 Found 224 hits of ki data for polymerid = 4064
Found 224 hits of ki data for polymerid = 4064 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034585
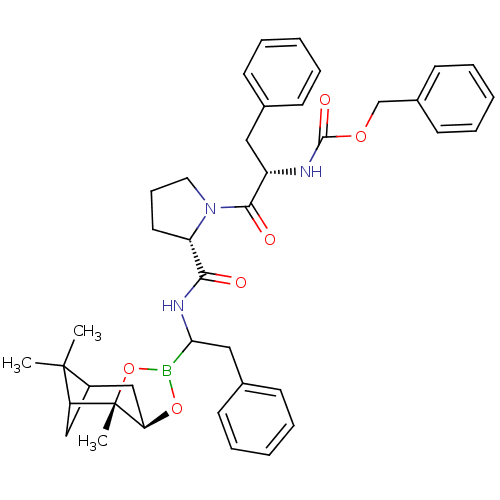
(CHEMBL285285 | Peptide boronate)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)C(Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C40H48BN3O6/c1-39(2)30-24-33(39)40(3)34(25-30)49-41(50-40)35(23-28-16-9-5-10-17-28)43-36(45)32-20-13-21-44(32)37(46)31(22-27-14-7-4-8-15-27)42-38(47)48-26-29-18-11-6-12-19-29/h4-12,14-19,30-35H,13,20-26H2,1-3H3,(H,42,47)(H,43,45)/t30?,31-,32-,33?,34+,35?,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034580
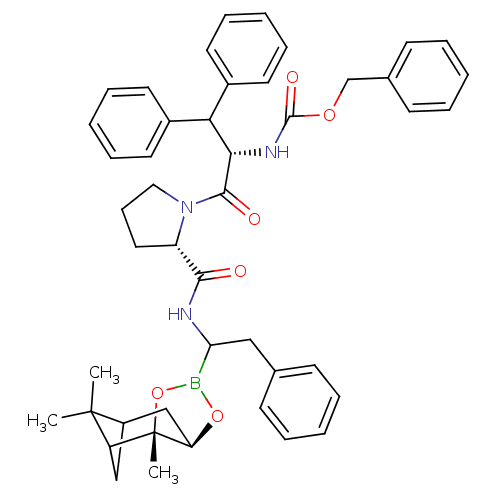
(CHEMBL418050 | Peptide boronate)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)C(Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C46H52BN3O6/c1-45(2)35-28-37(45)46(3)38(29-35)55-47(56-46)39(27-31-17-8-4-9-18-31)48-42(51)36-25-16-26-50(36)43(52)41(49-44(53)54-30-32-19-10-5-11-20-32)40(33-21-12-6-13-22-33)34-23-14-7-15-24-34/h4-15,17-24,35-41H,16,25-30H2,1-3H3,(H,48,51)(H,49,53)/t35?,36-,37?,38+,39?,41-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034577
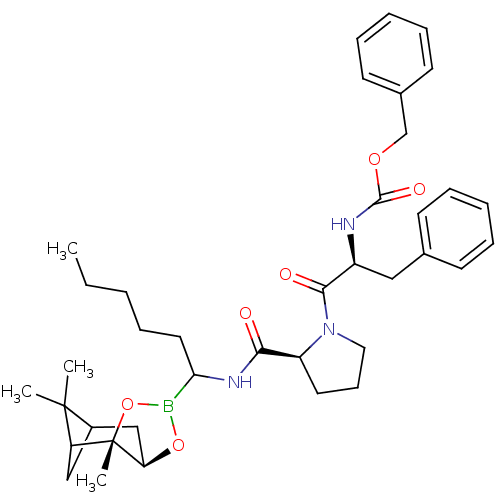
(CHEMBL291026 | Peptide boronate)Show SMILES CCCCCC(NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C38H52BN3O6/c1-5-6-9-20-33(39-47-32-24-28-23-31(37(28,2)3)38(32,4)48-39)41-34(43)30-19-14-21-42(30)35(44)29(22-26-15-10-7-11-16-26)40-36(45)46-25-27-17-12-8-13-18-27/h7-8,10-13,15-18,28-33H,5-6,9,14,19-25H2,1-4H3,(H,40,45)(H,41,43)/t28?,29-,30-,31?,32+,33?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034579
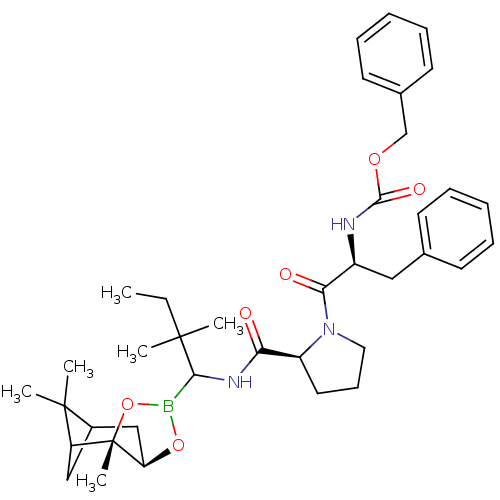
(CHEMBL290577 | Peptide boronate)Show SMILES CCC(C)(C)C(NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C38H52BN3O6/c1-7-36(2,3)34(39-47-31-23-27-22-30(37(27,4)5)38(31,6)48-39)41-32(43)29-19-14-20-42(29)33(44)28(21-25-15-10-8-11-16-25)40-35(45)46-24-26-17-12-9-13-18-26/h8-13,15-18,27-31,34H,7,14,19-24H2,1-6H3,(H,40,45)(H,41,43)/t27?,28-,29-,30?,31+,34?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034582
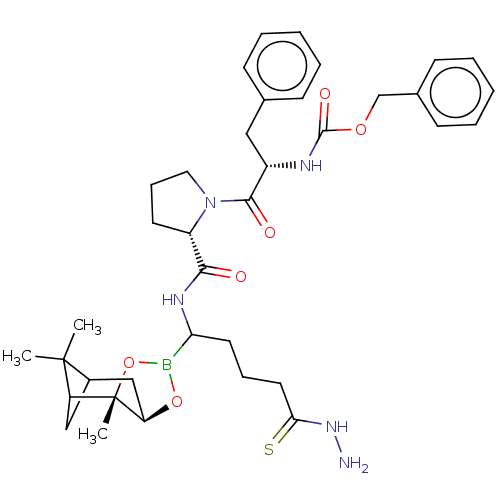
(CHEMBL2448441 | Peptide boronate)Show SMILES Br.[H][C@@]12CC3CC(C3(C)C)[C@]1(C)OB(O2)C(CCC\C(S)=N\N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |TLB:11:10:7:5,THB:12:10:7:5,14:2:7:5| Show InChI InChI=1S/C37H50BN5O6S.BrH/c1-36(2)26-21-29(36)37(3)30(22-26)48-38(49-37)31(17-10-18-32(50)42-39)41-33(44)28-16-11-19-43(28)34(45)27(20-24-12-6-4-7-13-24)40-35(46)47-23-25-14-8-5-9-15-25;/h4-9,12-15,26-31H,10-11,16-23,39H2,1-3H3,(H,40,46)(H,41,44)(H,42,50);1H/t26?,27-,28-,29?,30+,31?,37-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.848 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034583
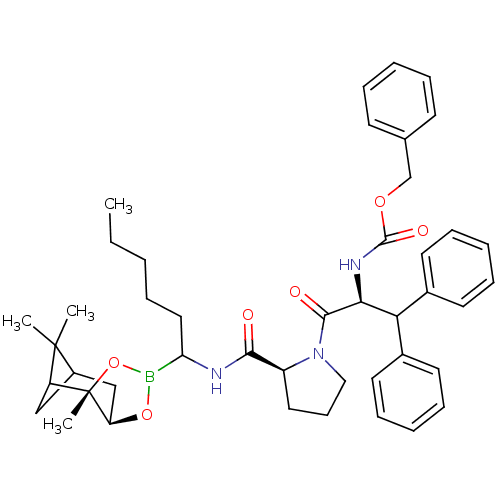
(CHEMBL287918 | Peptide boronate)Show SMILES CCCCCC(NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(c1ccccc1)c1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C44H56BN3O6/c1-5-6-10-25-37(45-53-36-28-33-27-35(43(33,2)3)44(36,4)54-45)46-40(49)34-24-17-26-48(34)41(50)39(47-42(51)52-29-30-18-11-7-12-19-30)38(31-20-13-8-14-21-31)32-22-15-9-16-23-32/h7-9,11-16,18-23,33-39H,5-6,10,17,24-29H2,1-4H3,(H,46,49)(H,47,51)/t33?,34-,35?,36+,37?,39-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034574
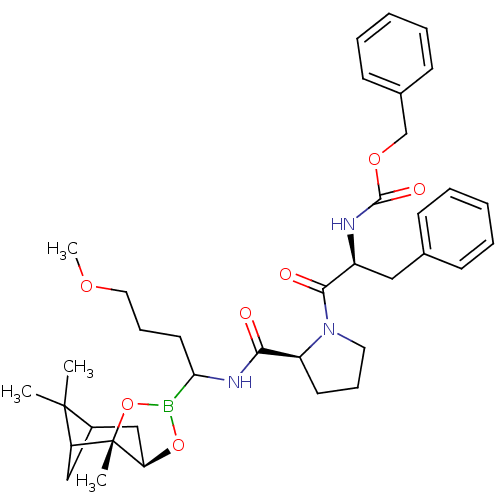
(CHEMBL288150 | Peptide boronate)Show SMILES COCCCC(NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C37H50BN3O7/c1-36(2)27-22-30(36)37(3)31(23-27)47-38(48-37)32(18-12-20-45-4)40-33(42)29-17-11-19-41(29)34(43)28(21-25-13-7-5-8-14-25)39-35(44)46-24-26-15-9-6-10-16-26/h5-10,13-16,27-32H,11-12,17-24H2,1-4H3,(H,39,44)(H,40,42)/t27?,28-,29-,30?,31+,32?,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034576
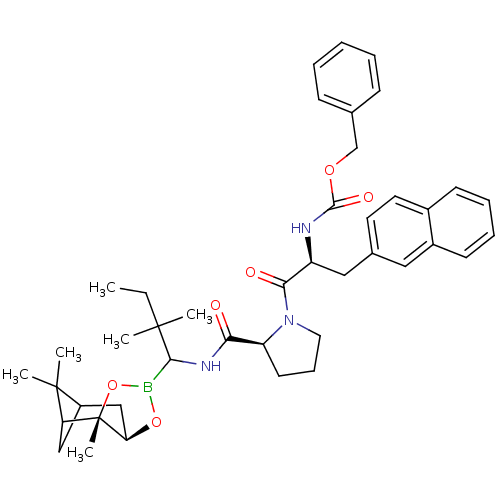
(CHEMBL288176 | Peptide boronate)Show SMILES CCC(C)(C)C(NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)OCc1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C42H54BN3O6/c1-7-40(2,3)38(43-51-35-25-31-24-34(41(31,4)5)42(35,6)52-43)45-36(47)33-18-13-21-46(33)37(48)32(44-39(49)50-26-27-14-9-8-10-15-27)23-28-19-20-29-16-11-12-17-30(29)22-28/h8-12,14-17,19-20,22,31-35,38H,7,13,18,21,23-26H2,1-6H3,(H,44,49)(H,45,47)/t31?,32-,33-,34?,35+,38?,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034573
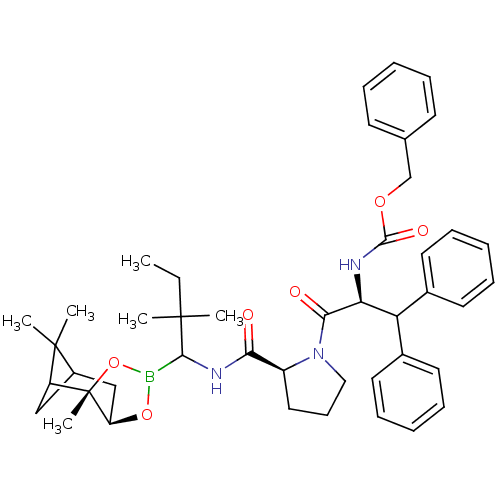
(CHEMBL291261 | Peptide boronate)Show SMILES CCC(C)(C)C(NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(c1ccccc1)c1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C44H56BN3O6/c1-7-42(2,3)40(45-53-35-27-32-26-34(43(32,4)5)44(35,6)54-45)47-38(49)33-24-17-25-48(33)39(50)37(46-41(51)52-28-29-18-11-8-12-19-29)36(30-20-13-9-14-21-30)31-22-15-10-16-23-31/h8-16,18-23,32-37,40H,7,17,24-28H2,1-6H3,(H,46,51)(H,47,49)/t32?,33-,34?,35+,37-,40?,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50034581

