

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactosidase (Escherichia coli) | BDBM50140062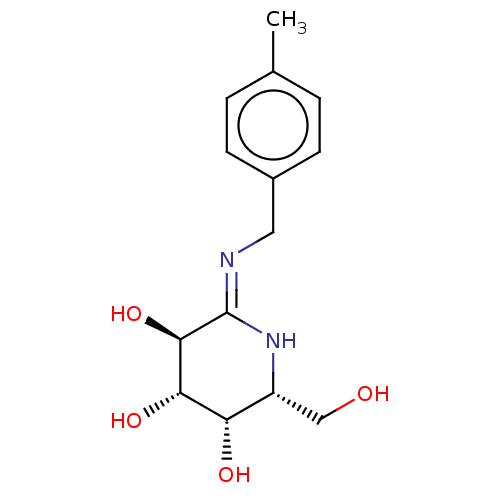 (CHEMBL3359672) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli beta-galactosidase assessed as p-nitrophenyl-beta-D-galactopyranoside substrate hydrolysis by UV/Vis spect... | Bioorg Med Chem 24: 661-71 (2016) Article DOI: 10.1016/j.bmc.2015.12.034 BindingDB Entry DOI: 10.7270/Q20P11WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50140061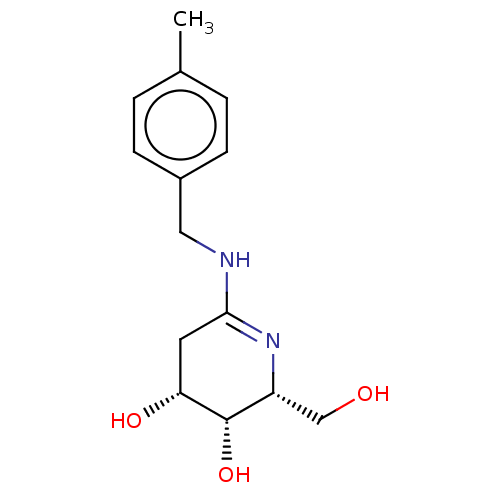 (CHEMBL3752112) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli beta-galactosidase assessed as p-nitrophenyl-beta-D-galactopyranoside substrate hydrolysis by UV/Vis spect... | Bioorg Med Chem 24: 661-71 (2016) Article DOI: 10.1016/j.bmc.2015.12.034 BindingDB Entry DOI: 10.7270/Q20P11WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50150470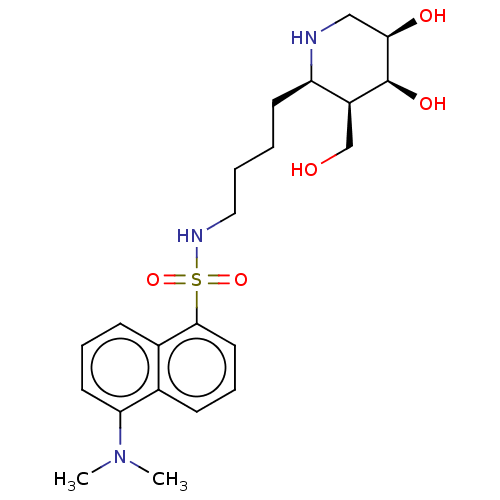 (CHEMBL3771185) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50350758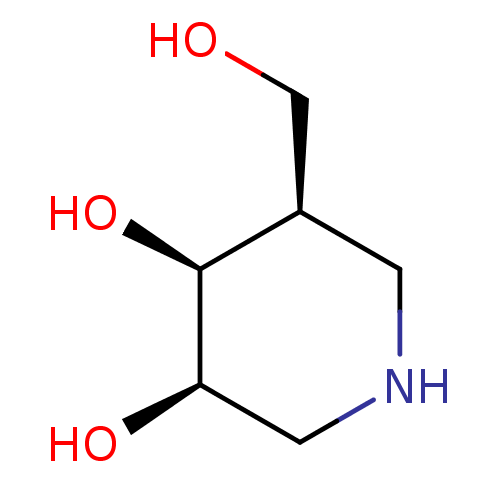 (CHEMBL1818433) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50140059 (CHEMBL3753015) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli beta-galactosidase assessed as p-nitrophenyl-beta-D-galactopyranoside substrate hydrolysis by UV/Vis spect... | Bioorg Med Chem 24: 661-71 (2016) Article DOI: 10.1016/j.bmc.2015.12.034 BindingDB Entry DOI: 10.7270/Q20P11WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50140060 (CHEMBL3754250) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli beta-galactosidase assessed as p-nitrophenyl-beta-D-galactopyranoside substrate hydrolysis by UV/Vis spect... | Bioorg Med Chem 24: 661-71 (2016) Article DOI: 10.1016/j.bmc.2015.12.034 BindingDB Entry DOI: 10.7270/Q20P11WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50150471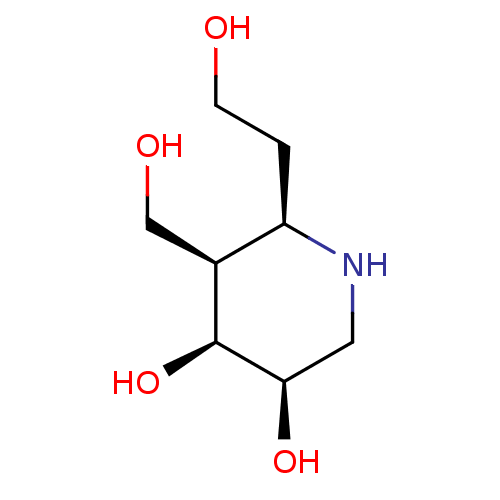 (CHEMBL3770764) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50150472 (CHEMBL3770736) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50254109 (CHEMBL4069909) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human fibroblast lysosomal beta-galactosidase using 4-methylumbelliferyl-beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50358321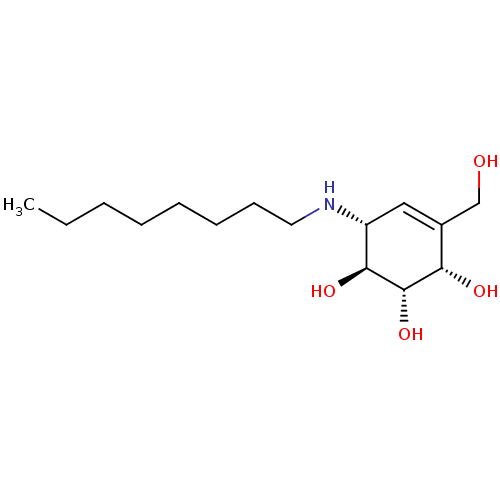 (CHEMBL1922579 | CHEMBL1922581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amicus Therapeutics Curated by ChEMBL | Assay Description Inhibition of human beta galactosidase | J Med Chem 56: 2705-25 (2013) Article DOI: 10.1021/jm301557k BindingDB Entry DOI: 10.7270/Q26111N4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50182795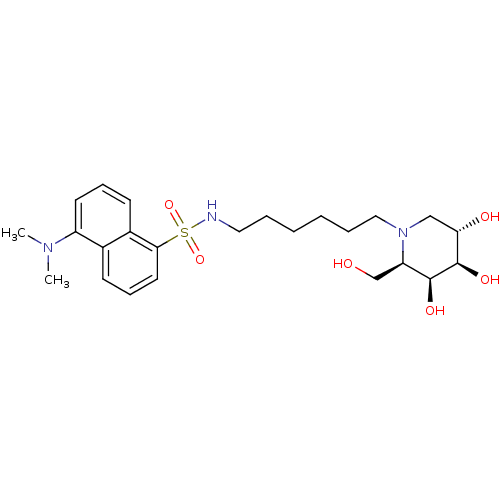 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50358321 (CHEMBL1922579 | CHEMBL1922581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human fibroblast lysosomal beta-galactosidase using 4-methylumbelliferyl-beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50246569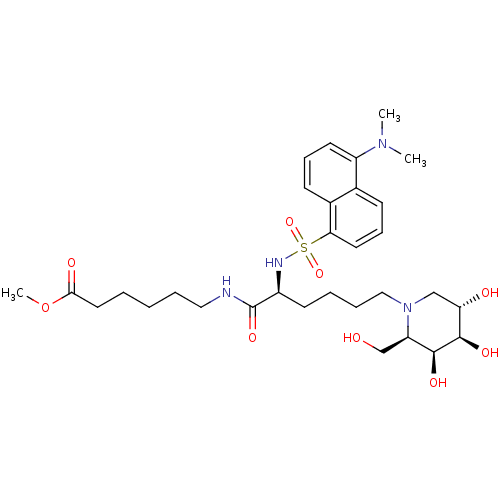 (CHEMBL505422 | Methyl 6-[N2-dansyl-N6-(1,5-dideoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50356096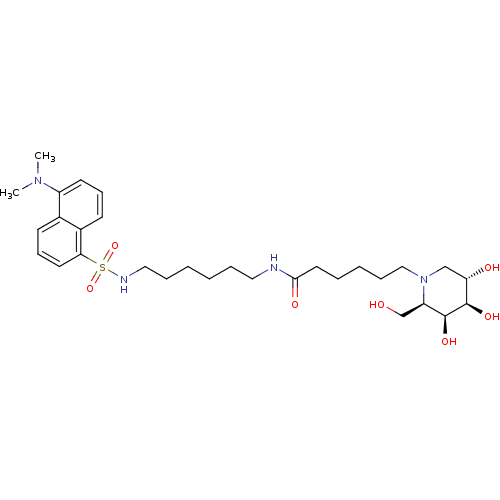 (CHEMBL1911831) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50356097 (CHEMBL461161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50403936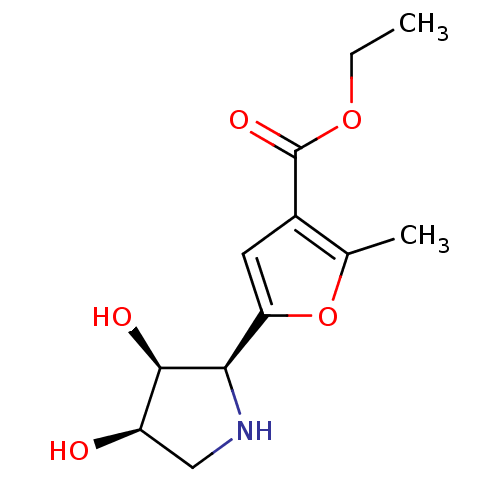 (CHEMBL2114148) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition constant against Beta-galactosidase from Jack beans; Mixed (Non competitive and competitive) | Bioorg Med Chem Lett 12: 2335-9 (2002) BindingDB Entry DOI: 10.7270/Q2GB23CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50403936 (CHEMBL2114148) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibitor concentration of the compound against alpha-L-Fucosidase from Bovine epididymis | Bioorg Med Chem Lett 12: 2335-9 (2002) BindingDB Entry DOI: 10.7270/Q2GB23CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50075942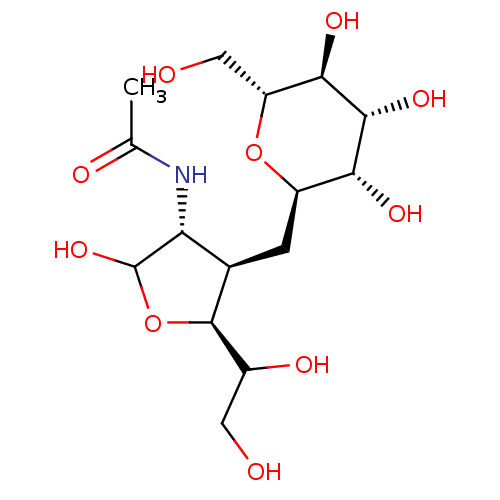 (CHEMBL165192 | N-[2,5-Dihydroxy-6-hydroxymethyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de chimie organique de l'Université de Lausanne Curated by ChEMBL | Assay Description Inhibition constant of the compound against beta-galactosidase enzyme of jack bean was reported | Bioorg Med Chem Lett 9: 793-6 (1999) BindingDB Entry DOI: 10.7270/Q2K073FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50254118 (CHEMBL4090899) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human fibroblast lysosomal beta-galactosidase using 4-methylumbelliferyl-beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50403937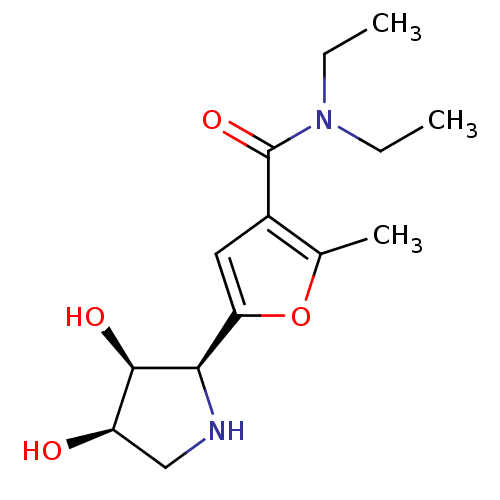 (CHEMBL2114149) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition constant (Competitive) of the compound against Beta-galactosidasee from Aspergillus niger was tested at a dose of 1 mM | Bioorg Med Chem Lett 12: 2335-9 (2002) BindingDB Entry DOI: 10.7270/Q2GB23CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50104296 (2-(2,2-Dihydroxy-ethyl)-pyrrolidine-3,4-diol | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-galactosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50481214 (CHEMBL593646) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase by Lineweaver-Burke plot analysis | Bioorg Med Chem 17: 6203-12 (2009) Article DOI: 10.1016/j.bmc.2009.07.055 BindingDB Entry DOI: 10.7270/Q2R2146V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50073992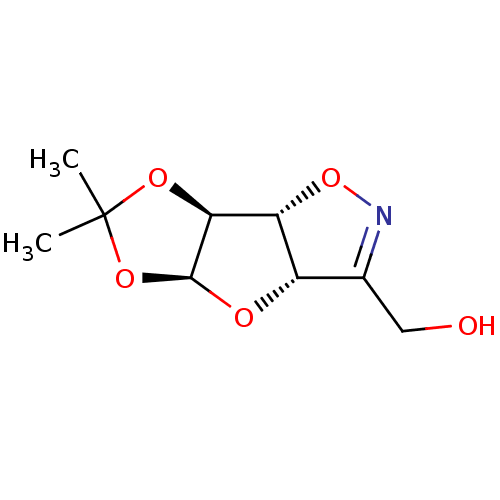 (((3aS,4aS,7aS,7bR)-6,6-Dimethyl-3a,4a,7a,7b-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de chimie organique de l'Université de Lausanne Curated by ChEMBL | Assay Description Inhibition of beta-glucosidase from Caldocellum saccharolyticum | Bioorg Med Chem Lett 9: 277-8 (1999) BindingDB Entry DOI: 10.7270/Q28051T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50403936 (CHEMBL2114148) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition constant (Competitive) of the compound against Beta-galactosidase from Escherichia coli | Bioorg Med Chem Lett 12: 2335-9 (2002) BindingDB Entry DOI: 10.7270/Q2GB23CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50481217 (CHEMBL596003) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase by Lineweaver-Burke plot analysis | Bioorg Med Chem 17: 6203-12 (2009) Article DOI: 10.1016/j.bmc.2009.07.055 BindingDB Entry DOI: 10.7270/Q2R2146V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50481216 (CHEMBL596230) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase by Lineweaver-Burke plot analysis | Bioorg Med Chem 17: 6203-12 (2009) Article DOI: 10.1016/j.bmc.2009.07.055 BindingDB Entry DOI: 10.7270/Q2R2146V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50298560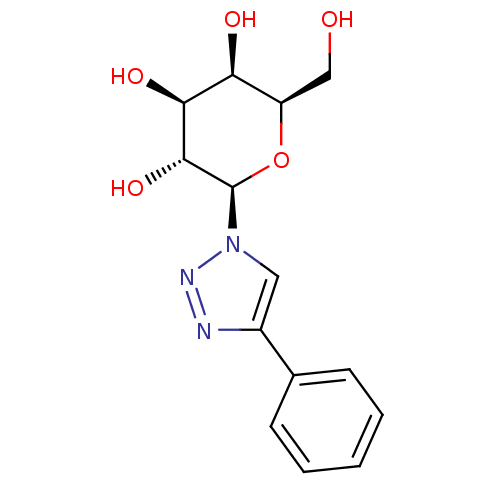 (1-(beta-D-galactopyranosyl)-4-phenyl-1,2,3-triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Katholieke Universiteit Leuven Curated by ChEMBL | Assay Description Inhibition of beta-galactosidase | Bioorg Med Chem 17: 5117-25 (2009) Article DOI: 10.1016/j.bmc.2009.05.056 BindingDB Entry DOI: 10.7270/Q2ZW1M03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50306549 ((2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-(4-phenyl-1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase at 1 mM | Bioorg Med Chem Lett 20: 4263-5 (2010) Article DOI: 10.1016/j.bmcl.2010.04.151 BindingDB Entry DOI: 10.7270/Q26D5V70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50481215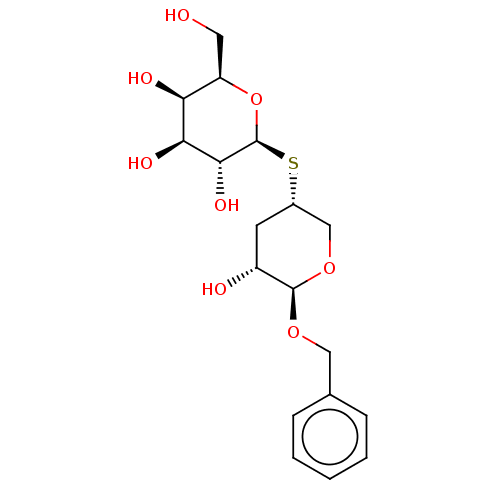 (CHEMBL594698) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase by Lineweaver-Burke plot analysis | Bioorg Med Chem 17: 6203-12 (2009) Article DOI: 10.1016/j.bmc.2009.07.055 BindingDB Entry DOI: 10.7270/Q2R2146V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50279833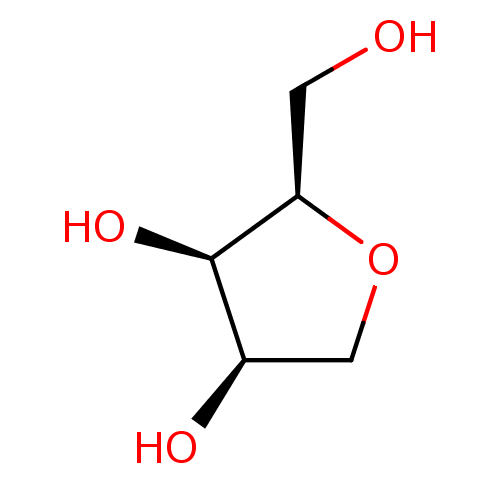 ((2R,3R,4R)-2-Hydroxymethyl-tetrahydro-furan-3,4-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards E-coli Beta-galactosidase | Bioorg Med Chem Lett 1: 667-672 (1991) Article DOI: 10.1016/S0960-894X(01)81044-1 BindingDB Entry DOI: 10.7270/Q26W9BKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50279832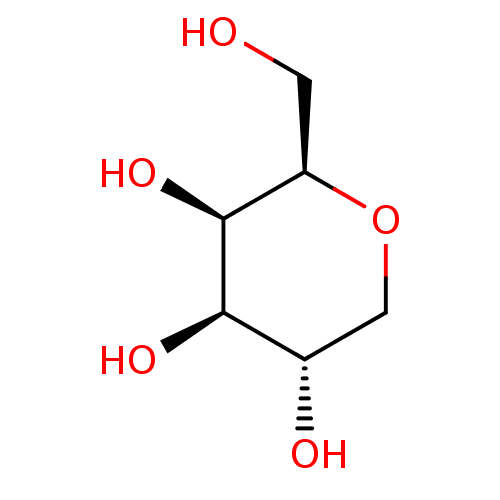 ((2R,3R,4R,5S)-2-Hydroxymethyl-tetrahydro-pyran-3,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 7.