 Found 10 hits of ki data for polymerid = 3678
Found 10 hits of ki data for polymerid = 3678 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50395272
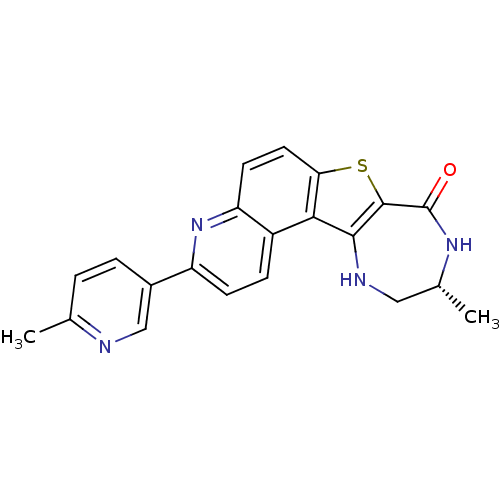
(CHEMBL1231206)Show SMILES C[C@@H]1CNc2c(sc3ccc4nc(ccc4c23)-c2ccc(C)nc2)C(=O)N1 |r| Show InChI InChI=1S/C21H18N4OS/c1-11-3-4-13(10-22-11)15-6-5-14-16(25-15)7-8-17-18(14)19-20(27-17)21(26)24-12(2)9-23-19/h3-8,10,12,23H,9H2,1-2H3,(H,24,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MK2 (45 to 400 residues) (unknown origin) using fluorescein isothiocyanate-KKKALSRQLSVAA as substrate |
Nat Rev Drug Discov 16: 424-440 (2017)
Article DOI: 10.1038/nrd.2016.266
BindingDB Entry DOI: 10.7270/Q2125VNC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50323635
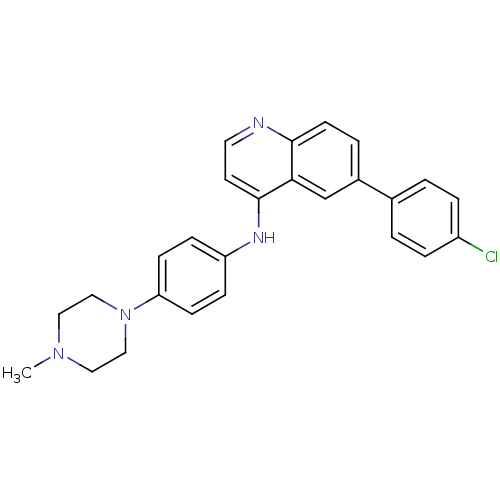
(6-(4-chlorophenyl)-N-(4-(4-methylpiperazin-1-yl)ph...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ccnc3ccc(cc23)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H25ClN4/c1-30-14-16-31(17-15-30)23-9-7-22(8-10-23)29-26-12-13-28-25-11-4-20(18-24(25)26)19-2-5-21(27)6-3-19/h2-13,18H,14-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Lund
Curated by ChEMBL
| Assay Description
Competitive inhibition of MK2 ATP-binding site |
Bioorg Med Chem Lett 20: 4738-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.107
BindingDB Entry DOI: 10.7270/Q2HX1CVQ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50323635

(6-(4-chlorophenyl)-N-(4-(4-methylpiperazin-1-yl)ph...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ccnc3ccc(cc23)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H25ClN4/c1-30-14-16-31(17-15-30)23-9-7-22(8-10-23)29-26-12-13-28-25-11-4-20(18-24(25)26)19-2-5-21(27)6-3-19/h2-13,18H,14-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Lund
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of MK2 ATP-binding site |
Bioorg Med Chem Lett 20: 4738-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.107
BindingDB Entry DOI: 10.7270/Q2HX1CVQ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50323635

(6-(4-chlorophenyl)-N-(4-(4-methylpiperazin-1-yl)ph...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ccnc3ccc(cc23)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H25ClN4/c1-30-14-16-31(17-15-30)23-9-7-22(8-10-23)29-26-12-13-28-25-11-4-20(18-24(25)26)19-2-5-21(27)6-3-19/h2-13,18H,14-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Lund
Curated by ChEMBL
| Assay Description
Mixed inhibition of MK2 ATP-binding site |
Bioorg Med Chem Lett 20: 4738-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.107
BindingDB Entry DOI: 10.7270/Q2HX1CVQ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50411445
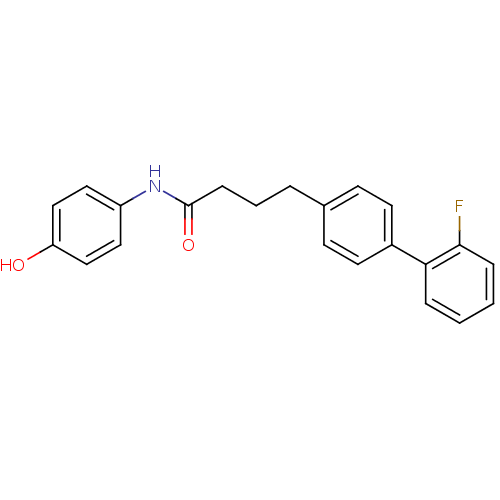
(CHEMBL235658)Show InChI InChI=1S/C22H20FNO2/c23-21-6-2-1-5-20(21)17-10-8-16(9-11-17)4-3-7-22(26)24-18-12-14-19(25)15-13-18/h1-2,5-6,8-15,25H,3-4,7H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of MK2 in human TERT immortalised HCA2 cells assessed as inhibition of anisomycin-induced HSP27 phosphorylation at 2.5 uM by ELISA |
Bioorg Med Chem Lett 17: 6832-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.036
BindingDB Entry DOI: 10.7270/Q2W095P4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50323635