(CHEMBL36744 | Peptide boronate)Show SMILES COCCCC(NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(c1ccccc1)c1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C43H54BN3O7/c1-42(2)32-26-34(42)43(3)35(27-32)53-44(54-43)36(23-15-25-51-4)45-39(48)33-22-14-24-47(33)40(49)38(46-41(50)52-28-29-16-8-5-9-17-29)37(30-18-10-6-11-19-30)31-20-12-7-13-21-31/h5-13,16-21,32-38H,14-15,22-28H2,1-4H3,(H,45,48)(H,46,50)/t32?,33-,34?,35+,36?,38-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Chymotrypsin |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50073123
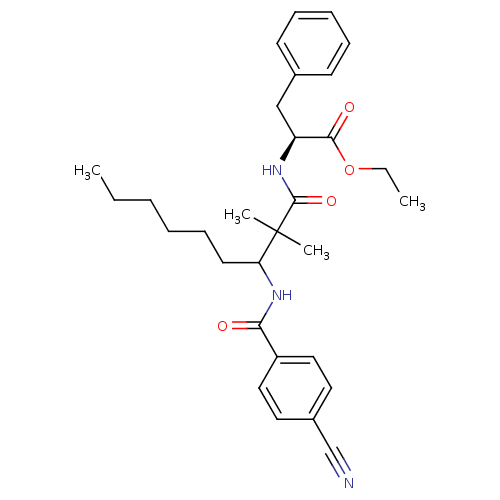
((S)-2-[3-(4-Cyano-benzoylamino)-2,2-dimethyl-nonan...)Show SMILES CCCCCCC(NC(=O)c1ccc(cc1)C#N)C(C)(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OCC Show InChI InChI=1S/C30H39N3O4/c1-5-7-8-12-15-26(33-27(34)24-18-16-23(21-31)17-19-24)30(3,4)29(36)32-25(28(35)37-6-2)20-22-13-10-9-11-14-22/h9-11,13-14,16-19,25-26H,5-8,12,15,20H2,1-4H3,(H,32,36)(H,33,34)/t25-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for inactivation of bovine alpha-Chymotrypsin Kinetic constant(Ki) |
J Med Chem 42: 312-23 (1999)
Article DOI: 10.1021/jm980562h
BindingDB Entry DOI: 10.7270/Q2930SBF |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50068892

