 Found 124 hits of ki data for polymerid = 49000267
Found 124 hits of ki data for polymerid = 49000267 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A
(RAT) | BDBM50240609
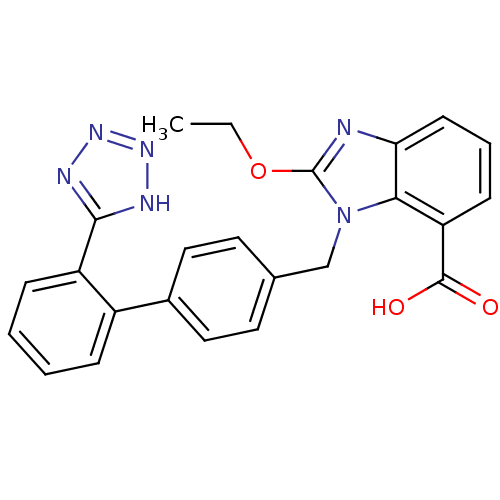
(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50030474
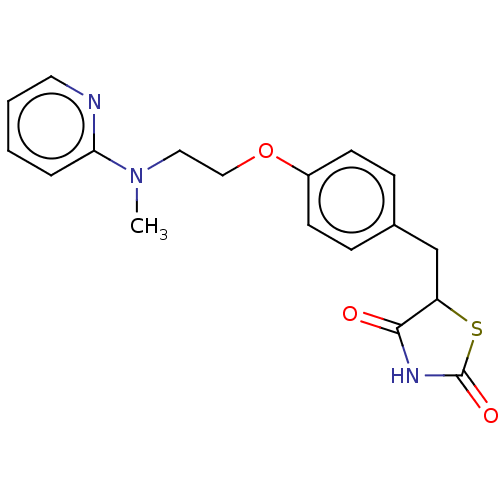
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9152-x
BindingDB Entry DOI: 10.7270/Q28K7CZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9153-9
BindingDB Entry DOI: 10.7270/Q24T6N8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM82077
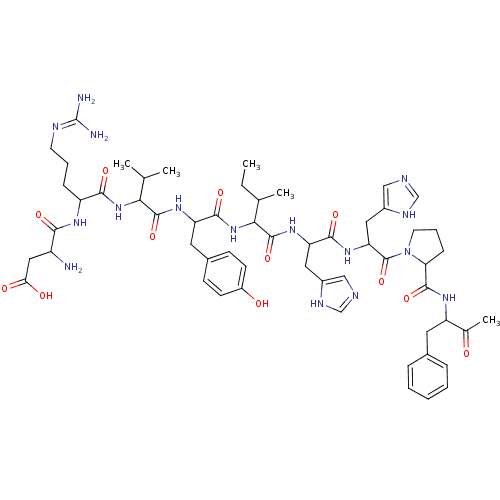
(Angiotensin II | CAS_11128-99-7 | NSC_439662)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)C(N)CC(O)=O)C(C)C)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(Cc1cnc[nH]1)C(=O)N1CCCC1C(=O)NC(Cc1ccccc1)C(C)=O |(1.13,.52,;1.58,1.99,;.53,3.12,;-.97,2.77,;.98,4.59,;2.48,4.94,;3.54,3.81,;3.08,2.34,;5.04,4.16,;5.49,5.63,;4.44,6.76,;2.93,6.41,;1.88,7.54,;2.33,9.01,;1.28,10.14,;3.84,9.36,;4.89,8.23,;6.09,3.03,;7.59,3.38,;8.04,4.85,;8.64,2.25,;10.14,2.6,;11.19,1.47,;10.74,-0,;12.69,1.82,;13.74,.69,;13.29,-.78,;14.34,-1.91,;13.89,-3.38,;12.39,-3.73,;11.94,-5.2,;11.34,-2.6,;13.14,3.29,;14.64,3.64,;15.69,2.51,;15.09,5.11,;14.04,6.24,;16.59,5.46,;17.04,6.93,;15.99,8.06,;18.54,7.28,;8.19,.78,;9.24,-.35,;6.69,.43,;-.07,5.72,;.38,7.19,;-1.57,5.37,;-2.62,6.5,;-2.17,7.97,;-3.22,9.1,;-2.92,10.61,;-4.27,11.35,;-5.4,10.3,;-4.75,8.91,;-4.12,6.15,;-4.57,4.68,;-5.17,7.28,;-6.67,6.93,;-6.64,5.39,;-7.96,4.6,;-9.38,5.2,;-10.39,4.04,;-9.6,2.72,;-8.1,3.06,;-7.72,8.06,;-7.27,9.53,;-9.22,7.71,;-10.38,8.72,;-11.7,7.93,;-11.36,6.43,;-9.82,6.29,;-10.42,4.87,;-10.87,3.4,;-11.95,4.69,;-12.55,3.27,;-14.08,3.08,;-14.68,1.66,;-13.76,.43,;-14.36,-.98,;-15.89,-1.17,;-16.81,.06,;-16.21,1.48,;-11.63,2.04,;-10.1,2.23,;-12.23,.62,)| Show InChI InChI=1S/C57H80N16O12/c1-6-32(4)48(72-52(81)42(23-35-16-18-38(75)19-17-35)68-54(83)47(31(2)3)71-50(79)40(14-10-20-63-57(59)60)66-49(78)39(58)26-46(76)77)55(84)69-43(24-36-27-61-29-64-36)51(80)70-44(25-37-28-62-30-65-37)56(85)73-21-11-15-45(73)53(82)67-41(33(5)74)22-34-12-8-7-9-13-34/h7-9,12-13,16-19,27-32,39-45,47-48,75H,6,10-11,14-15,20-26,58H2,1-5H3,(H,61,64)(H,62,65)(H,66,78)(H,67,82)(H,68,83)(H,69,84)(H,70,80)(H,71,79)(H,72,81)(H,76,77)(H4,59,60,63) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50009718
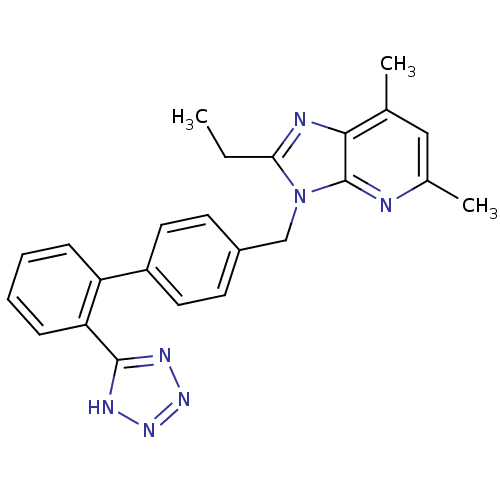
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50006909
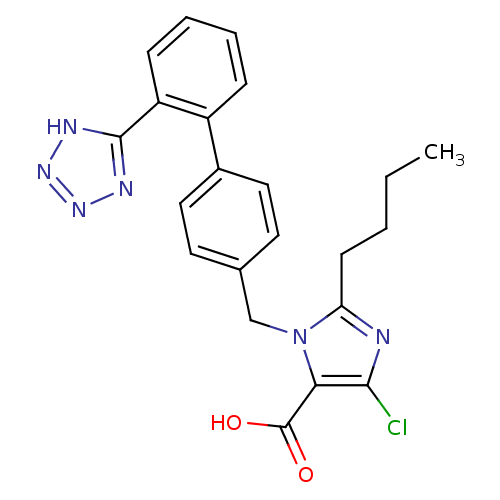
(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50003154
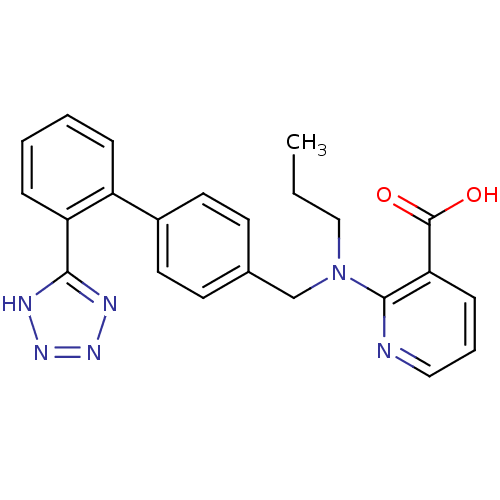
(2-{Propyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncccc1C(O)=O Show InChI InChI=1S/C23H22N6O2/c1-2-14-29(22-20(23(30)31)8-5-13-24-22)15-16-9-11-17(12-10-16)18-6-3-4-7-19(18)21-25-27-28-26-21/h3-13H,2,14-15H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM82259

