

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50024968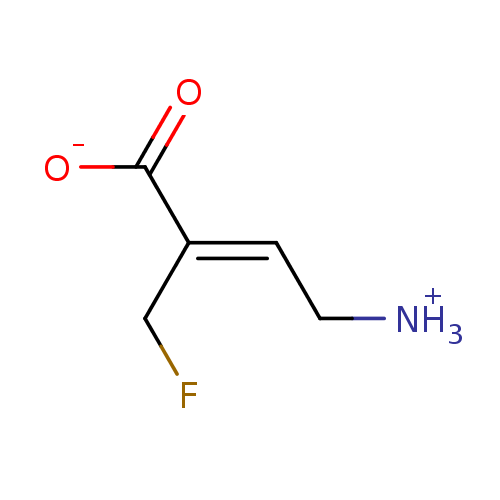 ((2Z)-4-ammonio-2-(fluoromethyl)but-2-enoate | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory effect against Gamma-amino-N-butyrate transaminase from bacteria | J Med Chem 29: 764-70 (1986) BindingDB Entry DOI: 10.7270/Q2668C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50021645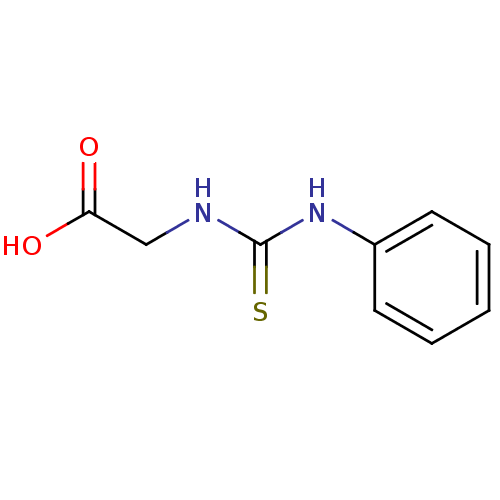 ((3-Phenyl-thioureido)-acetic acid | CHEMBL311337 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Gamma-amino-N-butyrate transaminase | J Med Chem 30: 239-49 (1987) BindingDB Entry DOI: 10.7270/Q2SF2V5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118885 (5-Amino-cyclohex-3-enecarboxylic acid | CHEMBL1379...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Binding affinity against Gamma-amino-N-butyrate transaminase | J Med Chem 45: 4531-9 (2002) BindingDB Entry DOI: 10.7270/Q2G1605T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118884 (2-Amino-cyclohex-3-enecarboxylic acid | CHEMBL1419...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Binding affinity against Gamma-amino-N-butyrate transaminase | J Med Chem 45: 4531-9 (2002) BindingDB Entry DOI: 10.7270/Q2G1605T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323799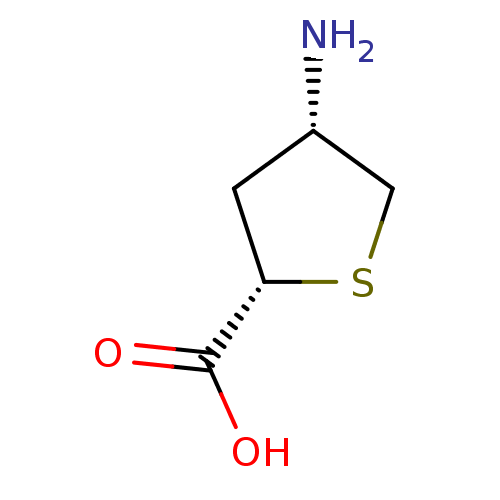 ((2S,4S)-4-Aminotetrahydrothiophene-2-carboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466532 (US10800753, Compound 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118886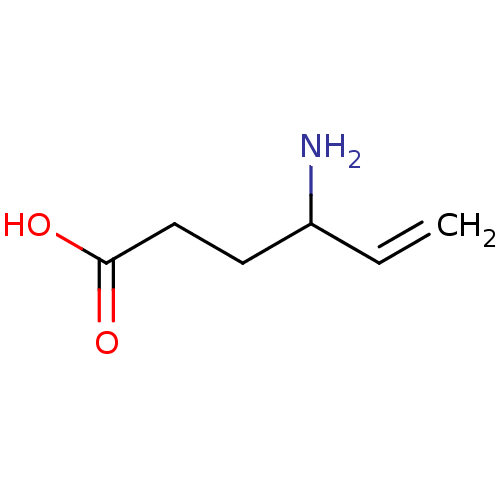 (4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Concentration dependent inactivation of Gamma-amino-N-butyrate transaminase | J Med Chem 45: 4531-9 (2002) BindingDB Entry DOI: 10.7270/Q2G1605T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323800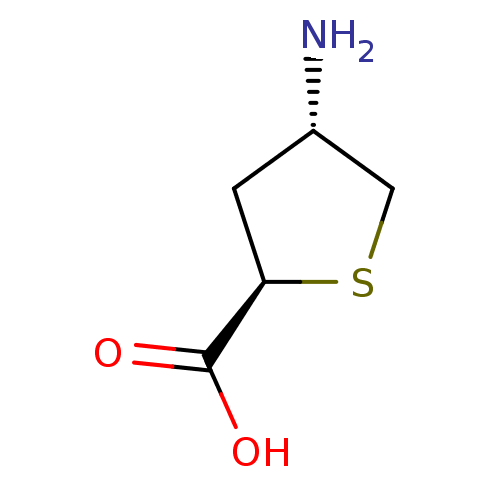 ((2R,4S)-4-Aminotetrahydrothiophene-2-carboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323800 ((2R,4S)-4-Aminotetrahydrothiophene-2-carboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118888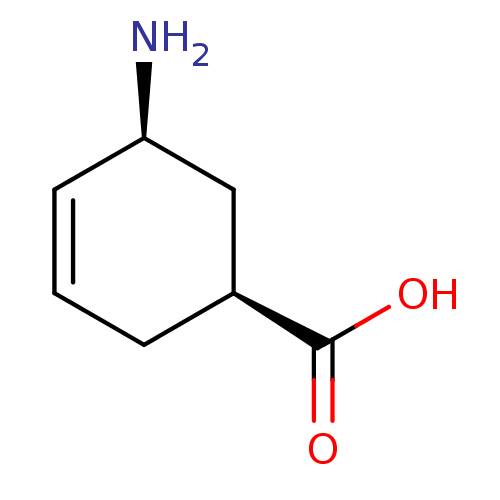 (5-Amino-cyclohex-3-enecarboxylic acid | CHEMBL3368...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Concentration dependent inactivation of gGamma-amino-N-butyrate transaminase | J Med Chem 45: 4531-9 (2002) BindingDB Entry DOI: 10.7270/Q2G1605T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118886 (4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inactivation of GABA-AT | J Med Chem 55: 357-66 (2012) Article DOI: 10.1021/jm201231w BindingDB Entry DOI: 10.7270/Q2QF8V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323801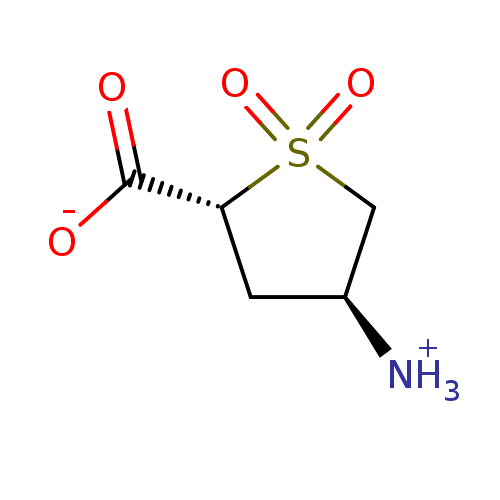 ((4S)-4-Aminotetrahydrothiophene-2-carboxylic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466536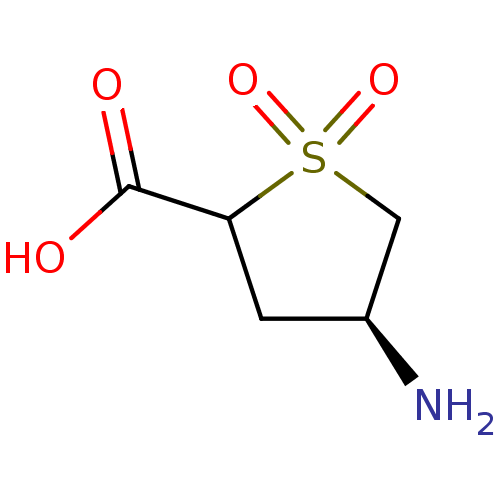 (US10800753, Compound 