(3-(4-{[(S)-1-((S)-2-Acetylamino-3-methyl-butyryl)-...)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C30H34F2N4O7/c1-17(2)24(33-18(3)37)27(40)36-14-8-13-23(36)26(39)35-22(15-19-9-5-4-6-10-19)25(38)30(31,32)29(43)34-21-12-7-11-20(16-21)28(41)42/h4-7,9-12,16-17,22-24H,8,13-15H2,1-3H3,(H,33,37)(H,34,43)(H,35,39)(H,41,42)/t22?,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Chymotrypsinogen |
Bioorg Med Chem Lett 8: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GQ6WXB |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50068901
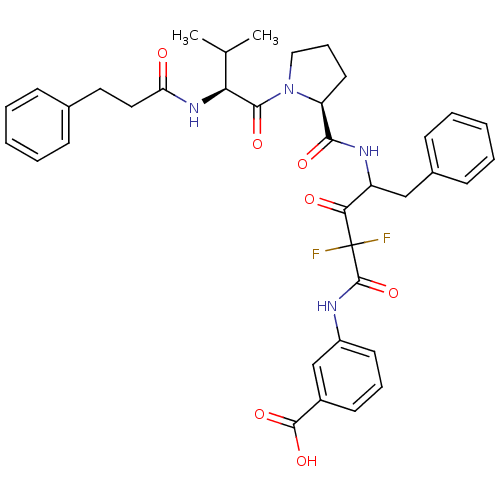
(3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C37H40F2N4O7/c1-23(2)31(42-30(44)19-18-24-11-5-3-6-12-24)34(47)43-20-10-17-29(43)33(46)41-28(21-25-13-7-4-8-14-25)32(45)37(38,39)36(50)40-27-16-9-15-26(22-27)35(48)49/h3-9,11-16,22-23,28-29,31H,10,17-21H2,1-2H3,(H,40,50)(H,41,46)(H,42,44)(H,48,49)/t28?,29-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Chymotrypsinogen |
Bioorg Med Chem Lett 8: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GQ6WXB |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098842
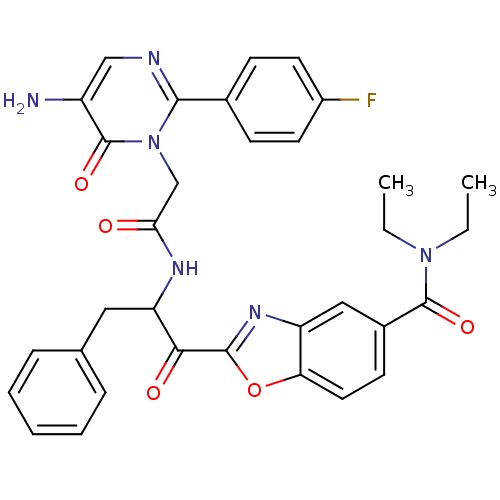
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES CCN(CC)C(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C33H31FN6O5/c1-3-39(4-2)32(43)22-12-15-27-25(17-22)38-31(45-27)29(42)26(16-20-8-6-5-7-9-20)37-28(41)19-40-30(36-18-24(35)33(40)44)21-10-13-23(34)14-11-21/h5-15,17-18,26H,3-4,16,19,35H2,1-2H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098862

(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES CCNC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C31H27FN6O5/c1-2-34-29(41)20-10-13-25-23(15-20)37-30(43-25)27(40)24(14-18-6-4-3-5-7-18)36-26(39)17-38-28(35-16-22(33)31(38)42)19-8-11-21(32)12-9-19/h3-13,15-16,24H,2,14,17,33H2,1H3,(H,34,41)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM26142
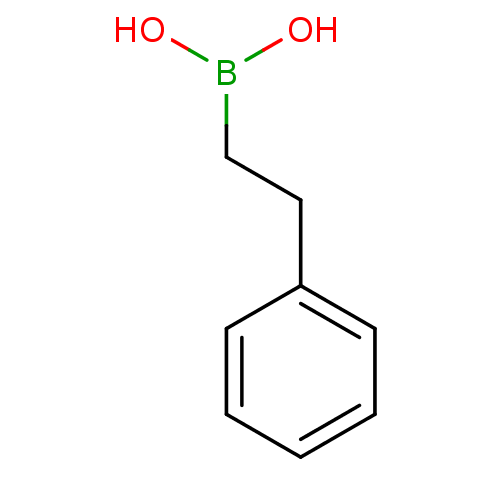
((2-phenylethyl)boranediol | Alkylboronic Acid, 21)Show InChI InChI=1S/C8H11BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-5,10-11H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of the compound was determined against Chymotrypsinogen |
Bioorg Med Chem Lett 2: 1391-1394 (1992)
Article DOI: 10.1016/S0960-894X(00)80519-3
BindingDB Entry DOI: 10.7270/Q23T9H47 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098839
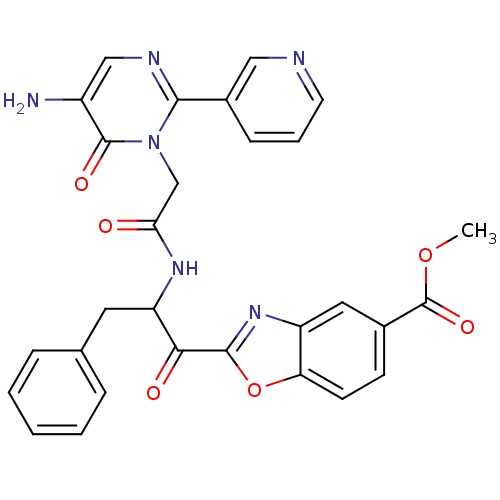
(2-{2-[2-(5-Amino-6-oxo-2-pyridin-3-yl-6H-pyrimidin...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccnc1 Show InChI InChI=1S/C29H24N6O6/c1-40-29(39)18-9-10-23-21(13-18)34-27(41-23)25(37)22(12-17-6-3-2-4-7-17)33-24(36)16-35-26(19-8-5-11-31-14-19)32-15-20(30)28(35)38/h2-11,13-15,22H,12,16,30H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098854

