 Found 356 hits of ki data for polymerid = 49000273,5550
Found 356 hits of ki data for polymerid = 49000273,5550 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50240609
(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Angiotensin 2 from human placental AT1 receptor expressed in African green monkey COS7 cell membranes after 90 mins by gamma cou... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50370684
(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM82077
(Angiotensin II | CAS_11128-99-7 | NSC_439662)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)C(N)CC(O)=O)C(C)C)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(Cc1cnc[nH]1)C(=O)N1CCCC1C(=O)NC(Cc1ccccc1)C(C)=O |(1.13,.52,;1.58,1.99,;.53,3.12,;-.97,2.77,;.98,4.59,;2.48,4.94,;3.54,3.81,;3.08,2.34,;5.04,4.16,;5.49,5.63,;4.44,6.76,;2.93,6.41,;1.88,7.54,;2.33,9.01,;1.28,10.14,;3.84,9.36,;4.89,8.23,;6.09,3.03,;7.59,3.38,;8.04,4.85,;8.64,2.25,;10.14,2.6,;11.19,1.47,;10.74,-0,;12.69,1.82,;13.74,.69,;13.29,-.78,;14.34,-1.91,;13.89,-3.38,;12.39,-3.73,;11.94,-5.2,;11.34,-2.6,;13.14,3.29,;14.64,3.64,;15.69,2.51,;15.09,5.11,;14.04,6.24,;16.59,5.46,;17.04,6.93,;15.99,8.06,;18.54,7.28,;8.19,.78,;9.24,-.35,;6.69,.43,;-.07,5.72,;.38,7.19,;-1.57,5.37,;-2.62,6.5,;-2.17,7.97,;-3.22,9.1,;-2.92,10.61,;-4.27,11.35,;-5.4,10.3,;-4.75,8.91,;-4.12,6.15,;-4.57,4.68,;-5.17,7.28,;-6.67,6.93,;-6.64,5.39,;-7.96,4.6,;-9.38,5.2,;-10.39,4.04,;-9.6,2.72,;-8.1,3.06,;-7.72,8.06,;-7.27,9.53,;-9.22,7.71,;-10.38,8.72,;-11.7,7.93,;-11.36,6.43,;-9.82,6.29,;-10.42,4.87,;-10.87,3.4,;-11.95,4.69,;-12.55,3.27,;-14.08,3.08,;-14.68,1.66,;-13.76,.43,;-14.36,-.98,;-15.89,-1.17,;-16.81,.06,;-16.21,1.48,;-11.63,2.04,;-10.1,2.23,;-12.23,.62,)| Show InChI InChI=1S/C57H80N16O12/c1-6-32(4)48(72-52(81)42(23-35-16-18-38(75)19-17-35)68-54(83)47(31(2)3)71-50(79)40(14-10-20-63-57(59)60)66-49(78)39(58)26-46(76)77)55(84)69-43(24-36-27-61-29-64-36)51(80)70-44(25-37-28-62-30-65-37)56(85)73-21-11-15-45(73)53(82)67-41(33(5)74)22-34-12-8-7-9-13-34/h7-9,12-13,16-19,27-32,39-45,47-48,75H,6,10-11,14-15,20-26,58H2,1-5H3,(H,61,64)(H,62,65)(H,66,78)(H,67,82)(H,68,83)(H,69,84)(H,70,80)(H,71,79)(H,72,81)(H,76,77)(H4,59,60,63) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50015662
(3-Amino-N-{1-[5-[2-[2-(1-carboxy-2-phenyl-ethylcar...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Asp-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-ValTyr-Ile-His-Pro-Phe-OH Tris(hydrotrifluoroacetate) from human AT1 re... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031962
