 Found 43 hits of ki data for polymerid = 50001107,8010
Found 43 hits of ki data for polymerid = 50001107,8010 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583383
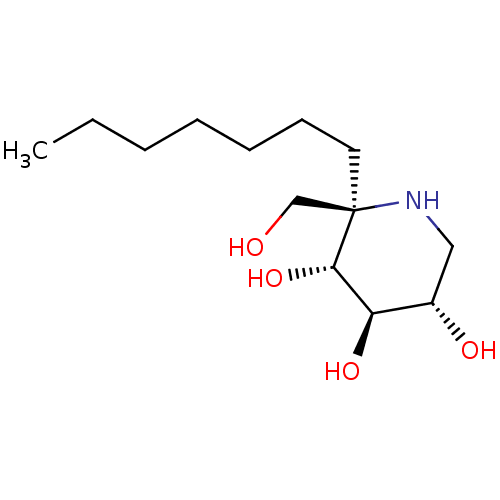
(CHEMBL5028005 | US20230339856, Compound (IIb3))Show SMILES CCCCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583382
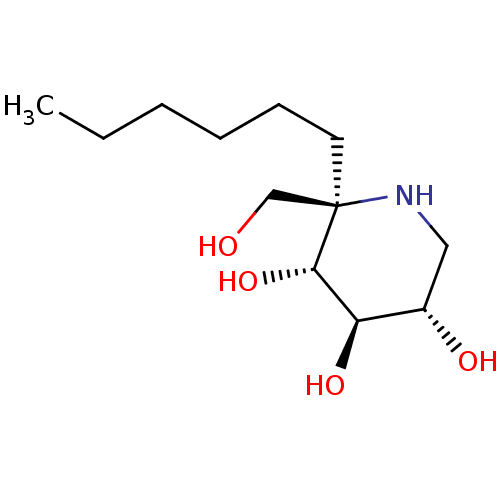
(CHEMBL5028265)Show SMILES CCCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583381
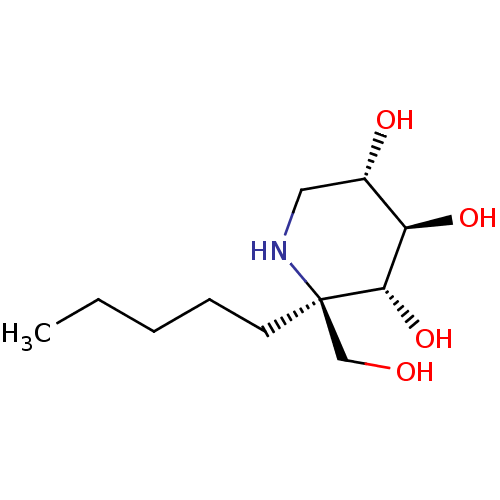
(CHEMBL5029066 | US20230339856, Compound (IIb1))Show SMILES CCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583380
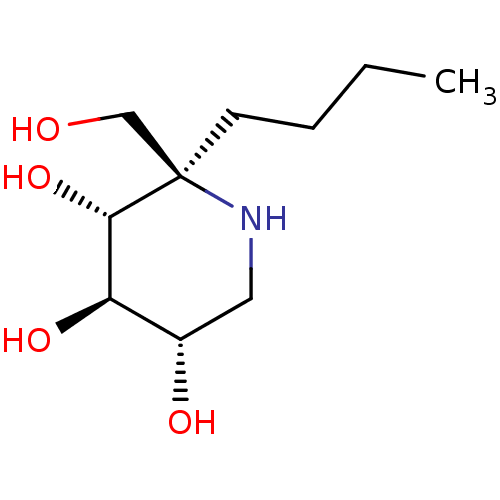
(CHEMBL5027974)Show SMILES CCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583379
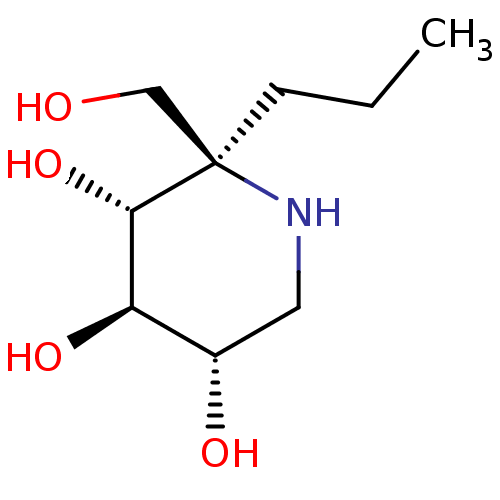
(CHEMBL5028138 | US20230339856, Compound (IIb))Show SMILES CCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50335398
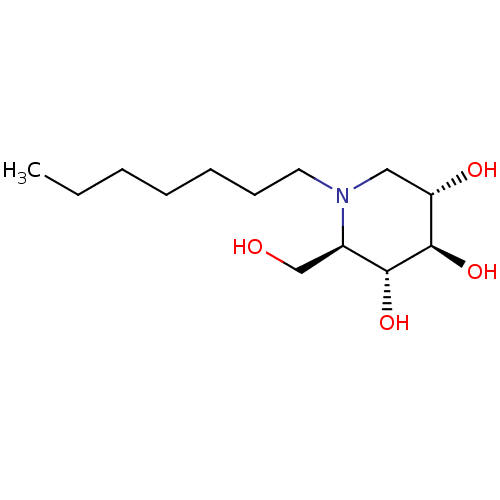
(CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin)Show SMILES CCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C13H27NO4/c1-2-3-4-5-6-7-14-8-11(16)13(18)12(17)10(14)9-15/h10-13,15-18H,2-9H2,1H3/t10-,11+,12-,13-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18356