(2-{2-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccccc1 Show InChI InChI=1S/C30H25N5O6/c1-40-30(39)20-12-13-24-22(15-20)34-28(41-24)26(37)23(14-18-8-4-2-5-9-18)33-25(36)17-35-27(19-10-6-3-7-11-19)32-16-21(31)29(35)38/h2-13,15-16,23H,14,17,31H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 46.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098838

(2-{2-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C31H27N5O6/c1-18-7-6-10-20(13-18)28-33-16-22(32)30(39)36(28)17-26(37)34-24(14-19-8-4-3-5-9-19)27(38)29-35-23-15-21(31(40)41-2)11-12-25(23)42-29/h3-13,15-16,24H,14,17,32H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098856
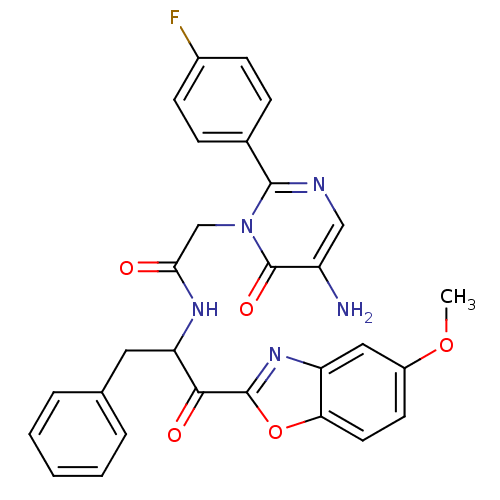
(2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...)Show SMILES COc1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C29H24FN5O5/c1-39-20-11-12-24-22(14-20)34-28(40-24)26(37)23(13-17-5-3-2-4-6-17)33-25(36)16-35-27(32-15-21(31)29(35)38)18-7-9-19(30)10-8-18/h2-12,14-15,23H,13,16,31H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098847
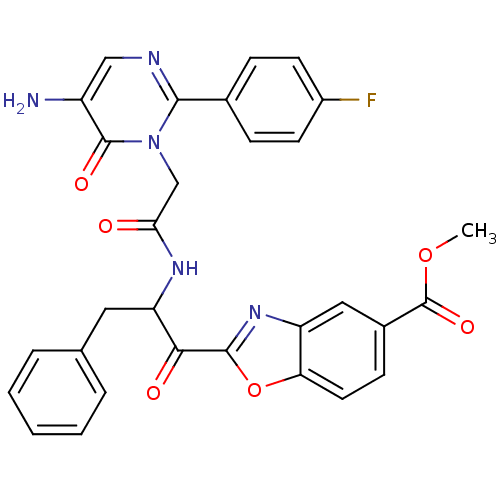
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C30H24FN5O6/c1-41-30(40)19-9-12-24-22(14-19)35-28(42-24)26(38)23(13-17-5-3-2-4-6-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-7-10-20(31)11-8-18/h2-12,14-15,23H,13,16,32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 52.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098858
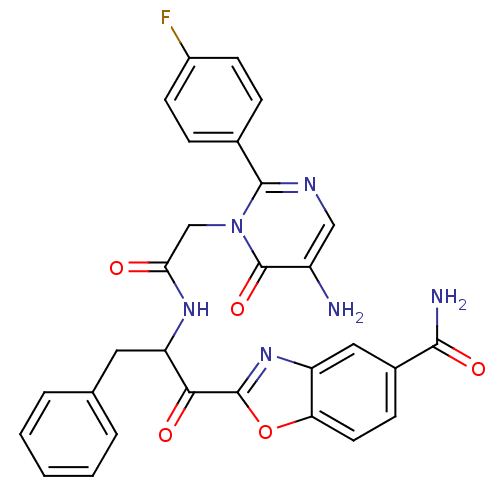
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES NC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C29H23FN6O5/c30-19-9-6-17(7-10-19)27-33-14-20(31)29(40)36(27)15-24(37)34-22(12-16-4-2-1-3-5-16)25(38)28-35-21-13-18(26(32)39)8-11-23(21)41-28/h1-11,13-14,22H,12,15,31H2,(H2,32,39)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 53.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50014579

(3-(2-Benzyloxycarbonylamino-3-methyl-butyrylamino)...)Show SMILES CCOC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C25H30N2O6/c1-4-32-24(30)22(28)20(15-18-11-7-5-8-12-18)26-23(29)21(17(2)3)27-25(31)33-16-19-13-9-6-10-14-19/h5-14,17,20-21H,4,15-16H2,1-3H3,(H,26,29)(H,27,31)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of alpha-chymotrypsin |
J Med Chem 33: 11-3 (1990)
BindingDB Entry DOI: 10.7270/Q2K93847 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50014578
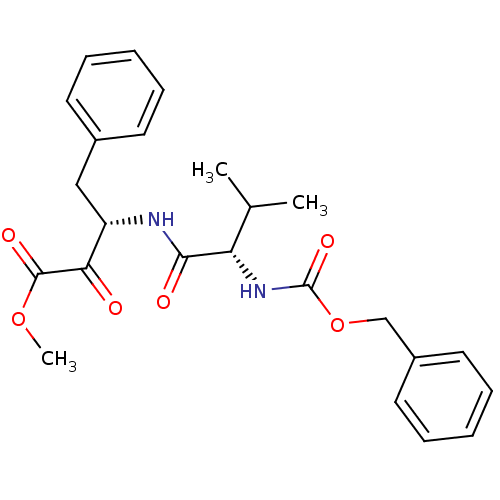
(3-(2-Benzyloxycarbonylamino-3-methyl-butyrylamino)...)Show SMILES COC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C24H28N2O6/c1-16(2)20(26-24(30)32-15-18-12-8-5-9-13-18)22(28)25-19(21(27)23(29)31-3)14-17-10-6-4-7-11-17/h4-13,16,19-20H,14-15H2,1-3H3,(H,25,28)(H,26,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of alpha-chymotrypsin |
J Med Chem 33: 11-3 (1990)
BindingDB Entry DOI: 10.7270/Q2K93847 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098855