(3-(5,7-Dimethyl-2-propyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCCc1nc2c(C)cc(C)nc2n1Cc1ccc2c(cc3ccccc3c(=O)c2c1)-c1nnn[nH]1 Show InChI InChI=1S/C28H25N7O/c1-4-7-24-30-25-16(2)12-17(3)29-28(25)35(24)15-18-10-11-21-22(13-18)26(36)20-9-6-5-8-19(20)14-23(21)27-31-33-34-32-27/h5-6,8-14H,4,7,15H2,1-3H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031954
(3-(2-Cyclopropyl-5,7-dimethyl-imidazo[4,5-b]pyridi...)Show SMILES Cc1cc(C)c2nc(C3CC3)n(Cc3ccc4c(cc5ccccc5c(=O)c4c3)-c3nnn[nH]3)c2n1 Show InChI InChI=1S/C28H23N7O/c1-15-11-16(2)29-28-24(15)30-27(18-8-9-18)35(28)14-17-7-10-21-22(12-17)25(36)20-6-4-3-5-19(20)13-23(21)26-31-33-34-32-26/h3-7,10-13,18H,8-9,14H2,1-2H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031953
(3-(2-Ethyl-6-methoxy-[1,5]naphthyridin-4-yloxymeth...)Show SMILES CCc1cc(OCc2ccc3c(cc4ccccc4c(=O)c3c2)-c2nnn[nH]2)c2nc(OC)ccc2n1 Show InChI InChI=1S/C28H22N6O3/c1-3-18-14-24(26-23(29-18)10-11-25(30-26)36-2)37-15-16-8-9-20-21(12-16)27(35)19-7-5-4-6-17(19)13-22(20)28-31-33-34-32-28/h4-14H,3,15H2,1-2H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031957
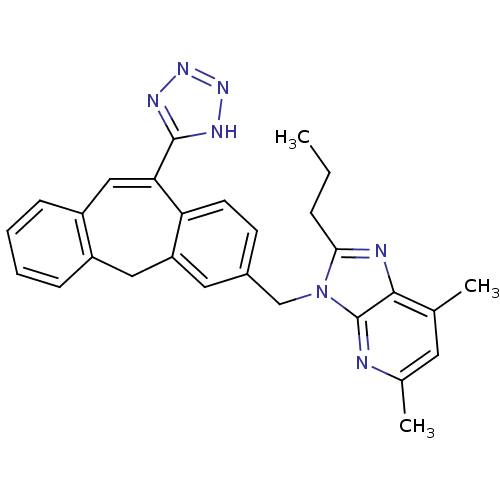
(5,7-Dimethyl-2-propyl-3-[11-(1H-tetrazol-5-yl)-5H-...)Show SMILES CCCc1nc2c(C)cc(C)nc2n1Cc1ccc2c(Cc3ccccc3C=C2c2nnn[nH]2)c1 |c:30| Show InChI InChI=1S/C28H27N7/c1-4-7-25-30-26-17(2)12-18(3)29-28(26)35(25)16-19-10-11-23-22(13-19)14-20-8-5-6-9-21(20)15-24(23)27-31-33-34-32-27/h5-6,8-13,15H,4,7,14,16H2,1-3H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287291

(CHEMBL35381 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)c2ccccc2C(O)=O)s1 |w:24.27| Show InChI InChI=1S/C26H21N7O3S/c1-2-22-30-33(26(37-22)27-24(34)20-9-5-6-10-21(20)25(35)36)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)23-28-31-32-29-23/h3-14H,2,15H2,1H3,(H,35,36)(H,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 0.619 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of binding of [125I]All at the angiotensin II type 1 receptor (AT1 receptor) in rat liver membranes |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1T6B |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031960
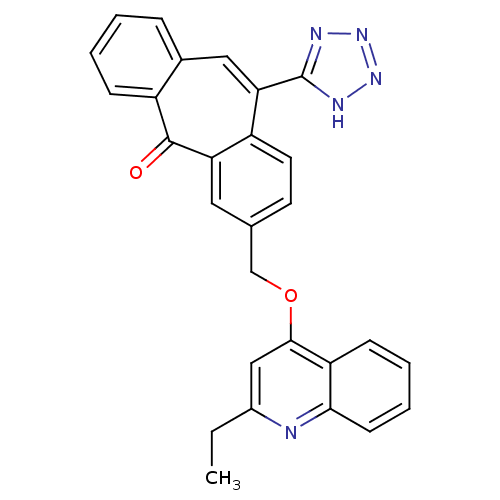
(3-(2-Ethyl-quinolin-4-yloxymethyl)-11-(1H-tetrazol...)Show SMILES CCc1cc(OCc2ccc3c(cc4ccccc4c(=O)c3c2)-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H21N5O2/c1-2-19-15-26(22-9-5-6-10-25(22)29-19)35-16-17-11-12-21-23(13-17)27(34)20-8-4-3-7-18(20)14-24(21)28-30-32-33-31-28/h3-15H,2,16H2,1H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030815
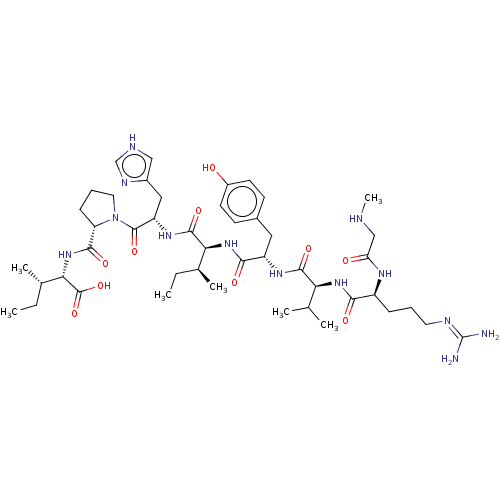
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031961
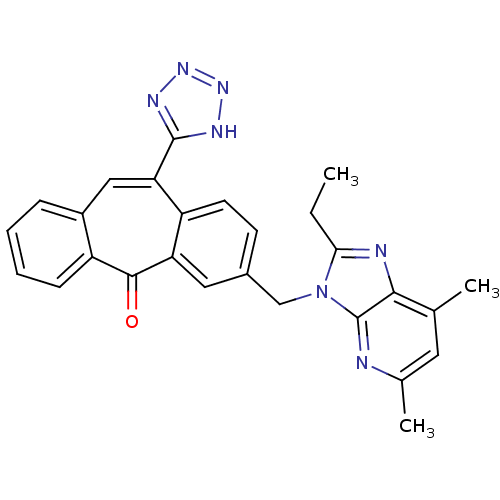