(CAS_123856 | L-158,809 | NSC_123856)Show SMILES CCc1nc2c(C)cc(C)nc2n1-c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7/c1-4-20-25-21-14(2)13-15(3)24-23(21)30(20)17-11-9-16(10-12-17)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-13H,4H2,1-3H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 963-9 (1995)
BindingDB Entry DOI: 10.7270/Q2J38R2N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50287290
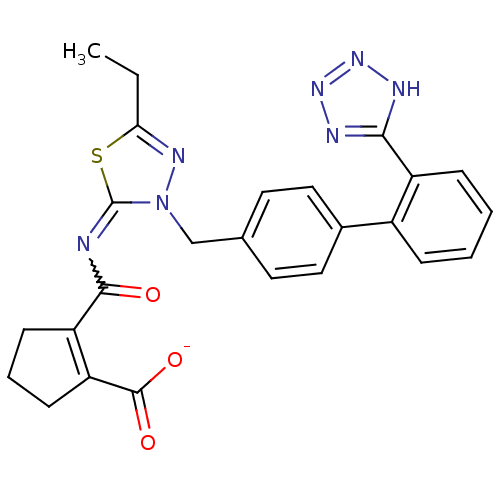
(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50049189
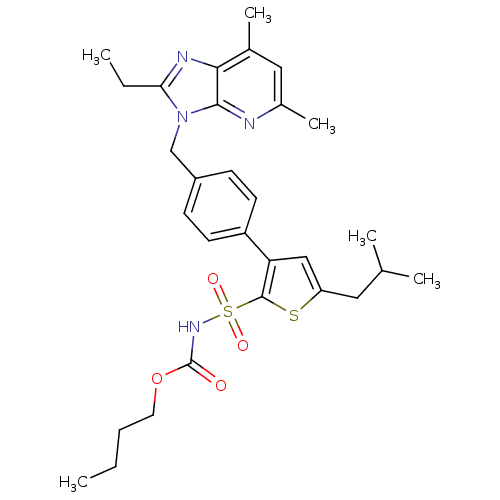
(3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C30H38N4O4S2/c1-7-9-14-38-30(35)33-40(36,37)29-25(17-24(39-29)15-19(3)4)23-12-10-22(11-13-23)18-34-26(8-2)32-27-20(5)16-21(6)31-28(27)34/h10-13,16-17,19H,7-9,14-15,18H2,1-6H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125]Ang2 from AT1 receptor in rat liver membrane |
Bioorg Med Chem 16: 6841-9 (2008)
Article DOI: 10.1016/j.bmc.2008.05.066
BindingDB Entry DOI: 10.7270/Q22F7N7C |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50082568