(CHEMBL23870 | N-[2-(5-Amino-benzooxazol-2-yl)-1-be...)Show SMILES Nc1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C28H23FN6O4/c29-18-8-6-17(7-9-18)26-32-14-20(31)28(38)35(26)15-24(36)33-22(12-16-4-2-1-3-5-16)25(37)27-34-21-13-19(30)10-11-23(21)39-27/h1-11,13-14,22H,12,15,30-31H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098857
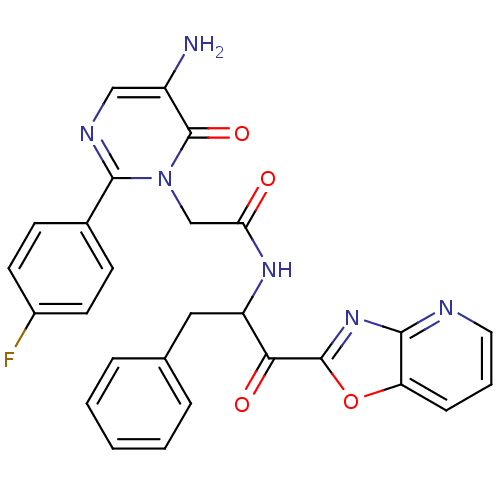
(2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...)Show SMILES Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(Cc2ccccc2)C(=O)c2nc3ncccc3o2)c1=O Show InChI InChI=1S/C27H21FN6O4/c28-18-10-8-17(9-11-18)25-31-14-19(29)27(37)34(25)15-22(35)32-20(13-16-5-2-1-3-6-16)23(36)26-33-24-21(38-26)7-4-12-30-24/h1-12,14,20H,13,15,29H2,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Chymotrypsin |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098851
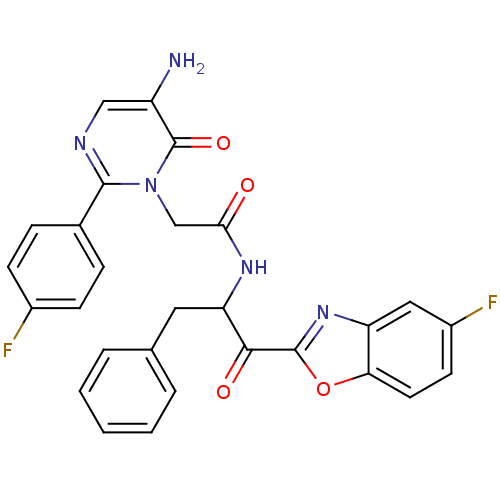
(2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...)Show SMILES Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(Cc2ccccc2)C(=O)c2nc3cc(F)ccc3o2)c1=O Show InChI InChI=1S/C28H21F2N5O4/c29-18-8-6-17(7-9-18)26-32-14-20(31)28(38)35(26)15-24(36)33-22(12-16-4-2-1-3-5-16)25(37)27-34-21-13-19(30)10-11-23(21)39-27/h1-11,13-14,22H,12,15,31H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 65.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50073127
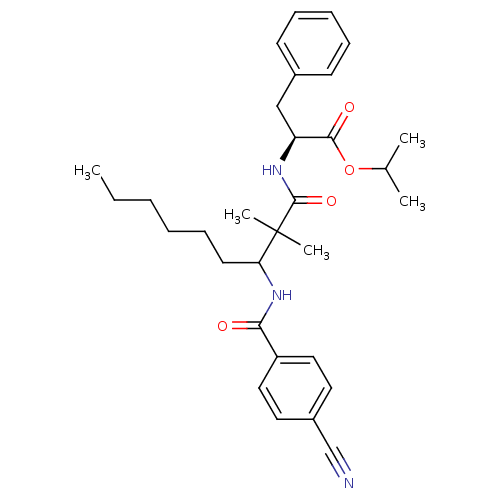
((S)-2-[3-(4-Cyano-benzoylamino)-2,2-dimethyl-nonan...)Show SMILES CCCCCCC(NC(=O)c1ccc(cc1)C#N)C(C)(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OC(C)C Show InChI InChI=1S/C31H41N3O4/c1-6-7-8-12-15-27(34-28(35)25-18-16-24(21-32)17-19-25)31(4,5)30(37)33-26(29(36)38-22(2)3)20-23-13-10-9-11-14-23/h9-11,13-14,16-19,22,26-27H,6-8,12,15,20H2,1-5H3,(H,33,37)(H,34,35)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for inactivation of bovine alpha-Chymotrypsin Kinetic constant(Ki) |
J Med Chem 42: 312-23 (1999)
Article DOI: 10.1021/jm980562h
BindingDB Entry DOI: 10.7270/Q2930SBF |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50068908
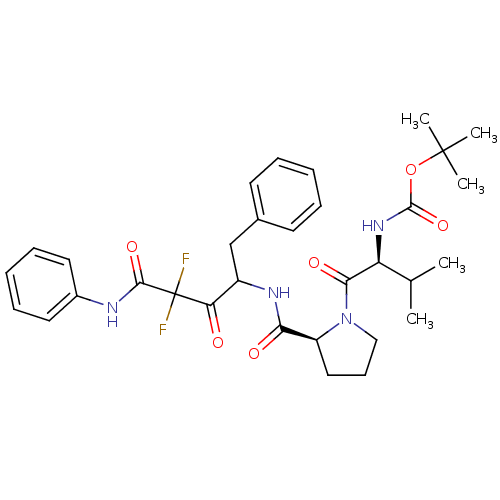
(CHEMBL352917 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1ccccc1 Show InChI InChI=1S/C32H40F2N4O6/c1-20(2)25(37-30(43)44-31(3,4)5)28(41)38-18-12-17-24(38)27(40)36-23(19-21-13-8-6-9-14-21)26(39)32(33,34)29(42)35-22-15-10-7-11-16-22/h6-11,13-16,20,23-25H,12,17-19H2,1-5H3,(H,35,42)(H,36,40)(H,37,43)/t23?,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Chymotrypsinogen |
Bioorg Med Chem Lett 8: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GQ6WXB |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098848

(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2nc(oc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C30H24FN5O6/c1-41-30(40)19-9-12-22-24(14-19)42-28(35-22)26(38)23(13-17-5-3-2-4-6-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-7-10-20(31)11-8-18/h2-12,14-15,23H,13,16,32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 71.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50031187
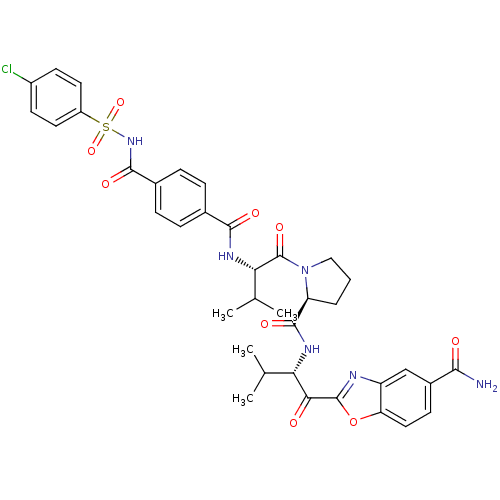
(2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nc2cc(ccc2o1)C(N)=O Show InChI InChI=1S/C37H39ClN6O9S/c1-19(2)29(31(45)36-40-26-18-23(32(39)46)11-16-28(26)53-36)41-35(49)27-6-5-17-44(27)37(50)30(20(3)4)42-33(47)21-7-9-22(10-8-21)34(48)43-54(51,52)25-14-12-24(38)13-15-25/h7-16,18-20,27,29-30H,5-6,17H2,1-4H3,(H2,39,46)(H,41,49)(H,42,47)(H,43,48)/t27-,29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards chymotrypsin |
J Med Chem 38: 3972-82 (1995)
BindingDB Entry DOI: 10.7270/Q2T152NN |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50462115