(3-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-yl...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc2c(cc3ccccc3c(=O)c2c1)-c1nnn[nH]1 Show InChI InChI=1S/C27H23N7O/c1-4-23-29-24-15(2)11-16(3)28-27(24)34(23)14-17-9-10-20-21(12-17)25(35)19-8-6-5-7-18(19)13-22(20)26-30-32-33-31-26/h5-13H,4,14H2,1-3H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50006909
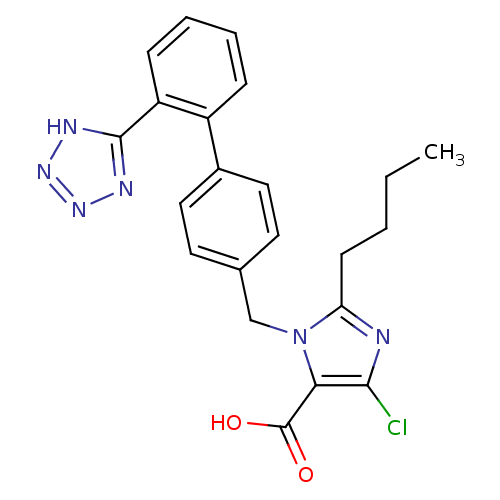
(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287290
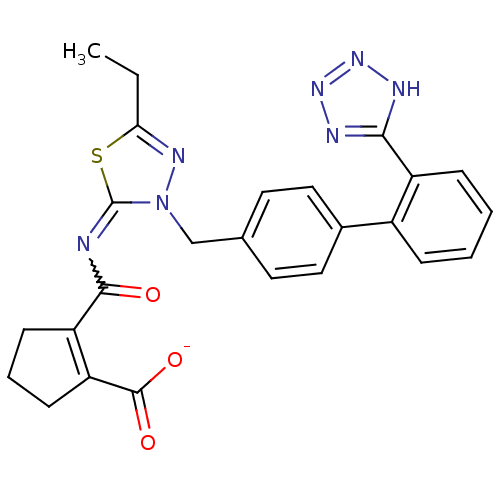
(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 1 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031955
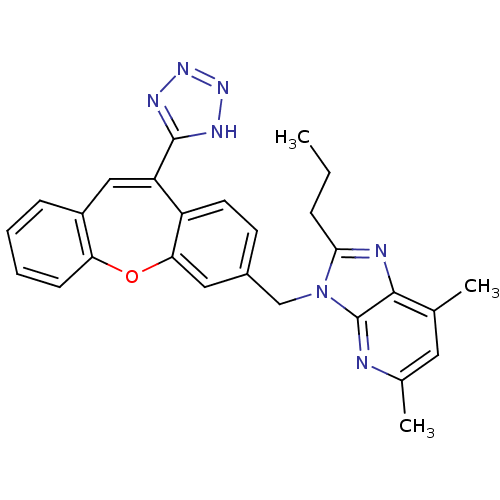
(5,7-Dimethyl-2-propyl-3-[11-(1H-tetrazol-5-yl)-dib...)Show SMILES CCCc1nc2c(C)cc(C)nc2n1Cc1ccc2c(Oc3ccccc3C=C2c2nnn[nH]2)c1 |c:30| Show InChI InChI=1S/C27H25N7O/c1-4-7-24-29-25-16(2)12-17(3)28-27(25)34(24)15-18-10-11-20-21(26-30-32-33-31-26)14-19-8-5-6-9-22(19)35-23(20)13-18/h5-6,8-14H,4,7,15H2,1-3H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313242
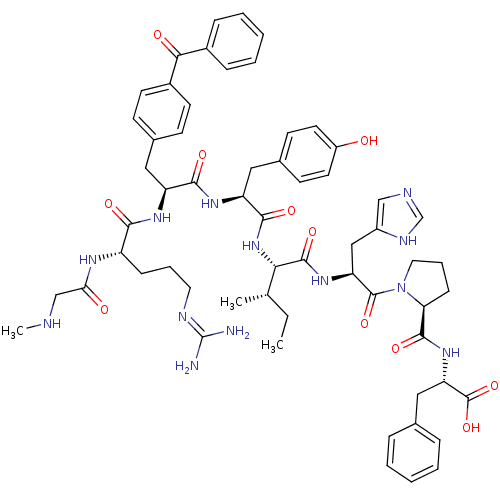
(CHEMBL1076603 | [Sar1,Bpa3]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,56.58,69.74,20.38,wD:2.2,39.49,73.77,8.17,(34.67,-36.98,;34.66,-38.57,;35.98,-39.34,;37.32,-38.56,;35.98,-40.87,;34.66,-41.63,;33.31,-40.85,;33.31,-39.33,;31.98,-41.61,;31.98,-43.17,;33.31,-43.93,;34.64,-43.18,;35.97,-43.96,;35.95,-45.5,;37.29,-46.29,;34.62,-46.26,;33.3,-45.48,;30.65,-40.84,;29.32,-41.62,;29.32,-43.17,;27.98,-40.84,;27.98,-39.31,;27.19,-37.97,;27.96,-36.62,;27.17,-35.29,;25.62,-35.3,;24.86,-36.66,;25.65,-37.99,;24.84,-33.97,;25.6,-32.63,;23.3,-33.98,;22.53,-32.66,;20.99,-32.67,;20.23,-34.01,;21.02,-35.34,;22.55,-35.32,;26.65,-41.61,;25.31,-40.84,;25.31,-39.31,;23.98,-41.61,;23.98,-43.16,;25.31,-43.92,;25.31,-45.46,;26.65,-46.23,;26.66,-47.77,;27.99,-48.53,;25.32,-48.54,;22.65,-40.82,;21.32,-41.59,;21.32,-43.14,;19.99,-40.83,;18.66,-41.59,;17.33,-40.82,;37.32,-41.64,;37.32,-43.19,;38.64,-40.88,;39.99,-41.65,;41.31,-40.89,;41.31,-39.36,;40.09,-38.45,;40.57,-36.99,;42.11,-37,;42.57,-38.47,;39.99,-43.21,;38.64,-43.98,;41.31,-43.97,;42.89,-43.43,;43.91,-44.76,;42.97,-46.14,;41.57,-45.46,;40.34,-46.39,;38.96,-45.72,;40.46,-47.92,;39.18,-48.77,;37.79,-48.1,;36.52,-48.95,;36.62,-50.49,;35.35,-51.35,;33.97,-50.68,;33.86,-49.14,;35.13,-48.28,;39.28,-50.31,;38.01,-51.17,;40.67,-50.98,)| Show InChI InChI=1S/C60H75N13O11/c1-4-36(2)51(57(81)70-47(32-42-33-64-35-66-42)58(82)73-28-12-18-49(73)56(80)71-48(59(83)84)31-37-13-7-5-8-14-37)72-55(79)46(30-39-21-25-43(74)26-22-39)69-54(78)45(29-38-19-23-41(24-20-38)52(76)40-15-9-6-10-16-40)68-53(77)44(67-50(75)34-63-3)17-11-27-65-60(61)62/h5-10,13-16,19-26,33,35-36,44-49,51,63,74H,4,11-12,17-18,27-32,34H2,1-3H3,(H,64,66)(H,67,75)(H,68,77)(H,69,78)(H,70,81)(H,71,80)(H,72,79)(H,83,84)(H4,61,62,65)/t36-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50159153

