

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204090 (CHEMBL3958859) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis | Eur J Med Chem 122: 326-338 (2016) Article DOI: 10.1016/j.ejmech.2016.06.036 BindingDB Entry DOI: 10.7270/Q2QJ7K8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50560609 (CHEMBL4749763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of electric eel AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by reciprocal Linew... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127477 BindingDB Entry DOI: 10.7270/Q2W099MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204086 (Avarol-3''-Thiosalicylate | CHEMBL238756) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis | Eur J Med Chem 122: 326-338 (2016) Article DOI: 10.1016/j.ejmech.2016.06.036 BindingDB Entry DOI: 10.7270/Q2QJ7K8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50557532 (CHEMBL4792421) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Electrophorus electricus AChE using varying levels of acetylthiocholine iodide as substrate preincubated for 15 mins follow... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116074 BindingDB Entry DOI: 10.7270/Q2HX1HBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204087 (CHEMBL3920392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis | Eur J Med Chem 122: 326-338 (2016) Article DOI: 10.1016/j.ejmech.2016.06.036 BindingDB Entry DOI: 10.7270/Q2QJ7K8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293451 (1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052204 (CHEMBL3318392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine iodide substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456708 (CHEMBL4204315) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456708 (CHEMBL4204315) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM26190 (1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... | Bioorg Med Chem 24: 2318-29 (2016) Article DOI: 10.1016/j.bmc.2016.04.002 BindingDB Entry DOI: 10.7270/Q2930W3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50124895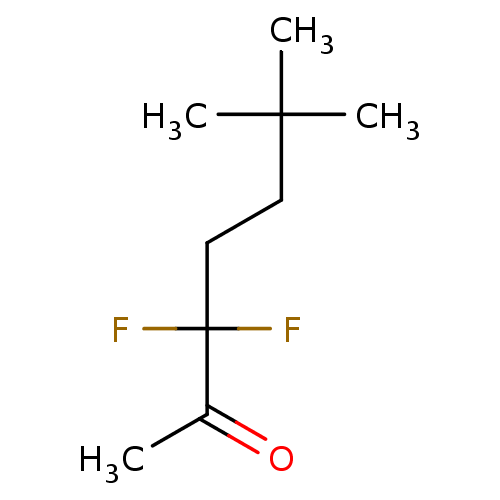 (CHEMBL3623568) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 25: 4405-11 (2015) Article DOI: 10.1016/j.bmcl.2015.09.019 BindingDB Entry DOI: 10.7270/Q2Z60QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016848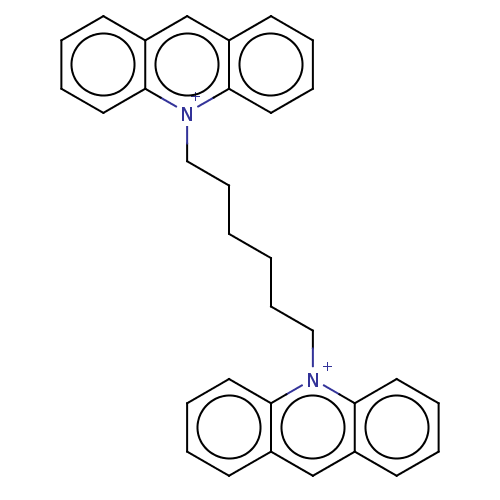 (CHEMBL3276408) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016848 (CHEMBL3276408) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456711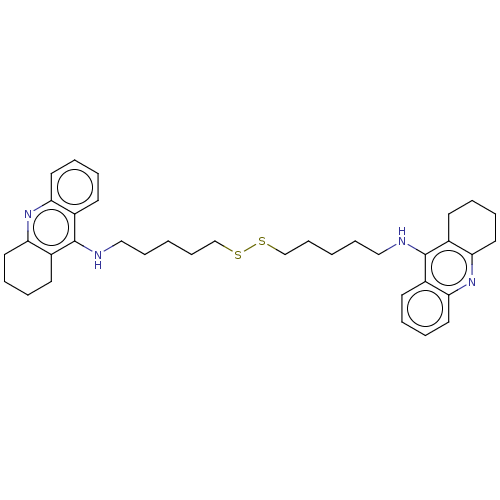 (CHEMBL4208641) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot anal... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13543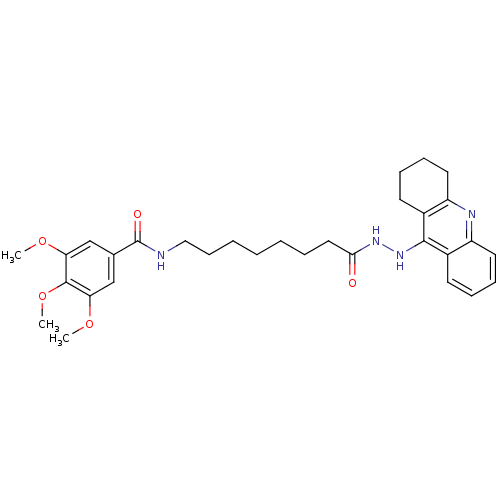 (3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.23 | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456702 (CHEMBL4213042) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456702 (CHEMBL4213042) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot | Eur J Med Chem 97: 181-9 (2015) Article DOI: 10.1016/j.ejmech.2015.04.055 BindingDB Entry DOI: 10.7270/Q2P84DM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070473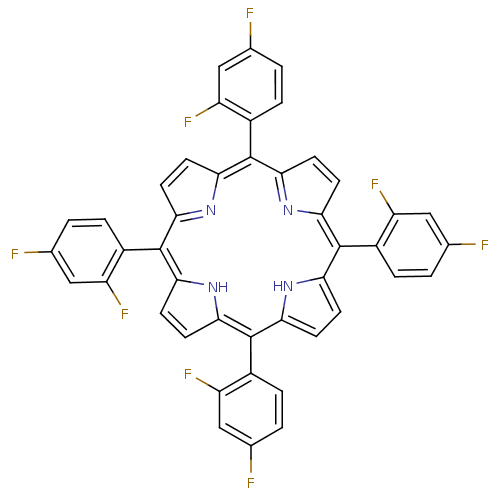 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,4-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taejon National University of Technology Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory constant for the electric eel Acetylcholinesterase (AChE) | Bioorg Med Chem Lett 8: 1467-70 (1999) BindingDB Entry DOI: 10.7270/Q21835ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340109 (CHEMBL1762827 | rac-4-[(13-Amino-10,11,12,14-tetra...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Mixed inhibition of Electrophorus electricus acetylcholinesterase using acetylcholine as substrate by Ellman's method | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070476 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,6-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456711 (CHEMBL4208641) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456713 (CHEMBL4214235) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016849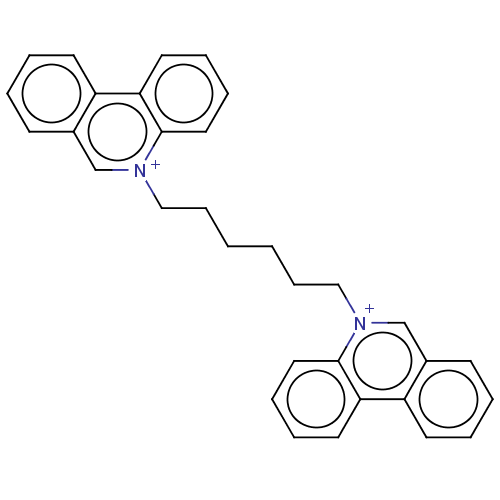 (CHEMBL3276410) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016849 (CHEMBL3276410) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50458333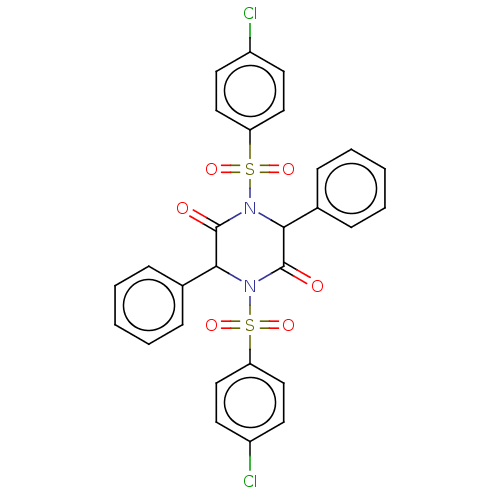 (CHEMBL4210820) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition b... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456712 (CHEMBL4204015) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456712 (CHEMBL4204015) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456710 (CHEMBL4213722) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456706 (CHEMBL4217176) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-B... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456701 (CHEMBL4218651) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456701 (CHEMBL4218651) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457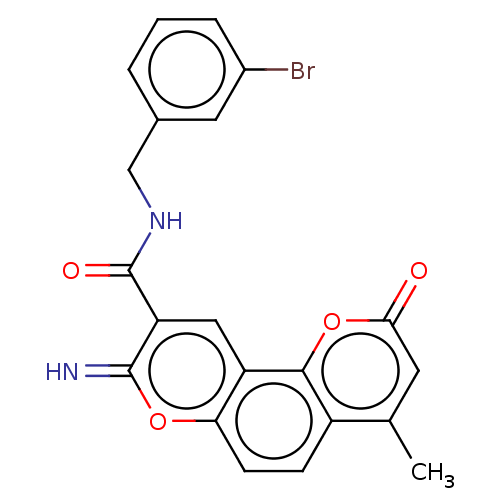 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as free enzyme preincubated for 5 mins followed by su... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for in vitro inhibition of acetylcholinesterase | Bioorg Med Chem Lett 5: 1131-1132 (1995) Article DOI: 10.1016/0960-894X(95)00179-W BindingDB Entry DOI: 10.7270/Q2W37WTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016850 (CHEMBL3276411) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070473 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,4-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234829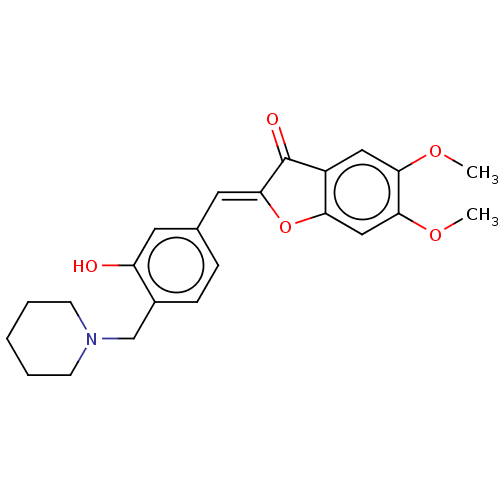 (CHEMBL4100760) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Mixed-type inhibition of Electrophorus electricus AChE pretreated for 15 mins followed by varying levels of acetylthiocholine iodide substrate additi... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070476 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,6-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taejon National University of Technology Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory constant for the electric eel Acetylcholinesterase (AChE) | Bioorg Med Chem Lett 8: 1467-70 (1999) BindingDB Entry DOI: 10.7270/Q21835ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457028 (CHEMBL4218303) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measur... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50185007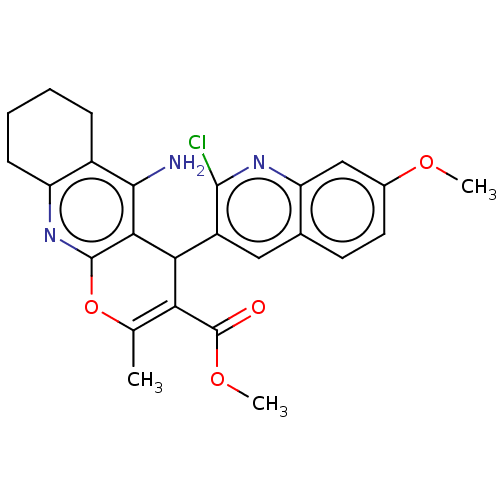 (CHEMBL3822686) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Complutense Madrid (UCM) Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 15 mins followed by substrate addition by Dixon plot... | Eur J Med Chem 118: 178-92 (2016) Article DOI: 10.1016/j.ejmech.2016.04.023 BindingDB Entry DOI: 10.7270/Q25H7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456714 (CHEMBL4217969) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456714 (CHEMBL4217969) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433313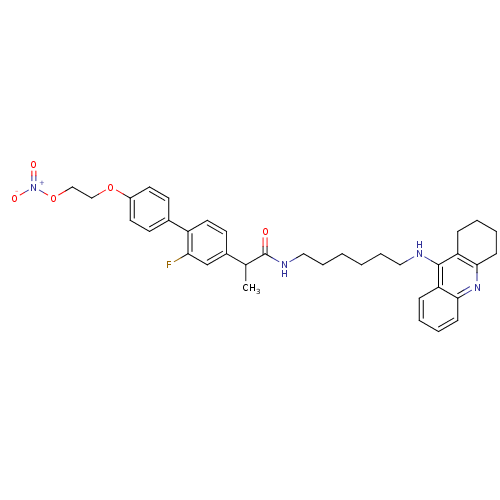 (CHEMBL2376474) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE by Lineweaver Burk reciprocal plot analysis in presence of acetylcholine | Bioorg Med Chem 21: 2462-70 (2013) Article DOI: 10.1016/j.bmc.2013.03.005 BindingDB Entry DOI: 10.7270/Q2319X82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443370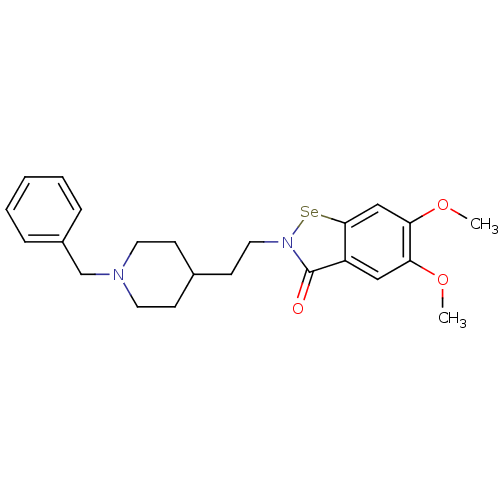 (CHEMBL3086276) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition by ... | J Med Chem 56: 9089-99 (2013) Article DOI: 10.1021/jm401047q BindingDB Entry DOI: 10.7270/Q21R6RZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50510681 (CHEMBL4451509) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zanjan Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition and measured after 15 mins by ... | Bioorg Med Chem 27: 2914-2922 (2019) Article DOI: 10.1016/j.bmc.2019.05.023 BindingDB Entry DOI: 10.7270/Q22F7RRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50439497 (CHEMBL2418125) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine chloride as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5830-40 (2013) Article DOI: 10.1016/j.bmc.2013.07.011 BindingDB Entry DOI: 10.7270/Q2VD70X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456703 (CHEMBL4209181) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate complex preincubated for 5 mins f... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 647 total ) | Next | Last >> |