(CHEMBL2311598)Show SMILES [#6]-[#6@H](-[#6][Si;v4]([#8])([#8])[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1)-[#6](-[#7])=O |r| Show InChI InChI=1S/C17H27N3O4Si/c1-12(16(18)21)11-25(23,24)15(10-13-6-3-2-4-7-13)20-17(22)14-8-5-9-19-14/h2-4,6-7,12,14-15,19,23-24H,5,8-11H2,1H3,(H2,18,21)(H,20,22)/t12-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of bovine alpha-chymotrypsin using succinyl Ala-Ala-Pro-Phe-p-nitroanilide as substrate after 1 hr by Dixon plot analysis |
J Med Chem 61: 3779-3798 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00718
BindingDB Entry DOI: 10.7270/Q2GT5QTK |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50104175
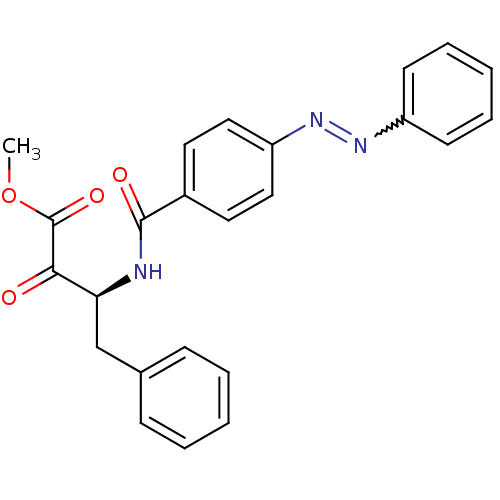
((S)-2-Oxo-4-phenyl-3-(4-phenylazo-benzoylamino)-bu...)Show SMILES COC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)N=Nc1ccccc1 |w:24.26| Show InChI InChI=1S/C24H21N3O4/c1-31-24(30)22(28)21(16-17-8-4-2-5-9-17)25-23(29)18-12-14-20(15-13-18)27-26-19-10-6-3-7-11-19/h2-15,21H,16H2,1H3,(H,25,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by ChEMBL
| Assay Description
Difference between inhibition of alpha-chymotrypsin by the UV light and inhibition of alpha-chymotrypsin by the ambient light |
Bioorg Med Chem Lett 11: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q2GB23BW |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50068898
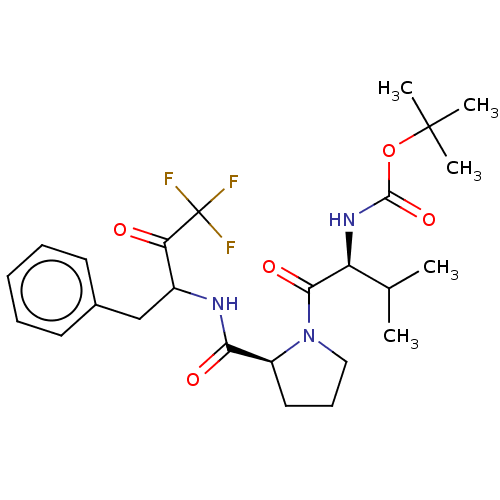
(BDBM50281588 | CHEMBL147013 | {1-[2-(1-Benzyl-3,3,...)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C25H34F3N3O5/c1-15(2)19(30-23(35)36-24(3,4)5)22(34)31-13-9-12-18(31)21(33)29-17(20(32)25(26,27)28)14-16-10-7-6-8-11-16/h6-8,10-11,15,17-19H,9,12-14H2,1-5H3,(H,29,33)(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Chymotrypsinogen |
Bioorg Med Chem Lett 8: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GQ6WXB |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50104175

((S)-2-Oxo-4-phenyl-3-(4-phenylazo-benzoylamino)-bu...)Show SMILES COC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)N=Nc1ccccc1 |w:24.26| Show InChI InChI=1S/C24H21N3O4/c1-31-24(30)22(28)21(16-17-8-4-2-5-9-17)25-23(29)18-12-14-20(15-13-18)27-26-19-10-6-3-7-11-19/h2-15,21H,16H2,1H3,(H,25,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by ChEMBL
| Assay Description
Inhibition constant for inhibition of alpha-chymotrypsin by the UV light |
Bioorg Med Chem Lett 11: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q2GB23BW |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50014576