(6-(4-chlorophenyl)-N-(4-(4-methylpiperazin-1-yl)ph...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ccnc3ccc(cc23)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H25ClN4/c1-30-14-16-31(17-15-30)23-9-7-22(8-10-23)29-26-12-13-28-25-11-4-20(18-24(25)26)19-2-5-21(27)6-3-19/h2-13,18H,14-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Lund
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of MK2 ATP-binding site |
Bioorg Med Chem Lett 20: 4738-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.107
BindingDB Entry DOI: 10.7270/Q2HX1CVQ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MAPKAPK2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50463483
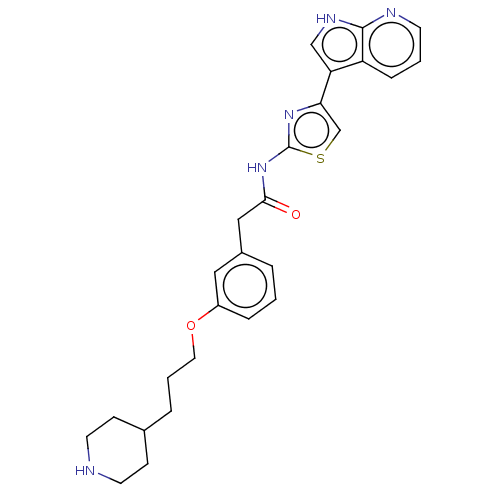
(CHEMBL4245242)Show SMILES O=C(Cc1cccc(OCCCC2CCNCC2)c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C26H29N5O2S/c32-24(31-26-30-23(17-34-26)22-16-29-25-21(22)7-2-10-28-25)15-19-4-1-6-20(14-19)33-13-3-5-18-8-11-27-12-9-18/h1-2,4,6-7,10,14,16-18,27H,3,5,8-9,11-13,15H2,(H,28,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MAPKAPK2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50224883
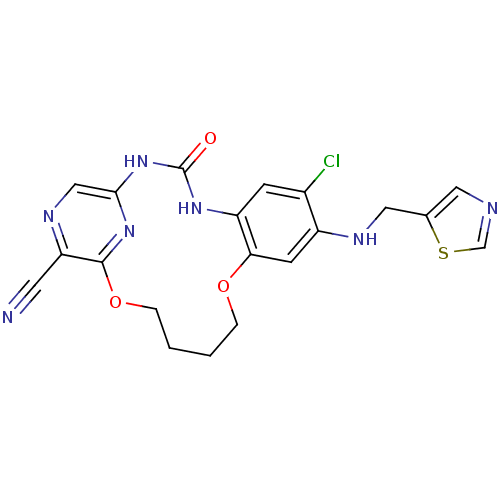
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50395262

(CHEMBL2163999)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1cc(Nc2ccc(F)c(Cl)c2)n2nccc2n1 |r,wU:4.7,wD:1.0,(19.34,-11.51,;18,-12.28,;16.67,-11.51,;15.33,-12.28,;15.33,-13.82,;16.67,-14.59,;18,-13.82,;14,-14.59,;12.67,-13.82,;12.67,-12.28,;11.33,-11.51,;11.33,-9.97,;12.67,-9.2,;12.67,-7.66,;14,-6.89,;15.33,-7.66,;16.67,-6.89,;15.33,-9.2,;16.67,-9.97,;14,-9.97,;10,-12.28,;8.54,-11.8,;7.63,-13.05,;8.54,-14.29,;10,-13.82,;11.33,-14.59,)| Show InChI InChI=1S/C18H20ClFN6/c19-14-9-13(5-6-15(14)20)24-18-10-16(25-17-7-8-22-26(17)18)23-12-3-1-11(21)2-4-12/h5-12,24H,1-4,21H2,(H,23,25)/t11-,12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Teijin Pharma Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of MAPKAP-K2 using KKLNRTLSVA as substrate and [33P]-gamma-ATP by Lineweaver-Burke plot analysis |
J Med Chem 55: 6700-15 (2012)
Article DOI: 10.1021/jm300411k
BindingDB Entry DOI: 10.7270/Q20K29PX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data