((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C12H25NO4/c1-2-3-4-5-6-13-7-10(15)12(17)11(16)9(13)8-14/h9-12,14-17H,2-8H2,1H3/t9-,10+,11-,12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibition constant against human lysosomal alpha-glucosidase |
Bioorg Med Chem Lett 7: 355-360 (1997)
Article DOI: 10.1016/S0960-894X(97)00012-7
BindingDB Entry DOI: 10.7270/Q2F18ZQ1 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50335399

(CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin)Show SMILES CCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23NO4/c1-2-3-4-5-12-6-9(14)11(16)10(15)8(12)7-13/h8-11,13-16H,2-7H2,1H3/t8-,9+,10-,11-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583384
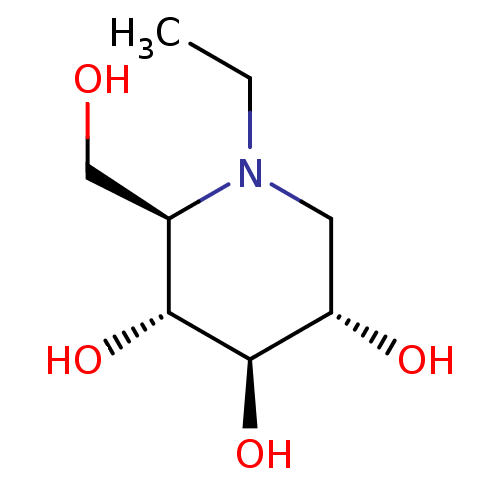
(CHEMBL5080975) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18354

