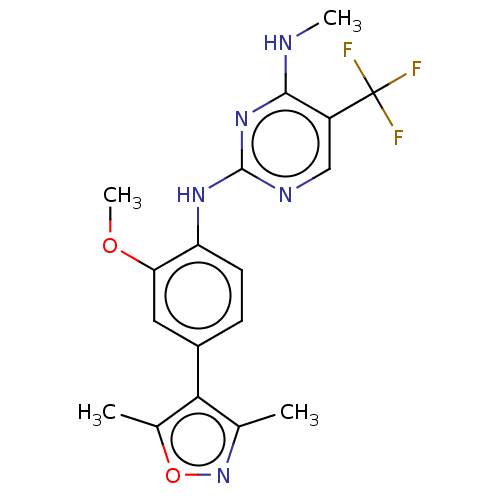
(US8791130, 26)Show SMILES CNc1nc(Nc2ccc(cc2OC)-c2c(C)noc2C)ncc1C(F)(F)F Show InChI InChI=1S/C18H18F3N5O2/c1-9-15(10(2)28-26-9)11-5-6-13(14(7-11)27-4)24-17-23-8-12(18(19,20)21)16(22-3)25-17/h5-8H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128274
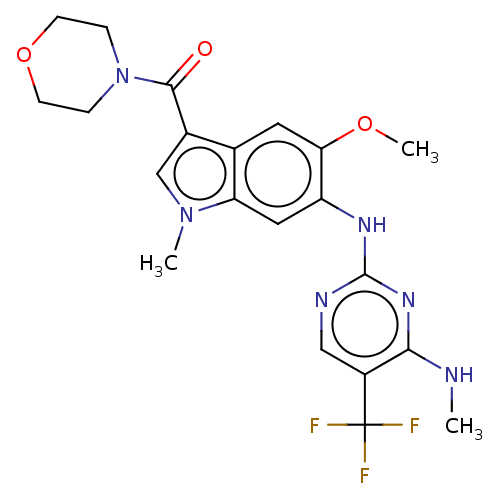
(US8796296, 30)Show SMILES CNc1nc(Nc2cc3n(C)cc(C(=O)N4CCOCC4)c3cc2OC)ncc1C(F)(F)F Show InChI InChI=1S/C21H23F3N6O3/c1-25-18-14(21(22,23)24)10-26-20(28-18)27-15-9-16-12(8-17(15)32-3)13(11-29(16)2)19(31)30-4-6-33-7-5-30/h8-11H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM182713
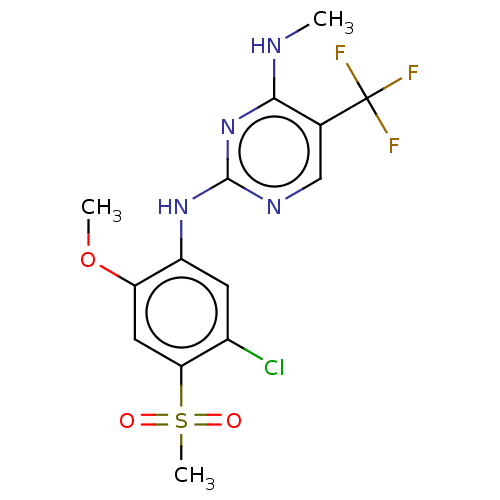
(US9145402, 20)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C14H14ClF3N4O3S/c1-19-12-7(14(16,17)18)6-20-13(22-12)21-9-4-8(15)11(26(3,23)24)5-10(9)25-2/h4-6H,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US9145402 (2015)
BindingDB Entry DOI: 10.7270/Q2J67FQ3 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128276
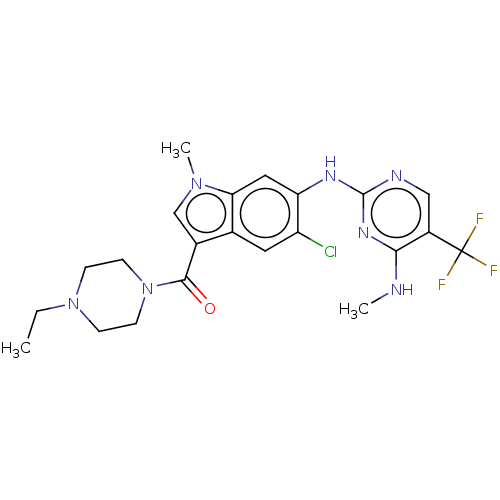
(US8796296, 32)Show SMILES CCN1CCN(CC1)C(=O)c1cn(C)c2cc(Nc3ncc(c(NC)n3)C(F)(F)F)c(Cl)cc12 Show InChI InChI=1S/C22H25ClF3N7O/c1-4-32-5-7-33(8-6-32)20(34)14-12-31(3)18-10-17(16(23)9-13(14)18)29-21-28-11-15(22(24,25)26)19(27-2)30-21/h9-12H,4-8H2,1-3H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 |
J Med Chem 55: 5536-45 (2012)
Article DOI: 10.1021/jm300452p
BindingDB Entry DOI: 10.7270/Q2RR20CQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150
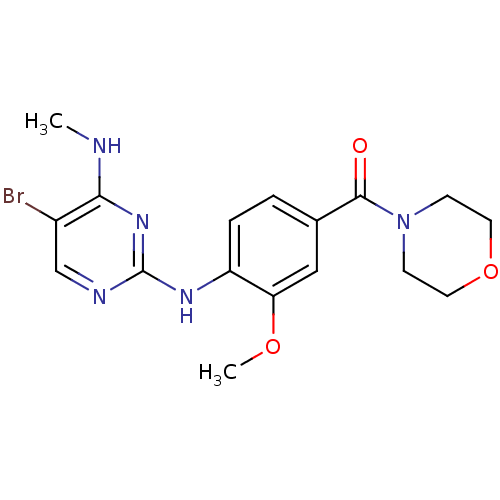
(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 |
J Med Chem 55: 5536-45 (2012)
Article DOI: 10.1021/jm300452p
BindingDB Entry DOI: 10.7270/Q2RR20CQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448117
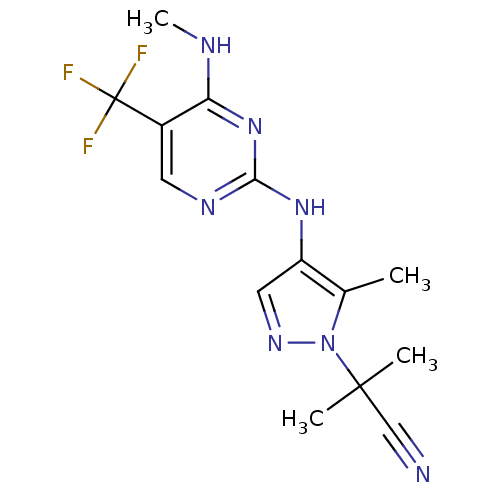
(CHEMBL3122114)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-21-24(8)13(2,3)7-18)22-12-20-5-9(14(15,16)17)11(19-4)23-12/h5-6H,1-4H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398662
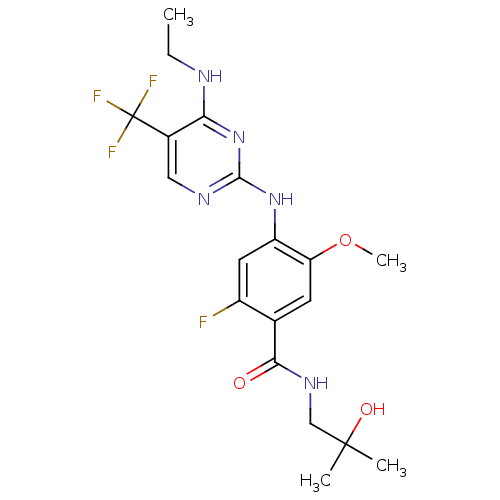
(CHEMBL2178140)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)NCC(C)(C)O)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-15-11(19(21,22)23)8-25-17(28-15)27-13-7-12(20)10(6-14(13)31-4)16(29)26-9-18(2,3)30/h6-8,30H,5,9H2,1-4H3,(H,26,29)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398667