(2-({3-[4-(2-Azido-benzenesulfonylaminocarbonyl)-be...)Show SMILES CCCCc1ncc(CNc2ccccc2C(O)=O)n1Cc1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1N=[N+]=[N-] Show InChI InChI=1S/C29H29N7O5S/c1-2-3-12-27-32-18-22(17-31-24-9-5-4-8-23(24)29(38)39)36(27)19-20-13-15-21(16-14-20)28(37)34-42(40,41)26-11-7-6-10-25(26)33-35-30/h4-11,13-16,18,31H,2-3,12,17,19H2,1H3,(H,34,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A.
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. |
J Med Chem 42: 4572-83 (1999)
BindingDB Entry DOI: 10.7270/Q2XW4J1J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50009338
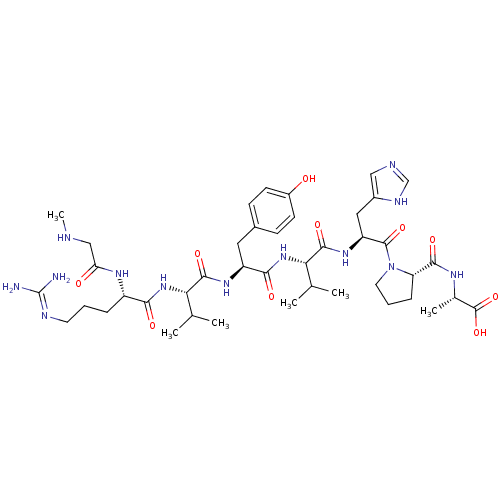
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 963-9 (1995)
BindingDB Entry DOI: 10.7270/Q2J38R2N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50003155

(4-{Butyl-[2''-(2H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncncc1C(O)=O Show InChI InChI=1S/C23H23N7O2/c1-2-3-12-30(22-20(23(31)32)13-24-15-25-22)14-16-8-10-17(11-9-16)18-6-4-5-7-19(18)21-26-28-29-27-21/h4-11,13,15H,2-3,12,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50161275

(CHEMBL3787059)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C23H21ClN4O4/c1-2-3-8-18-25-20(24)19(22(29)30)28(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-26-23(31)32-27-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,29,30)(H,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50141059
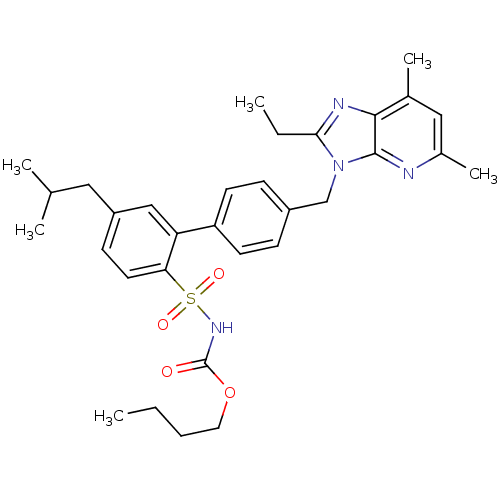
(CHEMBL289614 | L-162782 | N-Butyloxycarbonyl-4'-(2...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C32H40N4O4S/c1-7-9-16-40-32(37)35-41(38,39)28-15-12-25(17-21(3)4)19-27(28)26-13-10-24(11-14-26)20-36-29(8-2)34-30-22(5)18-23(6)33-31(30)36/h10-15,18-19,21H,7-9,16-17,20H2,1-6H3,(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125]Ang2 from AT1 receptor in rat liver membrane |
Bioorg Med Chem 16: 6841-9 (2008)
Article DOI: 10.1016/j.bmc.2008.05.066
BindingDB Entry DOI: 10.7270/Q22F7N7C |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50082567
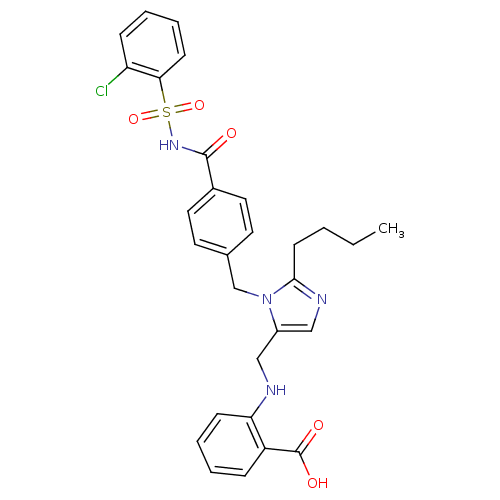
(2-({2-Butyl-3-[4-(2-chloro-benzenesulfonylaminocar...)Show SMILES CCCCc1ncc(CNc2ccccc2C(O)=O)n1Cc1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C29H29ClN4O5S/c1-2-3-12-27-32-18-22(17-31-25-10-6-4-8-23(25)29(36)37)34(27)19-20-13-15-21(16-14-20)28(35)33-40(38,39)26-11-7-5-9-24(26)30/h4-11,13-16,18,31H,2-3,12,17,19H2,1H3,(H,33,35)(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A.
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. |
J Med Chem 42: 4572-83 (1999)
BindingDB Entry DOI: 10.7270/Q2XW4J1J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50003158