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-propylpiperidine...)Show InChI InChI=1S/C9H19NO4/c1-2-3-10-4-7(12)9(14)8(13)6(10)5-11/h6-9,11-14H,2-5H2,1H3/t6-,7+,8-,9-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibition constant against human lysosomal alpha-glucosidase |
Bioorg Med Chem Lett 7: 355-360 (1997)
Article DOI: 10.1016/S0960-894X(97)00012-7
BindingDB Entry DOI: 10.7270/Q2F18ZQ1 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50291029
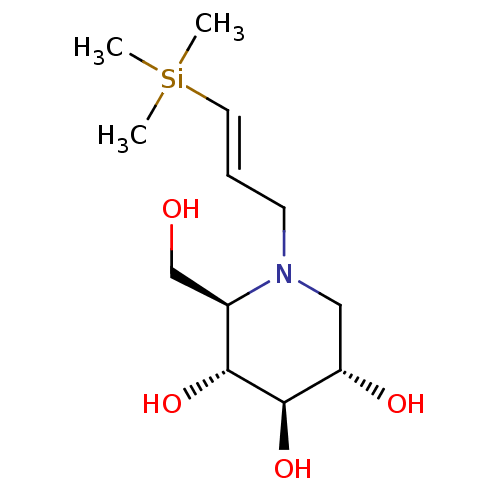
((2R,3R,4R,5S)-2-Hydroxymethyl-1-((E)-3-trimethylsi...)Show SMILES C[Si](C)(C)\C=C\CN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C12H25NO4Si/c1-18(2,3)6-4-5-13-7-10(15)12(17)11(16)9(13)8-14/h4,6,9-12,14-17H,5,7-8H2,1-3H3/b6-4+/t9-,10+,11-,12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibition constant against human lysosomal alpha-glucosidase |
Bioorg Med Chem Lett 7: 355-360 (1997)
Article DOI: 10.1016/S0960-894X(97)00012-7
BindingDB Entry DOI: 10.7270/Q2F18ZQ1 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583385

(CHEMBL5028105)Show SMILES CCCCCCC[C@@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Mus musculus (Mouse)) | BDBM243075
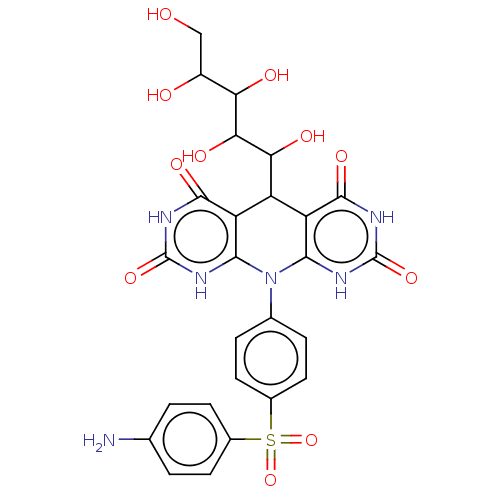
(α-Gl inhibitor, C3)Show SMILES Nc1ccc(cc1)S(=O)(=O)c1ccc(cc1)N1c2[nH]c(=O)[nH]c(=O)c2C(C(O)C(O)C(O)C(O)CO)c2c1[nH]c(=O)[nH]c2=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shiraz University
| Assay Description
In this study, the inhibition assay of yeast enzyme was performed in 100 mM phosphate buffer pH 7.0 at 25°C with minor changes, according to the meth... |
J Enzyme Inhib Med Chem 28: 1228-35 (2013)
Article DOI: 10.3109/14756366.2012.727812
BindingDB Entry DOI: 10.7270/Q2XP73V0 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50291031