(CHEMBL3787243)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN\C(N)=N\C(=O)NCCCCNC(=O)CC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C58H85N15O14.3C2HF3O2/c1-6-34(5)48(54(83)68-42(29-37-31-61-32-65-37)55(84)73-26-14-18-44(73)52(81)69-43(56(85)86)28-35-15-9-8-10-16-35)71-51(80)41(27-36-19-21-38(74)22-20-36)67-53(82)47(33(3)4)70-50(79)40(66-49(78)39(59)30-46(76)77)17-13-25-63-57(60)72-58(87)64-24-12-11-23-62-45(75)7-2;3*3-2(4,5)1(6)7/h8-10,15-16,19-22,31-34,39-44,47-48,74H,6-7,11-14,17-18,23-30,59H2,1-5H3,(H,61,65)(H,62,75)(H,66,78)(H,67,82)(H,68,83)(H,69,81)(H,70,79)(H,71,80)(H,76,77)(H,85,86)(H4,60,63,64,72,87);3*(H,6,7)/t34-,39-,40-,41-,42-,43-,44-,47-,48-;;;/m0.../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Asp-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-ValTyr-Ile-His-Pro-Phe-OH Tris(hydrotrifluoroacetate) from human AT1 re... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003154
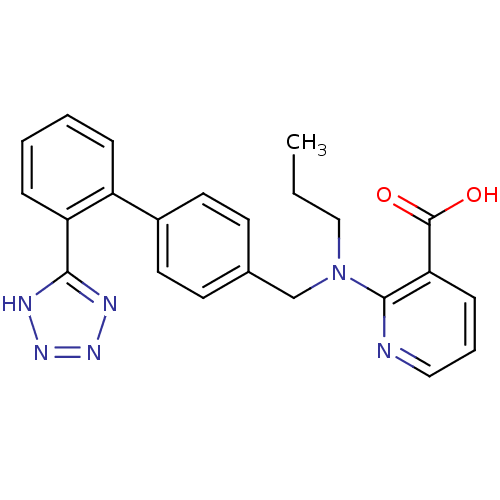
(2-{Propyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncccc1C(O)=O Show InChI InChI=1S/C23H22N6O2/c1-2-14-29(22-20(23(30)31)8-5-13-24-22)15-16-9-11-17(12-10-16)18-6-3-4-7-19(18)21-25-27-28-26-21/h3-13H,2,14-15H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against angiotensin II receptor, type 1 in rat liver |
J Med Chem 35: 3714-7 (1992)
BindingDB Entry DOI: 10.7270/Q2H41QCN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503902

(CHEMBL4588688)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1F)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H36FN3O4S/c1-5-25-26(15-31-27(34)21(17-38)13-18(3)4)33(29(32-25)37-6-2)16-20-12-11-19(14-24(20)30)22-9-7-8-10-23(22)28(35)36/h7-12,14,18,21,38H,5-6,13,15-17H2,1-4H3,(H,31,34)(H,35,36)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449917
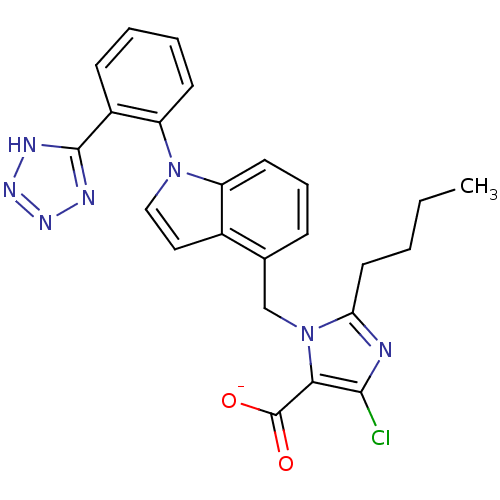
(BMS-180560 | CHEMBL2021417)Show SMILES [Li+].[Li]O.CCCCc1nc(Cl)c(C([O-])=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C24H22ClN7O2.2Li.H2O/c1-2-3-11-20-26-22(25)21(24(33)34)32(20)14-15-7-6-10-18-16(15)12-13-31(18)19-9-5-4-8-17(19)23-27-29-30-28-23;;;/h4-10,12-13H,2-3,11,14H2,1H3,(H,33,34)(H,27,28,29,30);;;1H2/q;2*+1;/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175523
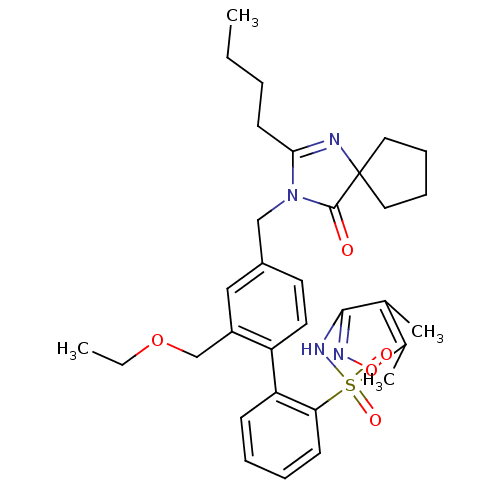
(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(COCC)c1)-c1ccccc1S(=O)(=O)Nc1noc(C)c1C |t:4| Show InChI InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313247