(2-Methyl-4-{propyl-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1nc(C)ncc1C(O)=O Show InChI InChI=1S/C23H23N7O2/c1-3-12-30(22-20(23(31)32)13-24-15(2)25-22)14-16-8-10-17(11-9-16)18-6-4-5-7-19(18)21-26-28-29-27-21/h4-11,13H,3,12,14H2,1-2H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50161276
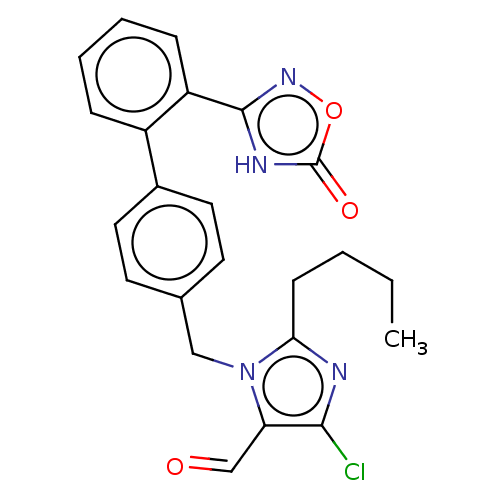
(CHEMBL3786570)Show SMILES CCCCc1nc(Cl)c(C=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C23H21ClN4O3/c1-2-3-8-20-25-21(24)19(14-29)28(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-26-23(30)31-27-22/h4-7,9-12,14H,2-3,8,13H2,1H3,(H,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50011977
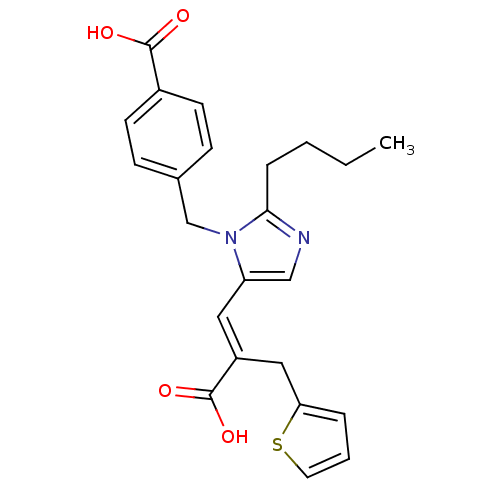
((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 963-9 (1995)
BindingDB Entry DOI: 10.7270/Q2J38R2N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50049189

(3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C30H38N4O4S2/c1-7-9-14-38-30(35)33-40(36,37)29-25(17-24(39-29)15-19(3)4)23-12-10-22(11-13-23)18-34-26(8-2)32-27-20(5)16-21(6)31-28(27)34/h10-13,16-17,19H,7-9,14-15,18H2,1-6H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang II from AT1 receptor in rat liver membranes |
J Med Chem 63: 1978-1995 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01780
BindingDB Entry DOI: 10.7270/Q20868NG |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50049189

(3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C30H38N4O4S2/c1-7-9-14-38-30(35)33-40(36,37)29-25(17-24(39-29)15-19(3)4)23-12-10-22(11-13-23)18-34-26(8-2)32-27-20(5)16-21(6)31-28(27)34/h10-13,16-17,19H,7-9,14-15,18H2,1-6H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116790
BindingDB Entry DOI: 10.7270/Q2ZW1QXF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50161277
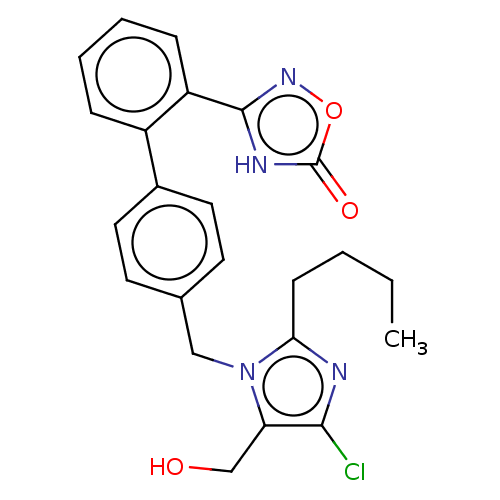
(CHEMBL3787050)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C23H23ClN4O3/c1-2-3-8-20-25-21(24)19(14-29)28(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-26-23(30)31-27-22/h4-7,9-12,29H,2-3,8,13-14H2,1H3,(H,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 963-9 (1995)
BindingDB Entry DOI: 10.7270/Q2J38R2N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50082570

(2-[2-butyl-1-(4-carboxybenzyl)-1H-5-imidazolylmeth...)Show SMILES CCCCc1ncc(CNc2ccccc2C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25N3O4/c1-2-3-8-21-25-14-18(13-24-20-7-5-4-6-19(20)23(29)30)26(21)15-16-9-11-17(12-10-16)22(27)28/h4-7,9-12,14,24H,2-3,8,13,15H2,1H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A.
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. |
J Med Chem 42: 4572-83 (1999)
BindingDB Entry DOI: 10.7270/Q2XW4J1J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303975
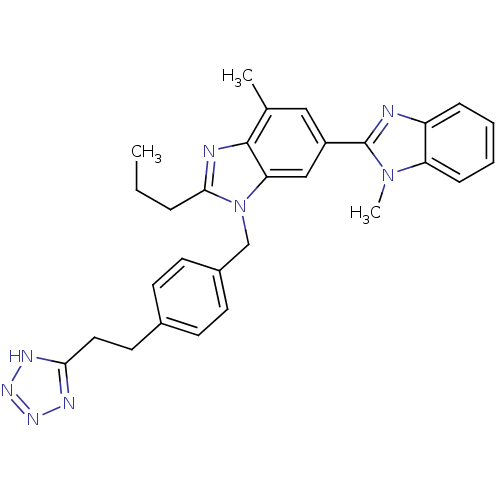
(3'-(4-(2-(1H-Tetrazol-5-yl)ethyl)benzyl)-1,7'-dime...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(CCc2nnn[nH]2)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C29H30N8/c1-4-7-27-31-28-19(2)16-22(29-30-23-8-5-6-9-24(23)36(29)3)17-25(28)37(27)18-21-12-10-20(11-13-21)14-15-26-32-34-35-33-26/h5-6,8-13,16-17H,4,7,14-15,18H2,1-3H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50119657