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118886 (4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank US Patent | 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50357219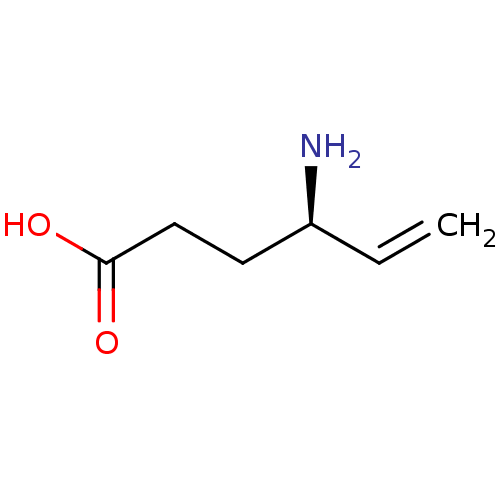 (CHEMBL1361446 | US10800753, Compound (S)-vigabatri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466538 (US10800753, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323803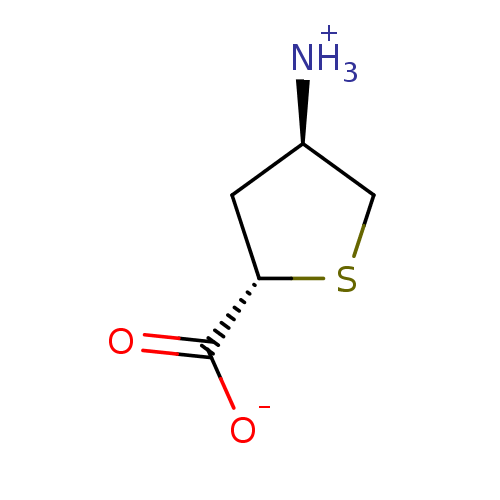 (US10189807, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466537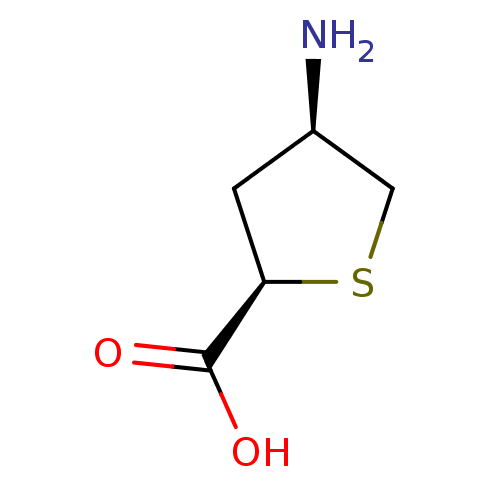 (US10800753, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323802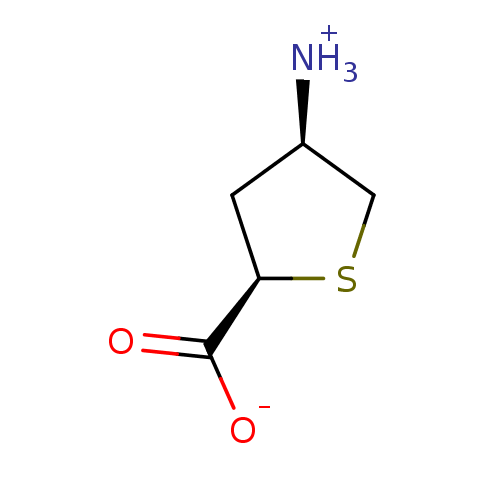 (US10189807, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50082127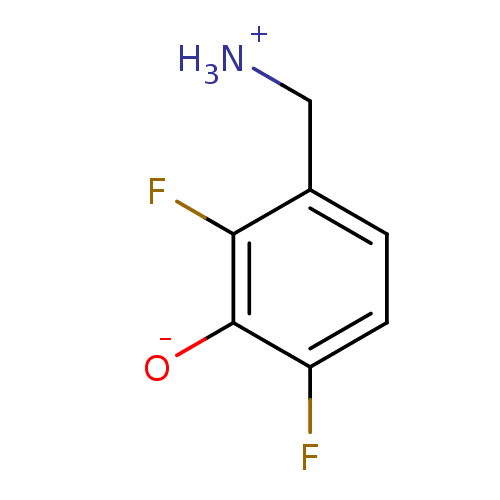 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Competitive inhibition of GABA aminotransferase (unknown origin) | J Med Chem 61: 5822-5880 (2018) Article DOI: 10.1021/acs.jmedchem.7b01788 BindingDB Entry DOI: 10.7270/Q232008T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50082127 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of GABA aminotransferase | J Med Chem 54: 2529-91 (2011) Article DOI: 10.1021/jm1013693 BindingDB Entry DOI: 10.7270/Q24M95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466539 (US10800753, Compound 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 7.