(CHEMBL1076632 | [Sar1,Bpa8]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(O)=O |r,wU:44.44,57.60,4.4,20.21,wD:2.2,24.32,8.17,61.63,(38.66,-15.79,;38.59,-17.33,;39.9,-18.15,;41.26,-17.44,;39.84,-19.68,;38.49,-20.39,;37.17,-19.56,;37.23,-18.03,;35.82,-20.27,;35.76,-21.82,;37.05,-22.64,;38.41,-21.94,;39.71,-22.77,;39.63,-24.31,;40.93,-25.15,;38.27,-25.02,;36.97,-24.19,;34.51,-19.45,;33.15,-20.17,;33.09,-21.73,;31.84,-19.34,;30.47,-20.06,;29.17,-19.24,;29.23,-17.71,;27.81,-19.95,;27.75,-21.5,;29.05,-22.32,;28.99,-23.86,;30.3,-24.67,;30.24,-26.21,;31.55,-27.03,;28.88,-26.93,;26.52,-19.12,;25.16,-19.84,;25.1,-21.38,;23.85,-19.02,;22.5,-19.73,;21.2,-18.91,;31.9,-17.81,;33.26,-17.1,;30.59,-16.99,;41.15,-20.51,;41.09,-22.06,;42.5,-19.8,;43.86,-20.56,;45.17,-19.78,;45.16,-18.24,;46.4,-17.34,;45.91,-15.88,;44.37,-15.89,;43.91,-17.36,;43.87,-22.12,;42.53,-22.91,;45.21,-22.86,;46.59,-22.21,;47.62,-23.33,;46.89,-24.66,;45.39,-24.37,;44.12,-25.24,;42.77,-24.5,;44.15,-26.78,;45.26,-27.84,;44.88,-29.33,;45.98,-30.41,;45.6,-31.9,;46.71,-32.97,;48.19,-32.55,;48.57,-31.06,;47.47,-29.99,;49.3,-33.63,;50.78,-33.21,;48.92,-35.12,;47.43,-35.53,;47.06,-37.03,;48.16,-38.1,;49.65,-37.68,;50.02,-36.18,;46.74,-27.42,;47.85,-28.5,;47.12,-25.93,)| Show InChI InChI=1S/C56H75N13O11/c1-6-33(4)47(68-50(74)41(26-35-18-22-39(70)23-19-35)64-52(76)46(32(2)3)67-49(73)40(63-45(71)30-59-5)14-10-24-61-56(57)58)53(77)65-42(28-38-29-60-31-62-38)54(78)69-25-11-15-44(69)51(75)66-43(55(79)80)27-34-16-20-37(21-17-34)48(72)36-12-8-7-9-13-36/h7-9,12-13,16-23,29,31-33,40-44,46-47,59,70H,6,10-11,14-15,24-28,30H2,1-5H3,(H,60,62)(H,63,71)(H,64,76)(H,65,77)(H,66,75)(H,67,73)(H,68,74)(H,79,80)(H4,57,58,61)/t33-,40-,41-,42-,43-,44-,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175523

(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(COCC)c1)-c1ccccc1S(=O)(=O)Nc1noc(C)c1C |t:4| Show InChI InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313240

(CHEMBL1076633 | [Sar1,Tdf8]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)C(O)=O |r,wU:44.44,57.60,4.4,20.21,wD:2.2,24.32,8.17,61.63,c:74,(7.79,3.66,;7.73,2.12,;9.03,1.3,;10.39,2.02,;8.97,-.22,;7.62,-.93,;6.31,-.11,;6.37,1.42,;4.95,-.81,;4.89,-2.36,;6.19,-3.18,;7.54,-2.48,;8.84,-3.32,;8.76,-4.86,;10.06,-5.69,;7.41,-5.56,;6.11,-4.73,;3.65,0,;2.28,-.72,;2.22,-2.27,;.97,.11,;-.39,-.61,;-1.69,.22,;-1.63,1.75,;-3.05,-.49,;-3.12,-2.04,;-1.82,-2.86,;-1.87,-4.4,;-.57,-5.22,;-.62,-6.75,;.68,-7.57,;-1.98,-7.48,;-4.35,.34,;-5.71,-.38,;-5.77,-1.92,;-7.01,.43,;-8.36,-.28,;-9.67,.55,;1.03,1.64,;2.39,2.36,;-.27,2.46,;10.28,-1.05,;10.22,-2.6,;11.63,-.34,;12.99,-1.1,;14.3,-.33,;14.29,1.21,;15.53,2