(8-Butyl-7-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN1N(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)C(=O)C2(CCCC2)CC1=O Show InChI InChI=1S/C26H30N6O2/c1-2-3-16-31-23(33)17-26(14-6-7-15-26)25(34)32(31)18-19-10-12-20(13-11-19)21-8-4-5-9-22(21)24-27-29-30-28-24/h4-5,8-13H,2-3,6-7,14-18H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1,Ile8-Ang2 from AT1 receptor in Wistar rat hepatic membrane |
J Med Chem 51: 2137-46 (2008)
Article DOI: 10.1021/jm7011563
BindingDB Entry DOI: 10.7270/Q20P10X0 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver; n=8 |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50003156
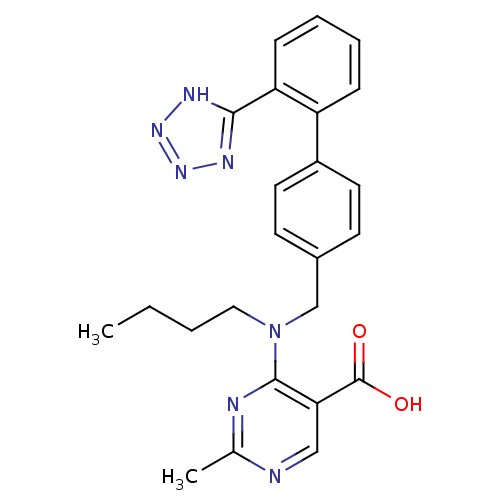
(4-{Butyl-[2''-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1nc(C)ncc1C(O)=O Show InChI InChI=1S/C24H25N7O2/c1-3-4-13-31(23-21(24(32)33)14-25-16(2)26-23)15-17-9-11-18(12-10-17)19-7-5-6-8-20(19)22-27-29-30-28-22/h5-12,14H,3-4,13,15H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50045358
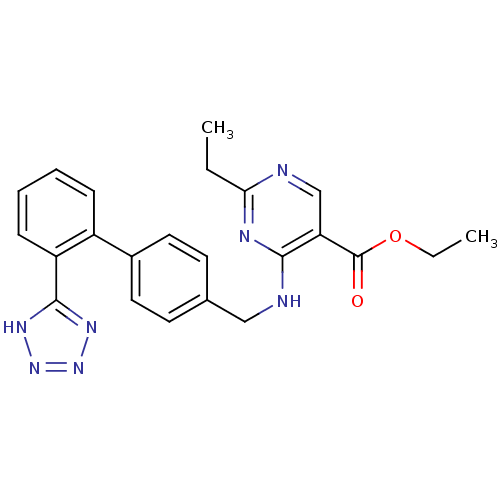
(2-Ethyl-4-{[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOC(=O)c1cnc(CC)nc1NCc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H23N7O2/c1-3-20-24-14-19(23(31)32-4-2)21(26-20)25-13-15-9-11-16(12-10-15)17-7-5-6-8-18(17)22-27-29-30-28-22/h5-12,14H,3-4,13H2,1-2H3,(H,24,25,26)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50082569
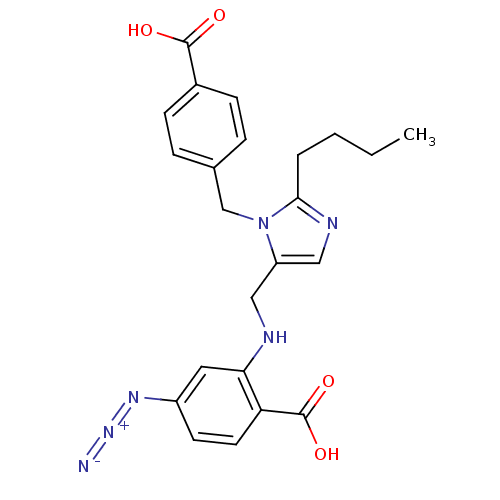
(4-Azido-2-{[2-butyl-3-(4-carboxy-benzyl)-3H-imidaz...)Show SMILES CCCCc1ncc(CNc2cc(ccc2C(O)=O)N=[N+]=[N-])n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O4/c1-2-3-4-21-26-13-18(29(21)14-15-5-7-16(8-6-15)22(30)31)12-25-20-11-17(27-28-24)9-10-19(20)23(32)33/h5-11,13,25H,2-4,12,14H2,1H3,(H,30,31)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A.
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. |
J Med Chem 42: 4572-83 (1999)
BindingDB Entry DOI: 10.7270/Q2XW4J1J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50089990
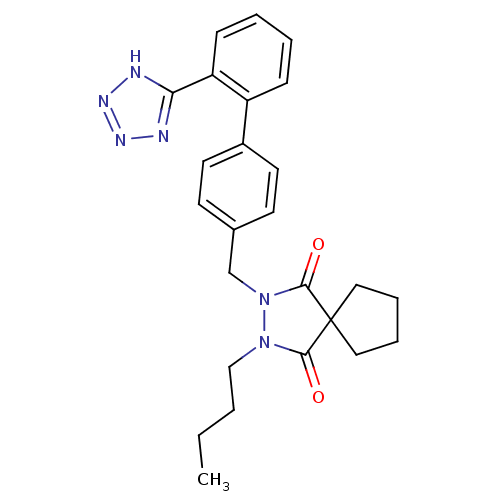
(2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN1N(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)C(=O)C2(CCCC2)C1=O Show InChI InChI=1S/C25H28N6O2/c1-2-3-16-30-23(32)25(14-6-7-15-25)24(33)31(30)17-18-10-12-19(13-11-18)20-8-4-5-9-21(20)22-26-28-29-27-22/h4-5,8-13H,2-3,6-7,14-17H2,1H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1,Ile8-Ang2 from AT1 receptor in Wistar rat hepatic membrane |