(3-Benzyloxycarbonylamino-2-oxo-4-phenyl-butyric ac...)Show SMILES CCOC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C20H21NO5/c1-2-25-19(23)18(22)17(13-15-9-5-3-6-10-15)21-20(24)26-14-16-11-7-4-8-12-16/h3-12,17H,2,13-14H2,1H3,(H,21,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of alpha-chymotrypsin |
J Med Chem 33: 11-3 (1990)
BindingDB Entry DOI: 10.7270/Q2K93847 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098850
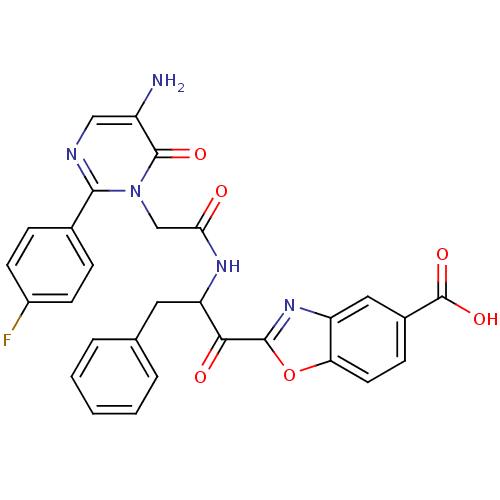
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(Cc2ccccc2)C(=O)c2nc3cc(ccc3o2)C(O)=O)c1=O Show InChI InChI=1S/C29H22FN5O6/c30-19-9-6-17(7-10-19)26-32-14-20(31)28(38)35(26)15-24(36)33-22(12-16-4-2-1-3-5-16)25(37)27-34-21-13-18(29(39)40)8-11-23(21)41-27/h1-11,13-14,22H,12,15,31H2,(H,33,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50014570
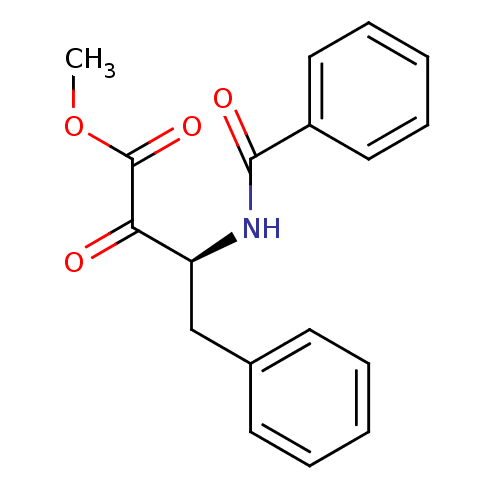
(3-Benzoylamino-2-oxo-4-phenyl-butyric acid methyl ...)Show InChI InChI=1S/C18H17NO4/c1-23-18(22)16(20)15(12-13-8-4-2-5-9-13)19-17(21)14-10-6-3-7-11-14/h2-11,15H,12H2,1H3,(H,19,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of alpha-chymotrypsin |
J Med Chem 33: 11-3 (1990)
BindingDB Entry DOI: 10.7270/Q2K93847 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098845
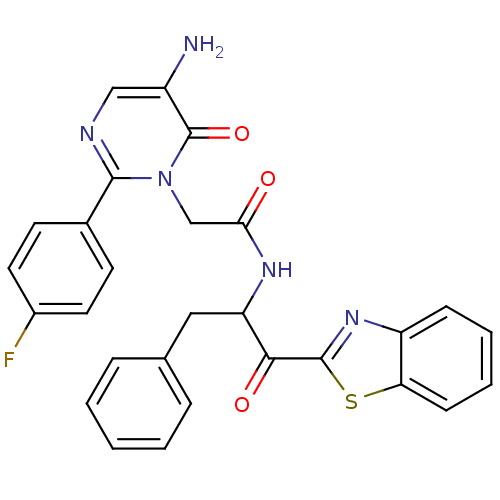
(2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...)Show SMILES Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(Cc2ccccc2)C(=O)c2nc3ccccc3s2)c1=O Show InChI InChI=1S/C28H22FN5O3S/c29-19-12-10-18(11-13-19)26-31-15-20(30)28(37)34(26)16-24(35)32-22(14-17-6-2-1-3-7-17)25(36)27-33-21-8-4-5-9-23(21)38-27/h1-13,15,22H,14,16,30H2,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50003737
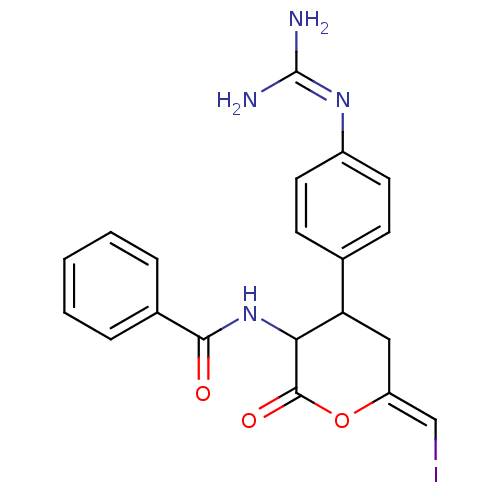
((Lactone4)N-[4-(4-Guanidino-phenyl)-6-iodomethylen...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(cc1)-[#6]-1-[#6]\[#6](-[#8]-[#6](=O)-[#6]-1-[#7]-[#6](=O)-c1ccccc1)=[#6]\I Show InChI InChI=1S/C20H19IN4O3/c21-11-15-10-16(12-6-8-14(9-7-12)24-20(22)23)17(19(27)28-15)25-18(26)13-4-2-1-3-5-13/h1-9,11,16-17H,10H2,(H,25,26)(H4,22,23,24)/b15-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against the enzyme alpha-chymotrypsin |
J Med Chem 35: 4297-305 (1992)
BindingDB Entry DOI: 10.7270/Q2TD9W9D |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50036079
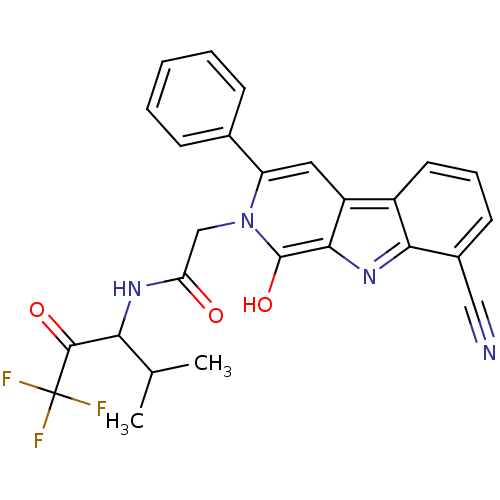
(2-(8-Cyano-1-oxo-3-phenyl-1,9-dihydro-beta-carboli...)Show SMILES CC(C)C(NC(=O)Cn1c(O)c2nc3c(cccc3c2cc1-c1ccccc1)C#N)C(=O)C(F)(F)F Show InChI InChI=1S/C26H21F3N4O3/c1-14(2)21(24(35)26(27,28)29)31-20(34)13-33-19(15-7-4-3-5-8-15)11-18-17-10-6-9-16(12-30)22(17)32-23(18)25(33)36/h3-11,14,21,36H,13H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine chymotrypsin |
J Med Chem 38: 86-97 (1995)
BindingDB Entry DOI: 10.7270/Q2TD9WDR |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50283074
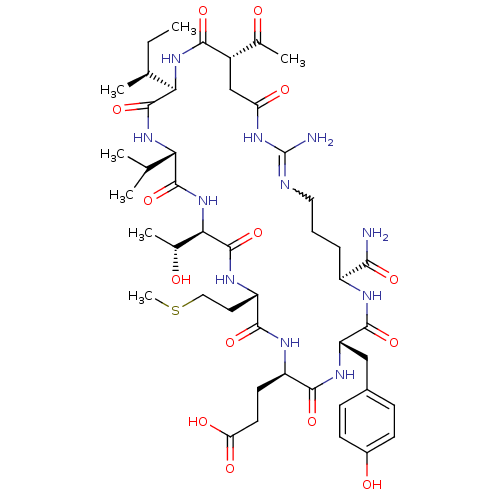
(CHEMBL413681 | Cyclic-Ac-Asp-Ile-Val-Thr-Met-Glu-T...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CC(=O)NC(N)=NCCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)[C@@H](C)O)C(N)=O)C(C)=O |w:15.15| Show InChI InChI=1S/C46H71N11O14S/c1-8-23(4)36-44(70)55-35(22(2)3)43(69)57-37(25(6)59)45(71)52-31(17-19-72-7)41(67)51-30(15-16-34(62)63)40(66)53-32(20-26-11-13-27(60)14-12-26)42(68)50-29(38(47)64)10-9-18-49-46(48)54-33(61)21-28(24(5)58)39(65)56-36/h11-14,22-23,25,28-32,35-37,59-60H,8-10,15-21H2,1-7H3,(H2,47,64)(H,50,68)(H,51,67)(H,52,71)(H,53,66)(H,55,70)(H,56,65)(H,57,69)(H,62,63)(H3,48,49,54,61)/t23-,25+,28-,29-,30+,31-,32-,35-,36+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of chymotrypsin at 120 nM |
Bioorg Med Chem Lett 4: 2123-2128 (1994)
Article DOI: 10.1016/S0960-894X(01)80114-1
BindingDB Entry DOI: 10.7270/Q2QC03F0 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50014571