(CHEMBL2178135)Show SMILES COc1cc(C(=O)N2CCOCC2)c(F)cc1Nc1ncc(c(NC2CC2)n1)C(F)(F)F Show InChI InChI=1S/C20H21F4N5O3/c1-31-16-8-12(18(30)29-4-6-32-7-5-29)14(21)9-15(16)27-19-25-10-13(20(22,23)24)17(28-19)26-11-2-3-11/h8-11H,2-7H2,1H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
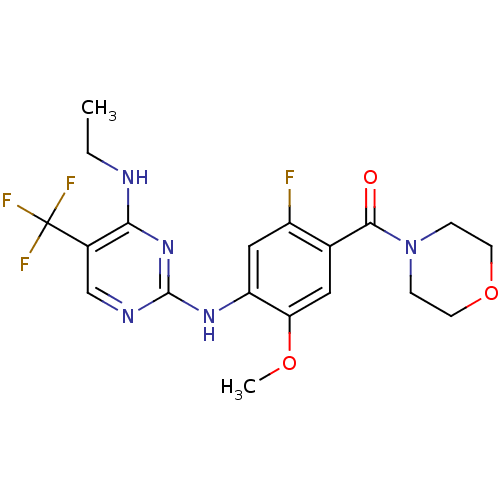
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398676
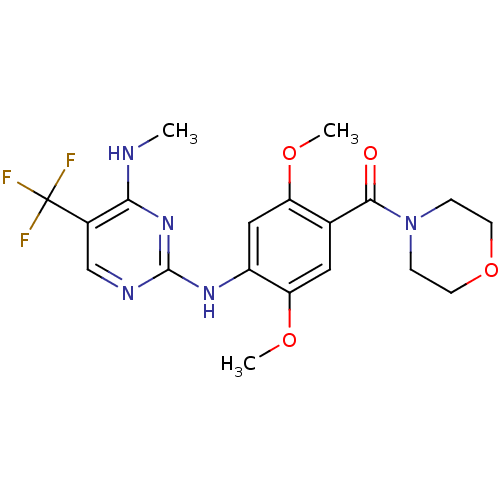
(CHEMBL2178125)Show SMILES CNc1nc(Nc2cc(OC)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O4/c1-23-16-12(19(20,21)22)10-24-18(26-16)25-13-9-14(29-2)11(8-15(13)30-3)17(28)27-4-6-31-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128275
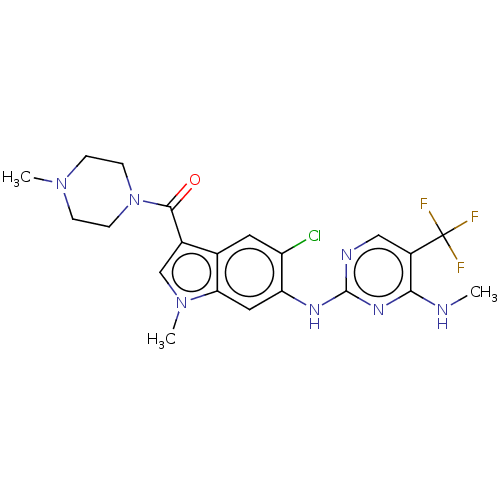
(US8796296, 31)Show SMILES CNc1nc(Nc2cc3n(C)cc(C(=O)N4CCN(C)CC4)c3cc2Cl)ncc1C(F)(F)F Show InChI InChI=1S/C21H23ClF3N7O/c1-26-18-14(21(23,24)25)10-27-20(29-18)28-16-9-17-12(8-15(16)22)13(11-31(17)3)19(33)32-6-4-30(2)5-7-32/h8-11H,4-7H2,1-3H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668