J Med Chem 51: 2137-46 (2008)
Article DOI: 10.1021/jm7011563
BindingDB Entry DOI: 10.7270/Q20P10X0 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50368649
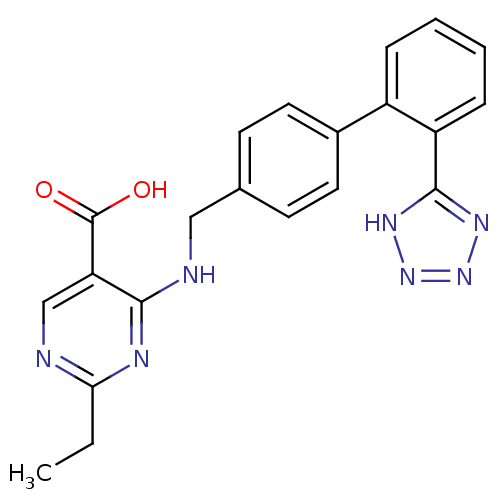
(CHEMBL1202852)Show SMILES CCc1ncc(C(O)=O)c(NCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C21H19N7O2/c1-2-18-22-12-17(21(29)30)19(24-18)23-11-13-7-9-14(10-8-13)15-5-3-4-6-16(15)20-25-27-28-26-20/h3-10,12H,2,11H2,1H3,(H,29,30)(H,22,23,24)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor from rat liver |
J Med Chem 36: 2676-88 (1993)
BindingDB Entry DOI: 10.7270/Q2QF8TGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50119658
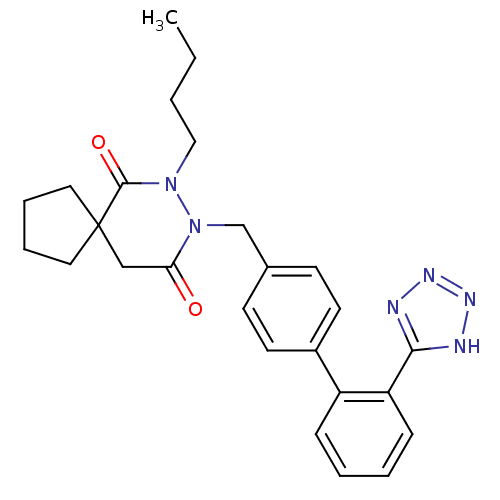
(7-Butyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN1N(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)C(=O)CC2(CCCC2)C1=O Show InChI InChI=1S/C26H30N6O2/c1-2-3-16-31-25(34)26(14-6-7-15-26)17-23(33)32(31)18-19-10-12-20(13-11-19)21-8-4-5-9-22(21)24-27-29-30-28-24/h4-5,8-13H,2-3,6-7,14-18H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1,Ile8-Ang2 from AT1 receptor in Wistar rat hepatic membrane |
J Med Chem 51: 2137-46 (2008)
Article DOI: 10.1021/jm7011563
BindingDB Entry DOI: 10.7270/Q20P10X0 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487564
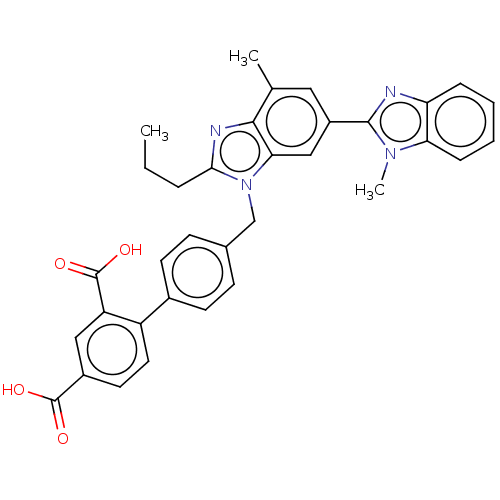
(CHEMBL2260199)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccc(cc1C(O)=O)C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C34H30N4O4/c1-4-7-30-36-31-20(2)16-24(32-35-27-8-5-6-9-28(27)37(32)3)18-29(31)38(30)19-21-10-12-22(13-11-21)25-15-14-23(33(39)40)17-26(25)34(41)42/h5-6,8-18H,4,7,19H2,1-3H3,(H,39,40)(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9153-9
BindingDB Entry DOI: 10.7270/Q24T6N8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487559
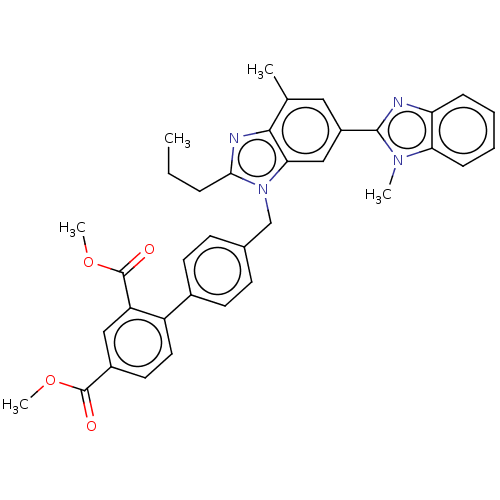
(CHEMBL2260198)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccc(cc1C(=O)OC)C(=O)OC)-c1nc2ccccc2n1C Show InChI InChI=1S/C36H34N4O4/c1-6-9-32-38-33-22(2)18-26(34-37-29-10-7-8-11-30(29)39(34)3)20-31(33)40(32)21-23-12-14-24(15-13-23)27-17-16-25(35(41)43-4)19-28(27)36(42)44-5/h7-8,10-20H,6,9,21H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9153-9
BindingDB Entry DOI: 10.7270/Q24T6N8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303976

