

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM18134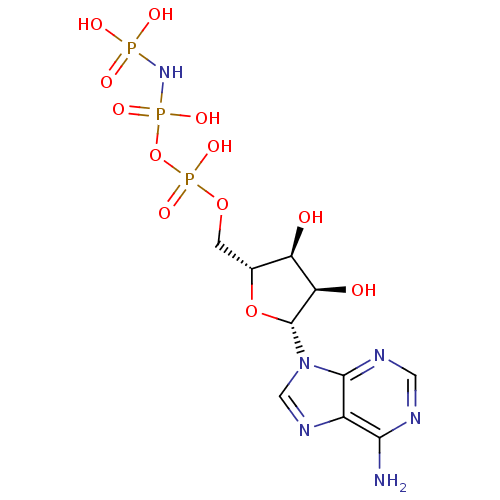 (({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM85447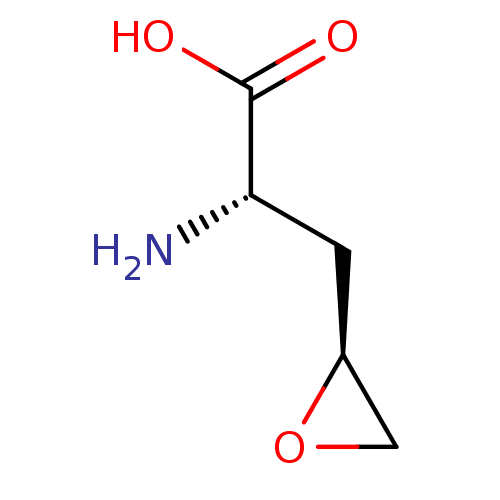 (Epoxy analogue, I(a)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 7.00E+3 | -30.6 | 5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Laboratoire de chimie bioorganique | Assay Description AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi... | J Enzym Inhib 13: 361-7 (1998) Article DOI: 10.3109/14756369809021481 BindingDB Entry DOI: 10.7270/Q2668BQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367328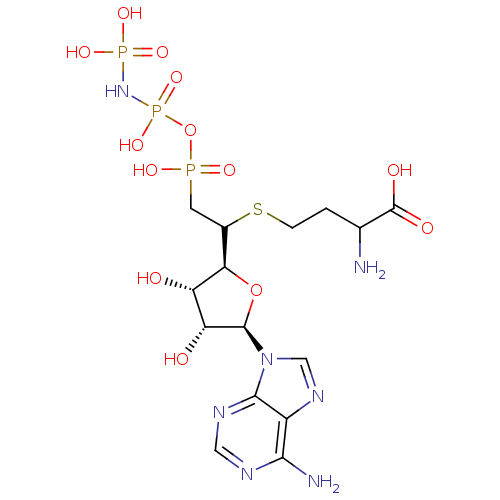 (CHEMBL1791415) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367329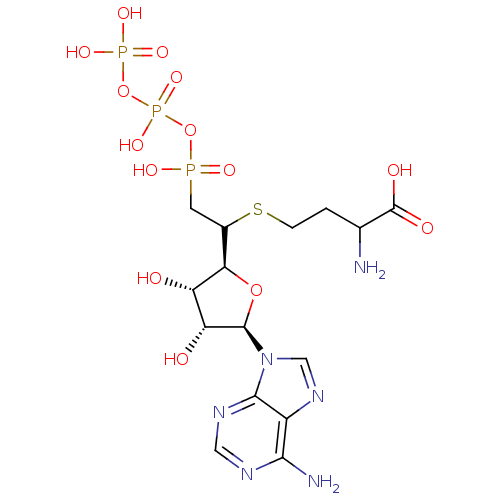 (CHEMBL1791416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367329 (CHEMBL1791416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367301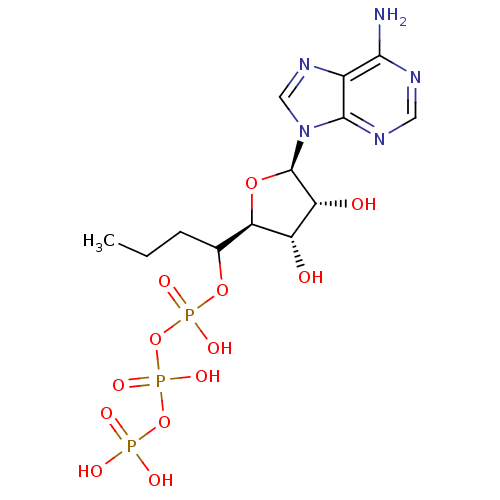 (CHEMBL1791432) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50452293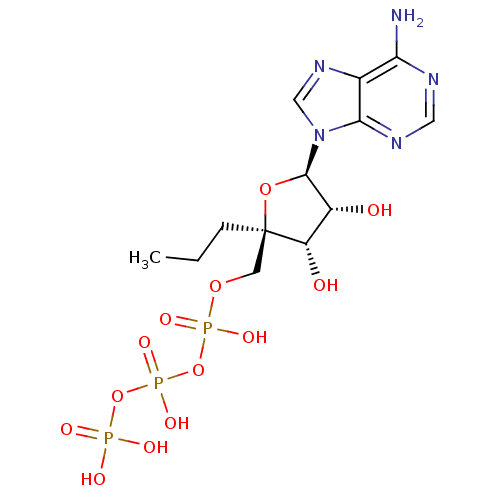 (CHEMBL2092766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50452293 (CHEMBL2092766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM85448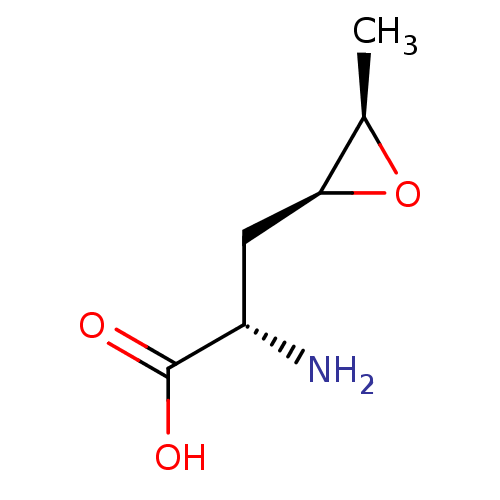 (Epoxy analogue, I(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.10E+4 | -25.0 | 1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Laboratoire de chimie bioorganique | Assay Description AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi... | J Enzym Inhib 13: 361-7 (1998) Article DOI: 10.3109/14756369809021481 