(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LRRK2 G2019S mutant (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582719

(CHEMBL5083563)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cn(C)nc3C)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582720

(CHEMBL5073900)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3c[nH]nc3C)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127911

(US8791130, 41)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)-c2cnn(C)c2C(=O)N(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C20H21F4N7O2/c1-25-17-12(20(22,23)24)9-26-19(29-17)28-14-7-13(21)10(6-15(14)33-5)11-8-27-31(4)16(11)18(32)30(2)3/h6-9H,1-5H3,(H2,25,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127915
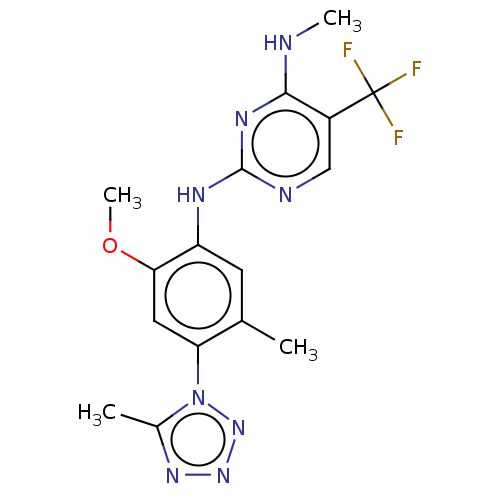
(US8791130, 45)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)-n2nnnc2C)ncc1C(F)(F)F Show InChI InChI=1S/C16H17F3N8O/c1-8-5-11(13(28-4)6-12(8)27-9(2)24-25-26-27)22-15-21-7-10(16(17,18)19)14(20-3)23-15/h5-7H,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127916
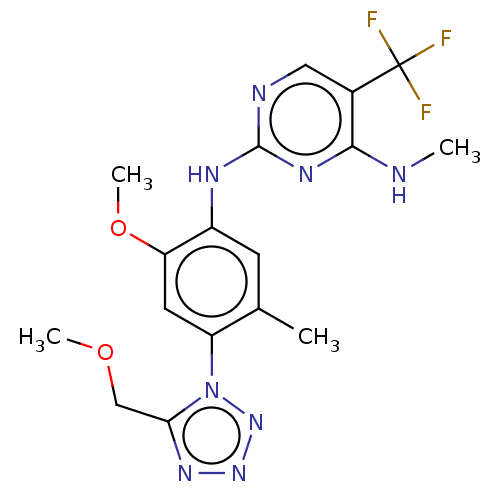
(US8791130, 46)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)-n2nnnc2COC)ncc1C(F)(F)F Show InChI InChI=1S/C17H19F3N8O2/c1-9-5-11(23-16-22-7-10(17(18,19)20)15(21-2)24-16)13(30-4)6-12(9)28-14(8-29-3)25-26-27-28/h5-7H,8H2,1-4H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data