(3'-(2-(4-(2-(1H-Tetrazol-5-yl)ethyl)phenoxy)ethyl)...)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CCc2nnn[nH]2)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C30H32N8O/c1-4-7-28-32-29-20(2)18-22(30-31-24-8-5-6-9-25(24)37(30)3)19-26(29)38(28)16-17-39-23-13-10-21(11-14-23)12-15-27-33-35-36-34-27/h5-6,8-11,13-14,18-19H,4,7,12,15-17H2,1-3H3,(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50514581

(CHEMBL1885579)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCNC(=O)[C@H](CCCN=C(N)N)NC(=O)OCc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cccnc1)C(O)=O |wD:15.23,4.4,25.54,55.67,33.42,8.7,2.2,(24.22,-16.21,;22.68,-16.15,;21.86,-17.46,;22.57,-18.82,;20.32,-17.4,;19.5,-18.7,;17.96,-18.64,;17.24,-17.28,;17.14,-19.94,;17.71,-21.37,;16.52,-22.36,;15.22,-21.54,;15.6,-20.04,;14.61,-18.86,;15.15,-17.42,;13.1,-19.12,;12.14,-20.33,;12.72,-21.76,;14.21,-22.14,;14.31,-23.68,;12.88,-24.25,;11.89,-23.07,;12.52,-17.69,;11,-17.47,;10.05,-18.68,;10.43,-16.04,;8.9,-15.82,;8.33,-14.39,;6.81,-14.17,;6.24,-12.74,;4.71,-12.52,;4.14,-11.09,;5.09,-9.88,;2.62,-10.87,;1.66,-12.08,;.14,-11.86,;-.81,-13.07,;-2.34,-12.85,;-3.29,-14.06,;-2.72,-15.49,;-4.81,-13.84,;2.05,-9.44,;.52,-9.22,;-.43,-10.43,;-.05,-7.79,;-1.57,-7.57,;-2.15,-6.14,;-3.67,-5.92,;-4.24,-4.49,;-3.29,-3.28,;-1.76,-3.5,;-1.19,-4.93,;11.38,-14.83,;12.91,-15.05,;13.48,-16.48,;13.86,-13.84,;13.29,-12.41,;11.76,-12.19,;10.81,-13.4,;9.29,-13.18,;8.71,-11.75,;7.19,-11.53,;9.67,-10.54,;11.19,-10.76,;15.38,-14.06,;16.34,-12.85,;15.76,-11.42,;17.86,-13.07,;18.81,-11.86,;20.34,-12.08,;20.91,-13.51,;19.96,-14.72,;18.43,-14.5,;19.6,-16.03,;20.62,-14.82,;18.06,-15.97,)| Show InChI InChI=1S/C52H69N13O11/c1-3-32(2)43(50(73)74)64-48(71)42-17-11-25-65(42)49(72)41(27-36-29-56-31-59-36)62-46(69)39(60-47(70)40(26-33-18-20-37(66)21-19-33)61-44(67)35-14-9-22-55-28-35)15-7-8-23-57-45(68)38(16-10-24-58-51(53)54)63-52(75)76-30-34-12-5-4-6-13-34/h4-6,9,12-14,18-22,28-29,31-32,38-43,66H,3,7-8,10-11,15-17,23-27,30H2,1-2H3,(H,56,59)(H,57,68)(H,60,70)(H,61,67)(H,62,69)(H,63,75)(H,64,71)(H,73,74)(H4,53,54,58)/t32-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang II from AT1 receptor in rat SMC membranes incubated for 60 mins by gamma counting method |
J Med Chem 63: 1978-1995 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01780
BindingDB Entry DOI: 10.7270/Q20868NG |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303977

(3-(4-(2-(1,7'-Dimethyl-2'-propyl-1H,3'H-2,5'-biben...)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C37H38N4O4/c1-5-11-33-39-34-25(2)22-27(35-38-30-14-9-10-15-31(30)40(35)4)23-32(34)41(33)20-21-44-28-18-16-26(17-19-28)24-37(3,36(42)43)45-29-12-7-6-8-13-29/h6-10,12-19,22-23H,5,11,20-21,24H2,1-4H3,(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303979

(CHEMBL571763 | Ethyl 3-(4-(2-(1,7'-Dimethyl-2'-pro...)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CC(C)(Oc2ccccc2)C(=O)OCC)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C39H42N4O4/c1-6-13-35-41-36-27(3)24-29(37-40-32-16-11-12-17-33(32)42(37)5)25-34(36)43(35)22-23-46-30-20-18-28(19-21-30)26-39(4,38(44)45-7-2)47-31-14-9-8-10-15-31/h8-12,14-21,24-25H,6-7,13,22-23,26H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303978
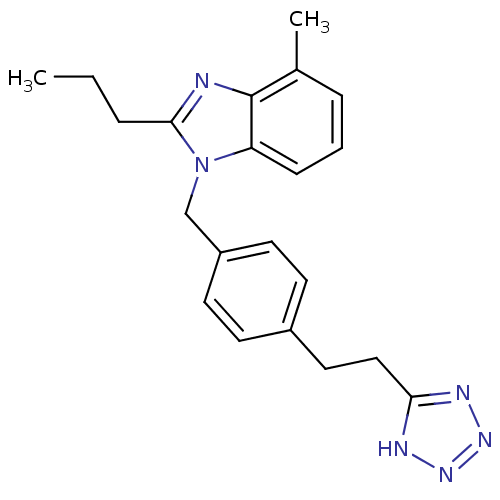
(1-(4-(2-(1H-tetrazol-5-yl)ethyl)benzyl)-4-methyl-2...)Show InChI InChI=1S/C21H24N6/c1-3-5-20-22-21-15(2)6-4-7-18(21)27(20)14-17-10-8-16(9-11-17)12-13-19-23-25-26-24-19/h4,6-11H,3,5,12-14H2,1-2H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303980
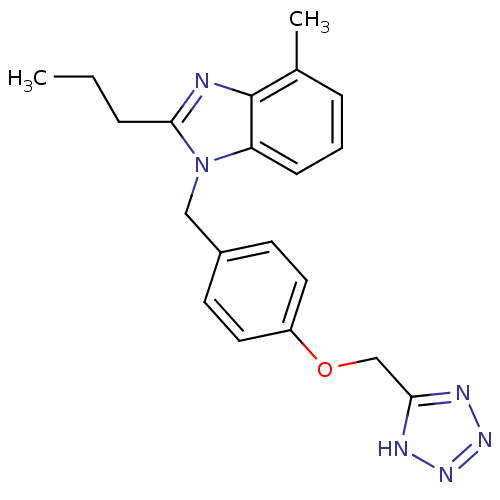
(1-(4-((1H-Tetrazol-5-yl)methoxy)benzyl)-4-methyl-2...)Show InChI InChI=1S/C20H22N6O/c1-3-5-19-21-20-14(2)6-4-7-17(20)26(19)12-15-8-10-16(11-9-15)27-13-18-22-24-25-23-18/h4,6-11H,3,5,12-13H2,1-2H3,(H,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303985