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... | US Patent US10800753 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM323805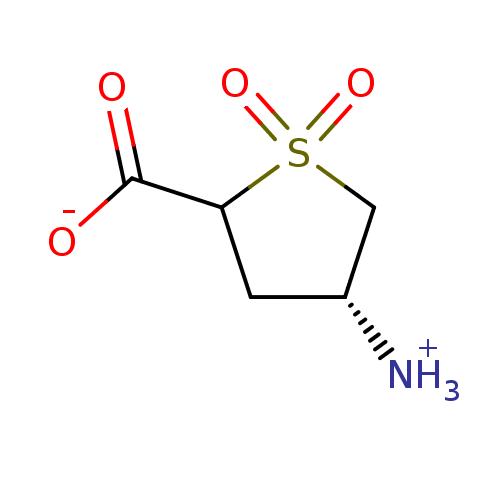 (US10189807, Compound 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 7.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... | US Patent US10189807 (2019) BindingDB Entry DOI: 10.7270/Q2C82CDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50073151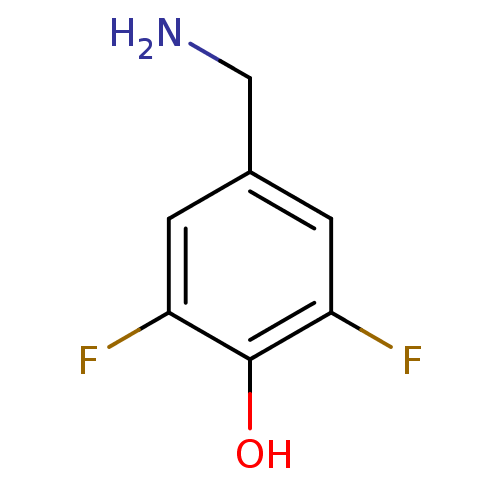 (4-(ammoniomethyl)-2,6-difluorobenzenolate | 4-Amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Competitive inhibition of GABA aminotransferase (unknown origin) | J Med Chem 61: 5822-5880 (2018) Article DOI: 10.1021/acs.jmedchem.7b01788 BindingDB Entry DOI: 10.7270/Q232008T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50073151 (4-(ammoniomethyl)-2,6-difluorobenzenolate | 4-Amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of GABA aminotransferase | J Med Chem 54: 2529-91 (2011) Article DOI: 10.1021/jm1013693 BindingDB Entry DOI: 10.7270/Q24M95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50118887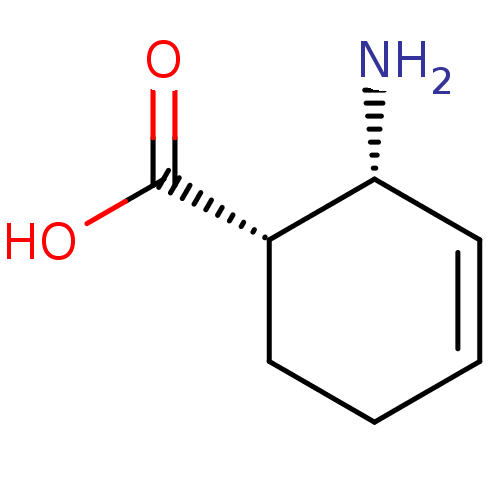 (2-Amino-cyclohex-3-enecarboxylic acid | CHEMBL1384...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Concentration dependent inactivation of Gamma-amino-N-butyrate transaminase | J Med Chem 45: 4531-9 (2002) BindingDB Entry DOI: 10.7270/Q2G1605T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||