.12,;15.04,3.58,;13.5,3.57,;13.04,2.1,;13,-2.66,;11.66,-3.45,;14.34,-3.4,;15.72,-2.76,;16.75,-3.87,;16.02,-5.2,;14.52,-4.91,;13.25,-5.78,;11.9,-5.04,;13.28,-7.32,;14.39,-8.38,;14.01,-9.87,;15.11,-10.95,;14.73,-12.44,;15.84,-13.51,;17.32,-13.09,;17.7,-11.6,;16.6,-10.53,;18.43,-14.17,;18.85,-15.65,;19.92,-14.54,;19.76,-13.38,;21.1,-14.14,;19.74,-11.84,;20.85,-12.29,;15.87,-7.97,;16.97,-9.04,;16.25,-6.48,)| Show InChI InChI=1S/C51H70F3N15O10/c1-6-28(4)41(66-43(73)35(21-30-13-17-33(70)18-14-30)62-45(75)40(27(2)3)65-42(72)34(61-39(71)25-57-5)9-7-19-59-49(55)56)46(76)63-36(23-32-24-58-26-60-32)47(77)69-20-8-10-38(69)44(74)64-37(48(78)79)22-29-11-15-31(16-12-29)50(67-68-50)51(52,53)54/h11-18,24,26-28,34-38,40-41,57,70H,6-10,19-23,25H2,1-5H3,(H,58,60)(H,61,71)(H,62,75)(H,63,76)(H,64,74)(H,65,72)(H,66,73)(H,78,79)(H4,55,56,59)/t28-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50006909

(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50004154

(2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(O)=O |c:7,t:4| Show InChI InChI=1S/C24H25ClN6O2/c1-3-4-9-20-26-22(25)21(24(32)33)15(2)31(20)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)23-27-29-30-28-23/h5-8,10-13,15H,3-4,9,14H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50039364
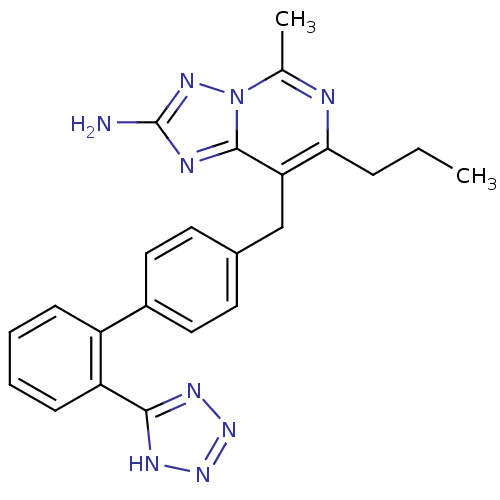
(5-Methyl-7-propyl-8-[2'-(1H-tetrazol-5-yl)-bipheny...)Show SMILES CCCc1nc(C)n2nc(N)nc2c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H23N9/c1-3-6-20-19(22-26-23(24)29-32(22)14(2)25-20)13-15-9-11-16(12-10-15)17-7-4-5-8-18(17)21-27-30-31-28-21/h4-5,7-12H,3,6,13H2,1-2H3,(H2,24,29)(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carpibem
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Sar1-Ile8-A II from rat adrenal Angiotensin-1 (AT-1) receptor |
J Med Chem 37: 2371-86 (1994)
BindingDB Entry DOI: 10.7270/Q2DR2TJS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004154

(2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(O)=O |c:7,t:4| Show InChI InChI=1S/C24H25ClN6O2/c1-3-4-9-20-26-22(25)21(24(32)33)15(2)31(20)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)23-27-29-30-28-23/h5-8,10-13,15H,3-4,9,14H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004155
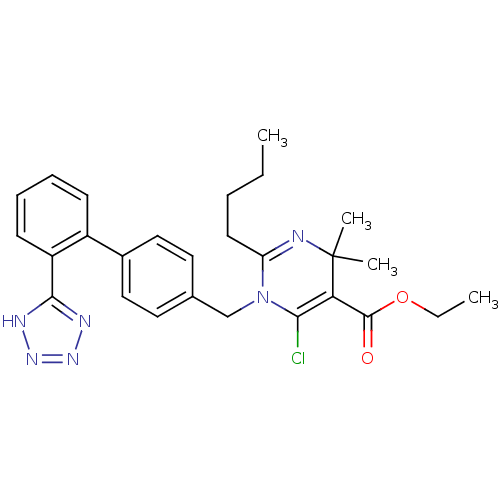
(2-Butyl-6-chloro-4,4-dimethyl-1-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C)C(C(=O)OCC)=C(Cl)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4,14| Show InChI InChI=1S/C27H31ClN6O2/c1-5-7-12-22-29-27(3,4)23(26(35)36-6-2)24(28)34(22)17-18-13-15-19(16-14-18)20-10-8-9-11-21(20)25-30-32-33-31-25/h8-11,13-16H,5-7,12,17H2,1-4H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031964