((2R,3R,4R,5S)-2-Hydroxymethyl-1-(3-trimethylsilany...)Show SMILES C[Si](C)(C)CCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C12H27NO4Si/c1-18(2,3)6-4-5-13-7-10(15)12(17)11(16)9(13)8-14/h9-12,14-17H,4-8H2,1-3H3/t9-,10+,11-,12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibition constant against human lysosomal alpha-glucosidase |
Bioorg Med Chem Lett 7: 355-360 (1997)
Article DOI: 10.1016/S0960-894X(97)00012-7
BindingDB Entry DOI: 10.7270/Q2F18ZQ1 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50291027
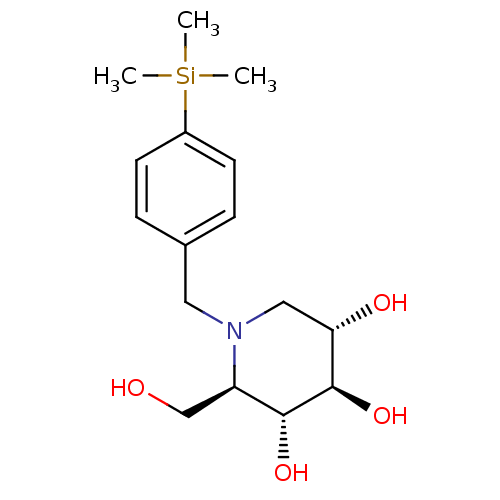
((2R,3R,4R,5S)-2-Hydroxymethyl-1-(4-trimethylsilany...)Show SMILES C[Si](C)(C)c1ccc(CN2C[C@H](O)[C@@H](O)[C@H](O)[C@H]2CO)cc1 Show InChI InChI=1S/C16H27NO4Si/c1-22(2,3)12-6-4-11(5-7-12)8-17-9-14(19)16(21)15(20)13(17)10-18/h4-7,13-16,18-21H,8-10H2,1-3H3/t13-,14+,15-,16-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibition constant against human lysosomal alpha-glucosidase |
Bioorg Med Chem Lett 7: 355-360 (1997)
Article DOI: 10.1016/S0960-894X(97)00012-7
BindingDB Entry DOI: 10.7270/Q2F18ZQ1 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50366376

(CHEMBL1159523)Show SMILES CC(=O)OCC(CO[C@H]1O[C@H](CS(O)(=O)=O)[C@@H](O)[C@H](O)[C@H]1O)OC(C)=O |r| Show InChI InChI=1S/C13H22O12S/c1-6(14)22-3-8(24-7(2)15)4-23-13-12(18)11(17)10(16)9(25-13)5-26(19,20)21/h8-13,16-18H,3-5H2,1-2H3,(H,19,20,21)/t8?,9-,10-,11+,12-,13+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory constant against yeast alpha-glucosidase |
Bioorg Med Chem Lett 5: 1241-1244 (1995)
Article DOI: 10.1016/0960-894X(95)00196-Z
BindingDB Entry DOI: 10.7270/Q2MP53SJ |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50285807
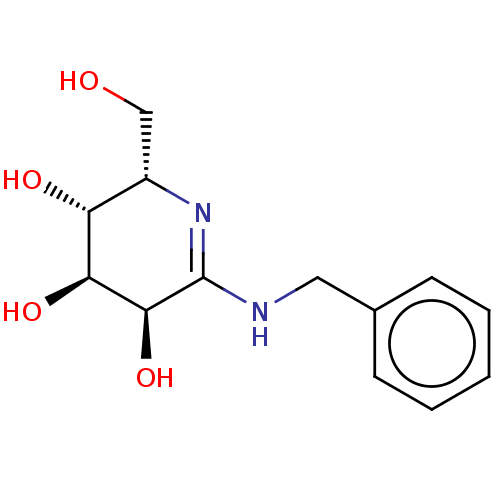
(CHEMBL2368787)Show SMILES OC[C@@H]1N=C(NCc2ccccc2)[C@@H](O)[C@@H](O)[C@@H]1O |r,t:3| Show InChI InChI=1S/C13H18N2O4/c16-7-9-10(17)11(18)12(19)13(15-9)14-6-8-4-2-1-3-5-8/h1-5,9-12,16-19H,6-7H2,(H,14,15)/t9-,10+,11-,12-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition against beta-glucosidase in sweet almond |
Bioorg Med Chem Lett 5: 2655-2660 (1995)
Article DOI: 10.1016/0960-894X(95)00474-8
BindingDB Entry DOI: 10.7270/Q21R6R0W |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583378
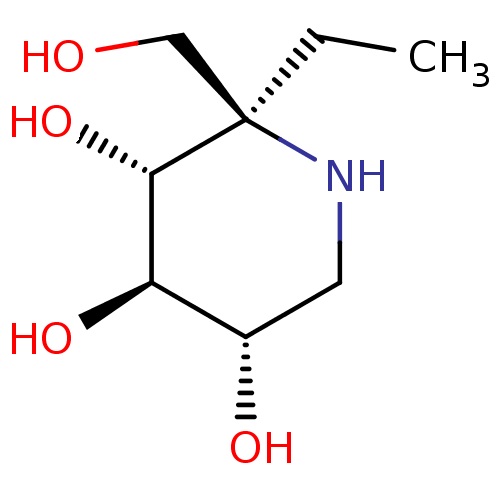
(CHEMBL5028084 | US20230339856, Compound (IIa)) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583381