(CHEMBL567269 | Ethyl 2,2-Dimethyl-3-(4-(2-(4-methy...)Show SMILES CCCc1nc2c(C)cccc2n1CCOc1ccc(CC(C)(C)C(=O)OCC)cc1 Show InChI InChI=1S/C26H34N2O3/c1-6-9-23-27-24-19(3)10-8-11-22(24)28(23)16-17-31-21-14-12-20(13-15-21)18-26(4,5)25(29)30-7-2/h8,10-15H,6-7,9,16-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303986

(CHEMBL584704 | Ethyl 2-Methyl-3-(4-(2-(4-methyl-2-...)Show SMILES CCCc1nc2c(C)cccc2n1CCOc1ccc(CC(C)(Oc2ccccc2)C(=O)OCC)cc1 Show InChI InChI=1S/C31H36N2O4/c1-5-11-28-32-29-23(3)12-10-15-27(29)33(28)20-21-36-25-18-16-24(17-19-25)22-31(4,30(34)35-6-2)37-26-13-8-7-9-14-26/h7-10,12-19H,5-6,11,20-22H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487549

(CHEMBL2260189)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CC2SC(=O)NC2=O)cc1C(=O)OC)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H33N5O5S/c1-5-8-28-35-29-19(2)15-21(30-34-23-9-6-7-10-24(23)37(30)3)18-25(29)38(28)13-14-43-26-12-11-20(16-22(26)32(40)42-4)17-27-31(39)36-33(41)44-27/h6-7,9-12,15-16,18,27H,5,8,13-14,17H2,1-4H3,(H,36,39,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9152-x
BindingDB Entry DOI: 10.7270/Q28K7CZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487550

(CHEMBL2260187)Show SMILES COC(=O)c1cc(CC2SC(=O)NC2=O)ccc1OCCn1c(CCCCC2CCCCC2)nc2ccccc12 Show InChI InChI=1S/C31H37N3O5S/c1-38-30(36)23-19-22(20-27-29(35)33-31(37)40-27)15-16-26(23)39-18-17-34-25-13-7-6-12-24(25)32-28(34)14-8-5-11-21-9-3-2-4-10-21/h6-7,12-13,15-16,19,21,27H,2-5,8-11,14,17-18,20H2,1H3,(H,33,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9152-x
BindingDB Entry DOI: 10.7270/Q28K7CZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487551

(CHEMBL2260186)Show SMILES CCCc1nc2c(C)cc(Br)cc2n1CCOc1ccc(CC2SC(=O)NC2=O)cc1C(=O)OC Show InChI InChI=1S/C25H26BrN3O5S/c1-4-5-21-27-22-14(2)10-16(26)13-18(22)29(21)8-9-34-19-7-6-15(11-17(19)24(31)33-3)12-20-23(30)28-25(32)35-20/h6-7,10-11,13,20H,4-5,8-9,12H2,1-3H3,(H,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9152-x
BindingDB Entry DOI: 10.7270/Q28K7CZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487552

(CHEMBL2260188)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CC2SC(=O)NC2=O)cc1C(=O)OC)-c1ccccc1 Show InChI InChI=1S/C31H31N3O5S/c1-4-8-27-32-28-19(2)15-22(21-9-6-5-7-10-21)18-24(28)34(27)13-14-39-25-12-11-20(16-23(25)30(36)38-3)17-26-29(35)33-31(37)40-26/h5-7,9-12,15-16,18,26H,4,8,13-14,17H2,1-3H3,(H,33,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9152-x
BindingDB Entry DOI: 10.7270/Q28K7CZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50487553

(CHEMBL2260185)Show SMILES COC(=O)c1cc(CC2SC(=O)NC2=O)ccc1OCCN(C)C(=O)CCCCC1CCCCC1 Show InChI InChI=1S/C26H36N2O6S/c1-28(23(29)11-7-6-10-18-8-4-3-5-9-18)14-15-34-21-13-12-19(16-20(21)25(31)33-2)17-22-24(30)27-26(32)35-22/h12-13,16,18,22H,3-11,14-15,17H2,1-2H3,(H,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-SI-Ang-2 from AT1 receptor in Rattus norvegicus Sprague-Dawley (rat) liver membranes after 2 hr |
Citation and Details
Article DOI: 10.1007/s00044-008-9152-x
BindingDB Entry DOI: 10.7270/Q28K7CZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data