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards E-coli Beta-galactosidase | Bioorg Med Chem Lett 1: 667-672 (1991) Article DOI: 10.1016/S0960-894X(01)81044-1 BindingDB Entry DOI: 10.7270/Q26W9BKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50279835 ((2R,3S,4R)-2-Hydroxymethyl-tetrahydro-furan-3,4-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 5.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-galactosidase | Bioorg Med Chem Lett 1: 667-672 (1991) Article DOI: 10.1016/S0960-894X(01)81044-1 BindingDB Entry DOI: 10.7270/Q26W9BKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50279834 ((2R,3S,4R,5S)-2-(hydroxymethyl)oxane-3,4,5-triol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 7.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards E-coli Beta-galactosidase | Bioorg Med Chem Lett 1: 667-672 (1991) Article DOI: 10.1016/S0960-894X(01)81044-1 BindingDB Entry DOI: 10.7270/Q26W9BKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50279836 ((3R,4R)-Tetrahydro-furan-3,4-diol | CHEMBL350524) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article | 7.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards E-coli Beta-galactosidase | Bioorg Med Chem Lett 1: 667-672 (1991) Article DOI: 10.1016/S0960-894X(01)81044-1 BindingDB Entry DOI: 10.7270/Q26W9BKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50279837 ((3S,4R,5R)-Tetrahydro-pyran-3,4,5-triol | CHEMBL16...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 8.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards E-coli Beta-galactosidase | Bioorg Med Chem Lett 1: 667-672 (1991) Article DOI: 10.1016/S0960-894X(01)81044-1 BindingDB Entry DOI: 10.7270/Q26W9BKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||