(CHEMBL5029066 | US20230339856, Compound (IIb1))Show SMILES CCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM628473
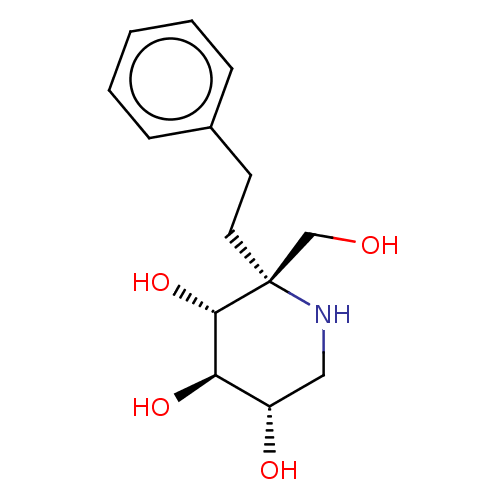
(US20230339856, Compound (IIc))Show SMILES OC[C@@]1(CCc2ccccc2)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583383

(CHEMBL5028005 | US20230339856, Compound (IIb3))Show SMILES CCCCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM628488

(US20230339856, Compound (IIc1))Show SMILES OC[C@@]1(CCCc2ccccc2)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583379

(CHEMBL5028138 | US20230339856, Compound (IIb))Show SMILES CCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM628474
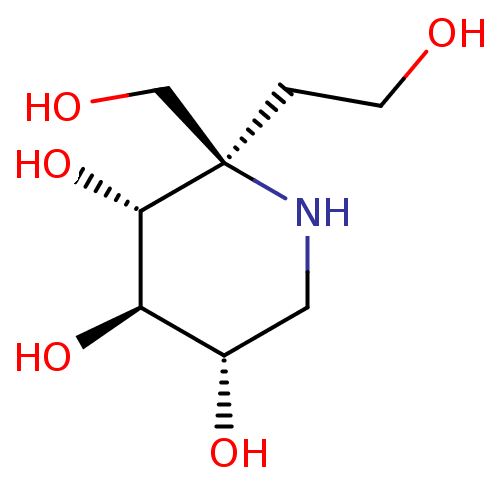
(US20230339856, Compound (IId))Show SMILES OCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM628489

(US20230339856, Compound (IIr))Show SMILES OC[C@@]1(CCC23CC4CC(CC(C4)C2)C3)NC[C@H](O)[C@@H](O)[C@@H]1O |r,TLB:12:11:14:8.7.6,12:7:14:11.13.10,THB:13:11:8:14.5.6,13:5:8:11.12.10,4:5:8:11.12.10| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha-glucosidase of yeast |
Bioorg Med Chem Lett 9: 615-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V40VQH |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50287006
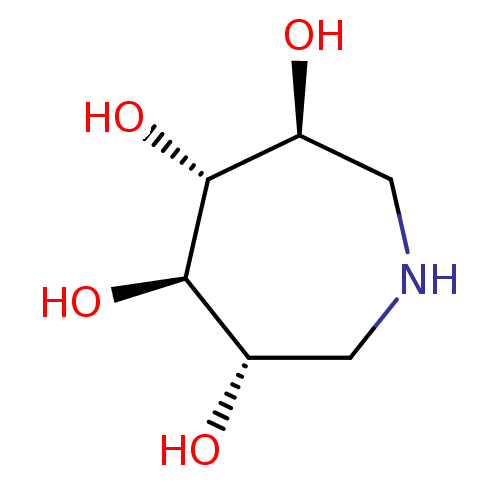
(Azepane-3,4,5,6-tetraol | CHEMBL13922)Show InChI InChI=1S/C6H13NO4/c8-3-1-7-2-4(9)6(11)5(3)10/h3-11H,1-2H2/t3-,4-,5+,6+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of alpha-glucosidase from yeast. |
Bioorg Med Chem Lett 6: 1117-1122 (1996)
Article DOI: 10.1016/0960-894X(96)00183-7
BindingDB Entry DOI: 10.7270/Q2M32VQR |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50583377