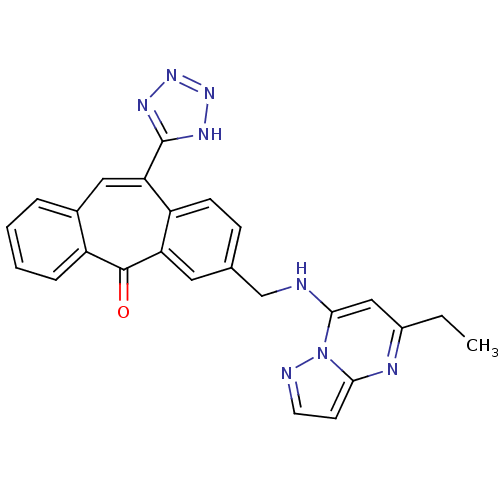
(3-[(5-Ethyl-pyrazolo[1,5-a]pyrimidin-7-ylamino)-me...)Show SMILES CCc1cc(NCc2ccc3c(cc4ccccc4c(=O)c3c2)-c2nnn[nH]2)n2nccc2n1 Show InChI InChI=1S/C25H20N8O/c1-2-17-13-23(33-22(28-17)9-10-27-33)26-14-15-7-8-19-20(11-15)24(34)18-6-4-3-5-16(18)12-21(19)25-29-31-32-30-25/h3-13,26H,2,14H2,1H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049189
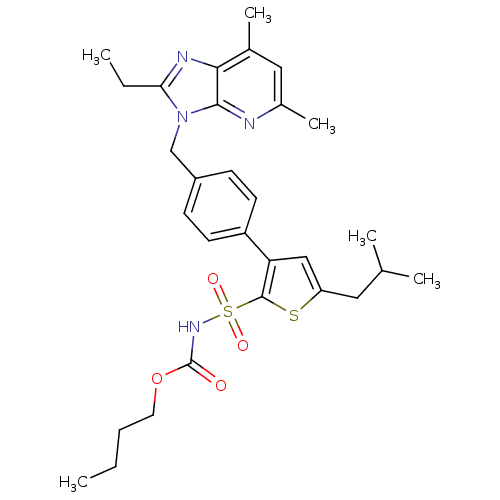
(3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C30H38N4O4S2/c1-7-9-14-38-30(35)33-40(36,37)29-25(17-24(39-29)15-19(3)4)23-12-10-22(11-13-23)18-34-26(8-2)32-27-20(5)16-21(6)31-28(27)34/h10-13,16-17,19H,7-9,14-15,18H2,1-6H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116790
BindingDB Entry DOI: 10.7270/Q2ZW1QXF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50287290

(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503894

(CHEMBL4591218)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H37N3O4S/c1-5-25-26(16-30-27(33)22(18-37)15-19(3)4)32(29(31-25)36-6-2)17-20-11-13-21(14-12-20)23-9-7-8-10-24(23)28(34)35/h7-14,19,22,37H,5-6,15-18H2,1-4H3,(H,30,33)(H,34,35)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503896
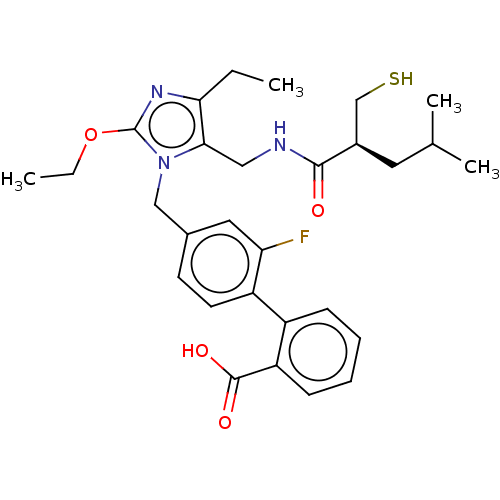
(CHEMBL4445778)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(c(F)c1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H36FN3O4S/c1-5-25-26(15-31-27(34)20(17-38)13-18(3)4)33(29(32-25)37-6-2)16-19-11-12-22(24(30)14-19)21-9-7-8-10-23(21)28(35)36/h7-12,14,18,20,38H,5-6,13,15-17H2,1-4H3,(H,31,34)(H,35,36)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313241

(CHEMBL1076604 | [Sar1,Tdf3]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,55.57,68.73,20.37,wD:2.2,38.48,72.76,8.17,c:31,(7.37,.5,;7.36,-1.08,;8.67,-1.85,;10.02,-1.07,;8.67,-3.38,;7.36,-4.13,;6.01,-3.36,;6.01,-1.84,;4.68,-4.12,;4.68,-5.67,;6.01,-6.44,;7.34,-5.69,;8.67,-6.47,;8.65,-8.01,;9.98,-8.8,;7.32,-8.76,;5.99,-7.99,;3.35,-3.35,;2.02,-4.12,;2.02,-5.68,;.68,-3.35,;.68,-1.82,;-.11,-.48,;.66,.87,;-.13,2.2,;-1.68,2.19,;-2.44,.83,;-1.65,-.5,;-2.47,3.52,;-2.48,5.06,;-3.81,4.27,;-3.81,2.76,;-5.13,3.53,;-3.82,1.22,;-5.14,1.99,;-.65,-4.12,;-1.99,-3.35,;-1.99,-1.82,;-3.32,-4.12,;-3.32,-5.67,;-1.99,-6.43,;-1.99,-7.97,;-.65,-8.73,;-.65,-10.27,;.69,-11.04,;-1.98,-11.05,;-4.65,-3.33,;-5.98,-4.1,;-5.98,-5.64,;-7.31,-3.34,;-8.63,-4.1,;-9.97,-3.33,;10.02,-4.15,;10.02,-5.7,;11.34,-3.39,;12.68,-4.16,;14.01,-3.4,;14.01,-1.87,;12.78,-.96,;13.27,.5,;14.81,.49,;15.27,-.98,;12.68,-5.72,;11.34,-6.49,;14.01,-6.48,;15.59,-5.94,;16.61,-7.27,;15.66,-8.65,;14.27,-7.97,;13.04,-8.9,;11.66,-8.22,;13.16,-10.43,;11.87,-11.28,;10.49,-10.61,;9.22,-11.46,;9.32,-13,;8.05,-13.86,;6.67,-13.19,;6.56,-11.65,;7.83,-10.79,;11.98,-12.81,;10.71,-13.68,;13.36,-13.49,)| Show