(CHEMBL24283 | [1-(1-Benzyl-2,3-dioxo-butylcarbamoy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(C)=O Show InChI InChI=1S/C24H28N2O5/c1-16(2)21(26-24(30)31-15-19-12-8-5-9-13-19)23(29)25-20(22(28)17(3)27)14-18-10-6-4-7-11-18/h4-13,16,20-21H,14-15H2,1-3H3,(H,25,29)(H,26,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of alpha-chymotrypsin |
J Med Chem 33: 11-3 (1990)
BindingDB Entry DOI: 10.7270/Q2K93847 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50068887

(4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C33H40F2N4O8/c1-19(2)25(38-31(46)47-32(3,4)5)28(42)39-17-9-12-24(39)27(41)37-23(18-20-10-7-6-8-11-20)26(40)33(34,35)30(45)36-22-15-13-21(14-16-22)29(43)44/h6-8,10-11,13-16,19,23-25H,9,12,17-18H2,1-5H3,(H,36,45)(H,37,41)(H,38,46)(H,43,44)/t23?,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Chymotrypsinogen |
Bioorg Med Chem Lett 8: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GQ6WXB |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM26996
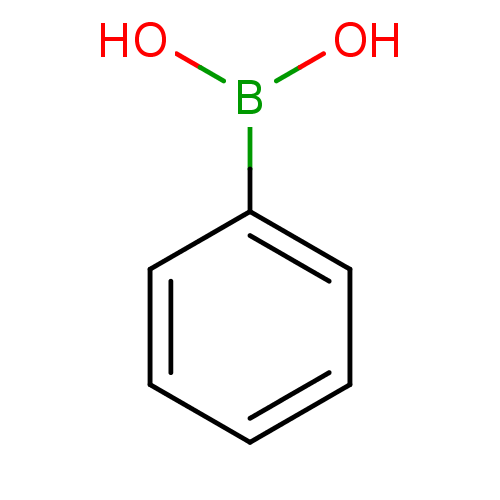
(CHEMBL21485 | PhB(OH)2 | Phenyl-boronic acid | Phe...)Show InChI InChI=1S/C6H7BO2/c8-7(9)6-4-2-1-3-5-6/h1-5,8-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of the compound was determined against Chymotrypsinogen |
Bioorg Med Chem Lett 2: 1391-1394 (1992)
Article DOI: 10.1016/S0960-894X(00)80519-3
BindingDB Entry DOI: 10.7270/Q23T9H47 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50283074

(CHEMBL413681 | Cyclic-Ac-Asp-Ile-Val-Thr-Met-Glu-T...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CC(=O)NC(N)=NCCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)[C@@H](C)O)C(N)=O)C(C)=O |w:15.15| Show InChI InChI=1S/C46H71N11O14S/c1-8-23(4)36-44(70)55-35(22(2)3)43(69)57-37(25(6)59)45(71)52-31(17-19-72-7)41(67)51-30(15-16-34(62)63)40(66)53-32(20-26-11-13-27(60)14-12-26)42(68)50-29(38(47)64)10-9-18-49-46(48)54-33(61)21-28(24(5)58)39(65)56-36/h11-14,22-23,25,28-32,35-37,59-60H,8-10,15-21H2,1-7H3,(H2,47,64)(H,50,68)(H,51,67)(H,52,71)(H,53,66)(H,55,70)(H,56,65)(H,57,69)(H,62,63)(H3,48,49,54,61)/t23-,25+,28-,29-,30+,31-,32-,35-,36+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of chymotrypsin at 69 nM |
Bioorg Med Chem Lett 4: 2123-2128 (1994)
Article DOI: 10.1016/S0960-894X(01)80114-1
BindingDB Entry DOI: 10.7270/Q2QC03F0 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50068909
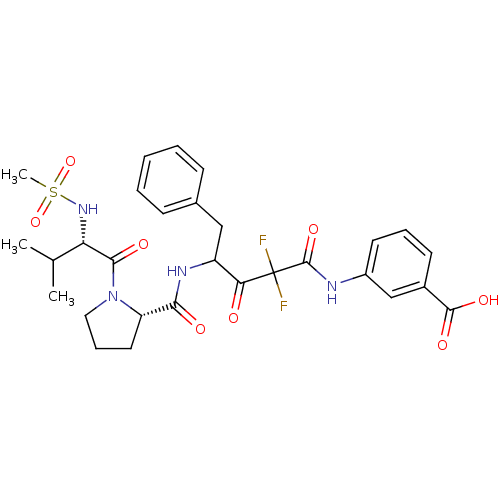
(3-(2,2-Difluoro-4-{[(S)-1-((S)-2-methanesulfonylam...)Show SMILES CC(C)[C@H](NS(C)(=O)=O)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C29H34F2N4O8S/c1-17(2)23(34-44(3,42)43)26(38)35-14-8-13-22(35)25(37)33-21(15-18-9-5-4-6-10-18)24(36)29(30,31)28(41)32-20-12-7-11-19(16-20)27(39)40/h4-7,9-12,16-17,21-23,34H,8,13-15H2,1-3H3,(H,32,41)(H,33,37)(H,39,40)/t21?,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Chymotrypsinogen |
Bioorg Med Chem Lett 8: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GQ6WXB |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098860

(2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...)Show SMILES Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(Cc2ccccc2)C(=O)c2nc3ccccc3o2)c1=O Show InChI InChI=1S/C28H22FN5O4/c29-19-12-10-18(11-13-19)26-31-15-20(30)28(37)34(26)16-24(35)32-22(14-17-6-2-1-3-7-17)25(36)27-33-21-8-4-5-9-23(21)38-27/h1-13,15,22H,14,16,30H2,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50104175

((S)-2-Oxo-4-phenyl-3-(4-phenylazo-benzoylamino)-bu...)Show SMILES COC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)N=Nc1ccccc1 |w:24.26| Show InChI InChI=1S/C24H21N3O4/c1-31-24(30)22(28)21(16-17-8-4-2-5-9-17)25-23(29)18-12-14-20(15-13-18)27-26-19-10-6-3-7-11-19/h2-15,21H,16H2,1H3,(H,25,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by ChEMBL
| Assay Description
Inhibition constant for inhibition of alpha-chymotrypsin by the UV light |
Bioorg Med Chem Lett 11: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q2GB23BW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data