(CHEMBL5028072) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01673
BindingDB Entry DOI: 10.7270/Q23J3HVM |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50076963
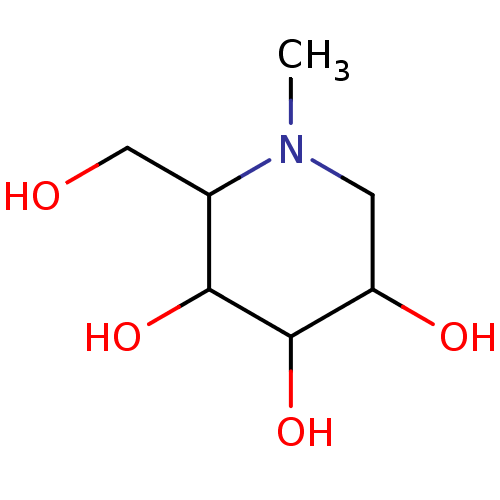
((3R,5R)-2-Hydroxymethyl-1-methyl-piperidine-3,4,5-...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.55 | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibitory potency for brewer's yeast alpha-glucosidase in 0.1 M sodium phosphate buffer at pH 6.55. |
Bioorg Med Chem Lett 9: 1255-60 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C2S |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50070029
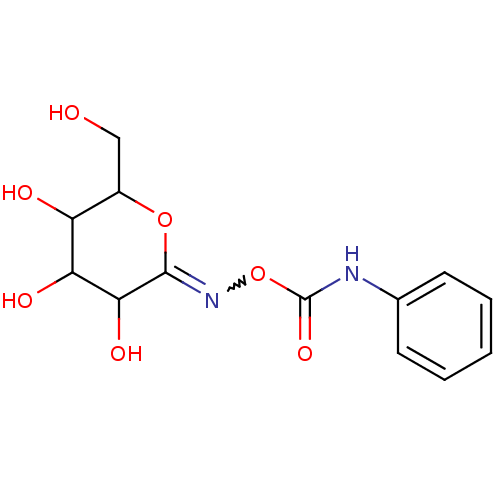
((2Z)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-...)Show SMILES OCC1OC(=NOC(=O)Nc2ccccc2)C(O)C(O)C1O |w:5.5| Show InChI InChI=1S/C13H16N2O7/c16-6-8-9(17)10(18)11(19)12(21-8)15-22-13(20)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,20) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against Alpha-glucosidase |
Bioorg Med Chem Lett 8: 371-2 (1999)
BindingDB Entry DOI: 10.7270/Q2PN9642 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50350757
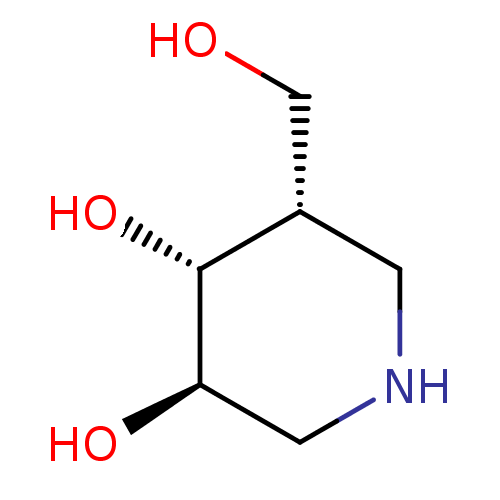
(CHEMBL1818432)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5+,6+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha-glucosidase of yeast |
Bioorg Med Chem Lett 9: 615-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V40VQH |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50285809