InChI InChI=1S/C55H70F3N15O10/c1-4-31(2)45(50(80)68-41(27-36-28-62-30-64-36)51(81)73-23-9-13-43(73)49(79)69-42(52(82)83)26-32-10-6-5-7-11-32)70-48(78)40(25-34-16-20-37(74)21-17-34)67-47(77)39(24-33-14-18-35(19-15-33)54(71-72-54)55(56,57)58)66-46(76)38(65-44(75)29-61-3)12-8-22-63-53(59)60/h5-7,10-11,14-21,28,30-31,38-43,45,61,74H,4,8-9,12-13,22-27,29H2,1-3H3,(H,62,64)(H,65,75)(H,66,76)(H,67,77)(H,68,80)(H,69,79)(H,70,78)(H,82,83)(H4,59,60,63)/t31-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503875
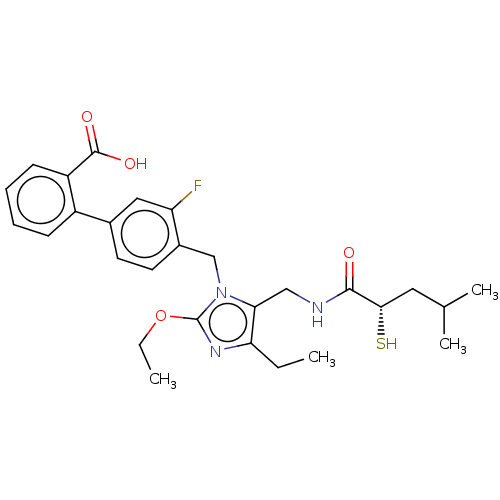
(CHEMBL4435800)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](S)CC(C)C)n1Cc1ccc(cc1F)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C28H34FN3O4S/c1-5-23-24(15-30-26(33)25(37)13-17(3)4)32(28(31-23)36-6-2)16-19-12-11-18(14-22(19)29)20-9-7-8-10-21(20)27(34)35/h7-12,14,17,25,37H,5-6,13,15-16H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031956
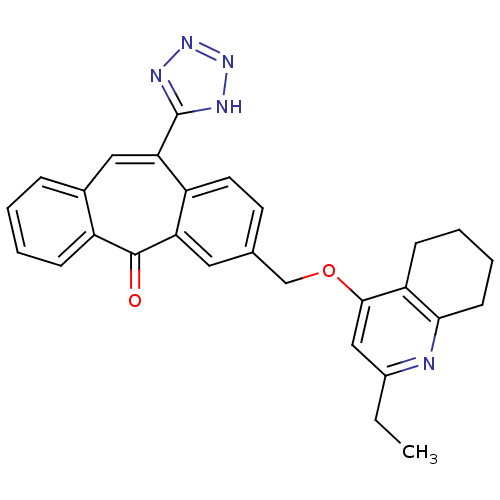
(3-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...)Show SMILES CCc1cc(OCc2ccc3c(cc4ccccc4c(=O)c3c2)-c2nnn[nH]2)c2CCCCc2n1 Show InChI InChI=1S/C28H25N5O2/c1-2-19-15-26(22-9-5-6-10-25(22)29-19)35-16-17-11-12-21-23(13-17)27(34)20-8-4-3-7-18(20)14-24(21)28-30-32-33-31-28/h3-4,7-8,11-15H,2,5-6,9-10,16H2,1H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 |
J Med Chem 38: 2728-41 (1995)
BindingDB Entry DOI: 10.7270/Q23B5Z5Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50291668

(CHEMBL4160691)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(C)C)[C@@H](C)CC)C(O)=O |r| Show InChI InChI=1S/C46H73N13O11/c1-7-25(5)36(43(67)55-33(20-28-21-50-23-52-28)44(68)59-18-10-12-34(59)41(65)58-37(45(69)70)26(6)8-2)57-40(64)32(19-27-13-15-29(61)16-14-27)54-42(66)35(24(3)4)56-39(63)31(11-9-17-51-46(48)49)53-38(62)30(47)22-60/h13-16,21,23-26,30-37,60-61H,7-12,17-20,22,47H2,1-6H3,(H,50,52)(H,53,62)(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,58,65)(H,69,70)(H4,48,49,51)/t25-,26-,30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahcesehir University (BAU)
Curated by ChEMBL
| Assay Description
Displacement of [125I-Sar1-Ile8]-Ang2 from human angiotensin 2 receptor type 1 receptor expressed in HEK293 cells after 1 hr by gamma counting analys... |
Eur J Med Chem 145: 273-290 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.021
BindingDB Entry DOI: 10.7270/Q2639S81 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030727
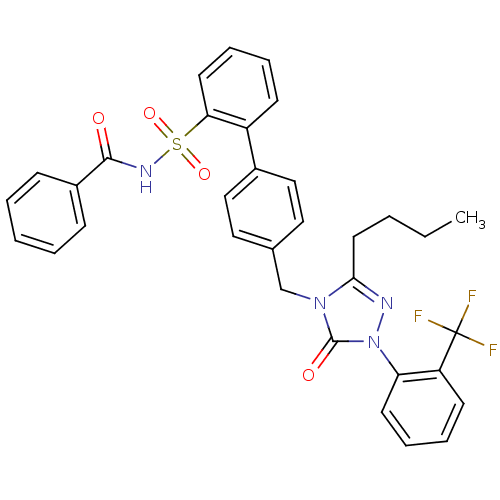
(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data