(CHEMBL2368788)Show SMILES CO[C@H]1O[C@@H](CNC2=N[C@@H](CO)[C@@H](O)[C@H](O)[C@@H]2O)[C@@H](O)[C@H](O)[C@H]1O |r,t:7| Show InChI InChI=1S/C13H24N2O9/c1-23-13-11(22)9(20)7(18)5(24-13)2-14-12-10(21)8(19)6(17)4(3-16)15-12/h4-11,13,16-22H,2-3H2,1H3,(H,14,15)/t4-,5-,6+,7+,8-,9-,10-,11+,13-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition against beta-glucosidase in sweet almond |
Bioorg Med Chem Lett 5: 2655-2660 (1995)
Article DOI: 10.1016/0960-894X(95)00474-8
BindingDB Entry DOI: 10.7270/Q21R6R0W |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50076963

((3R,5R)-2-Hydroxymethyl-1-methyl-piperidine-3,4,5-...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibitory potency for brewer's yeast alpha-glucosidase in 0.01 mM EDTA, 0.01 mM PIPES and 0.2 M NaOAc at pH 6.5. |
Bioorg Med Chem Lett 9: 1255-60 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C2S |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50285810

(6-benzylamino-2,3,4,5-tetrahydro-2-pyridiniumylmet...)Show InChI InChI=1S/C13H18N2O/c16-10-12-7-4-8-13(15-12)14-9-11-5-2-1-3-6-11/h1-3,5-6,12,16H,4,7-10H2,(H,14,15)/p+1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition against beta-glucosidase in sweet almond |
Bioorg Med Chem Lett 5: 2655-2660 (1995)
Article DOI: 10.1016/0960-894X(95)00474-8
BindingDB Entry DOI: 10.7270/Q21R6R0W |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50285808
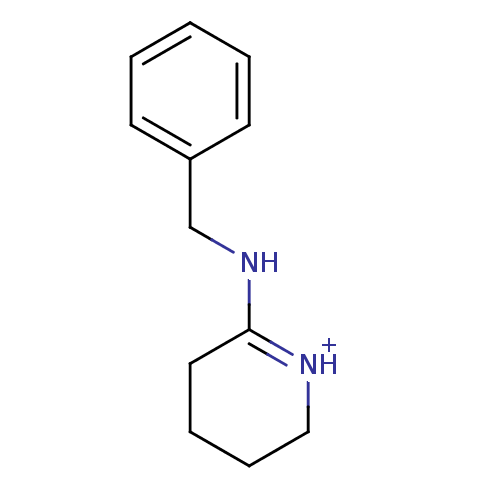
(6N-benzyl-2,3,4,5-tetrahydro-6-pyridiniumamine)Show InChI InChI=1S/C12H16N2/c1-2-6-11(7-3-1)10-14-12-8-4-5-9-13-12/h1-3,6-7H,4-5,8-10H2,(H,13,14)/p+1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
| 3.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition against beta-glucosidase in sweet almond |
Bioorg Med Chem Lett 5: 2655-2660 (1995)
Article DOI: 10.1016/0960-894X(95)00474-8
BindingDB Entry DOI: 10.7270/Q21R6R0W |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50285806

(5-benzylamino-3,4-dihydro-2H-2-pyrroliumylmethanol)Show InChI InChI=1S/C12H16N2O/c15-9-11-6-7-12(14-11)13-8-10-4-2-1-3-5-10/h1-5,11,15H,6-9H2,(H,13,14)/p+1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition against beta-glucosidase in sweet almond |
Bioorg Med Chem Lett 5: 2655-2660 (1995)
Article DOI: 10.1016/0960-894X(95)00474-8
BindingDB Entry DOI: 10.7270/Q21R6R0W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data