BindingDB Entry DOI: 10.7270/Q2668BQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367042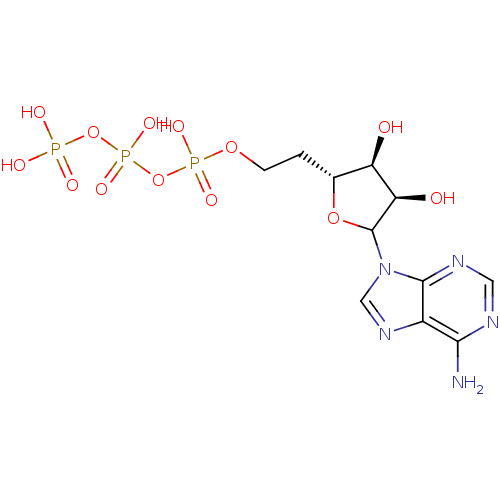 (CHEMBL606221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM85450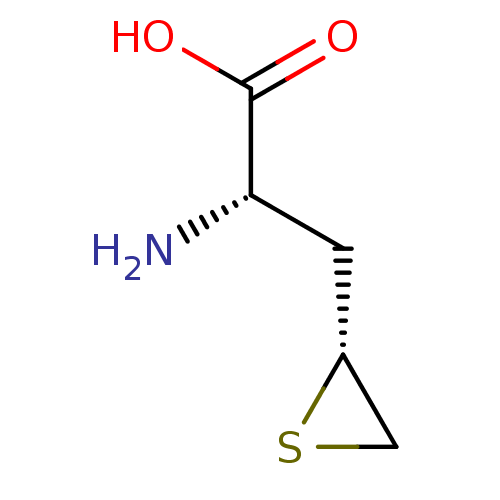 (Epithioamino acid analogue, II(a)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | -23.7 | 1.40E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Laboratoire de chimie bioorganique | Assay Description AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi... | J Enzym Inhib 13: 361-7 (1998) Article DOI: 10.3109/14756369809021481 BindingDB Entry DOI: 10.7270/Q2668BQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM85449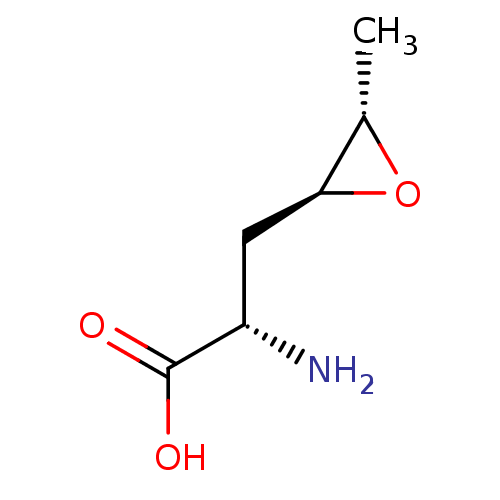 (Epoxy analogue, I(c)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | -23.6 | 1.10E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Laboratoire de chimie bioorganique | Assay Description AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi... | J Enzym Inhib 13: 361-7 (1998) Article DOI: 10.3109/14756369809021481 BindingDB Entry DOI: 10.7270/Q2668BQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM85451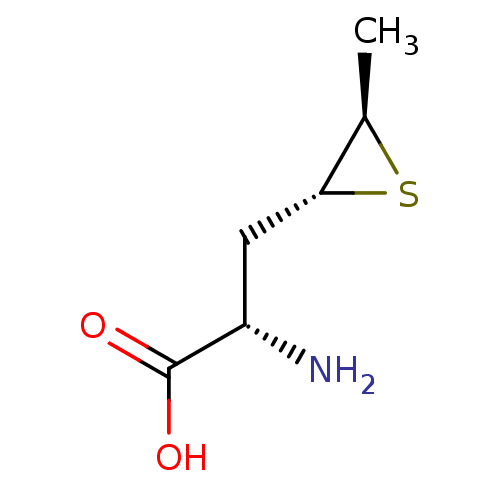 (Epithioamino acid analogue, II(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | -22.9 | 3.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Laboratoire de chimie bioorganique | Assay Description AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi... | J Enzym Inhib 13: 361-7 (1998) Article DOI: 10.3109/14756369809021481 BindingDB Entry DOI: 10.7270/Q2668BQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM85452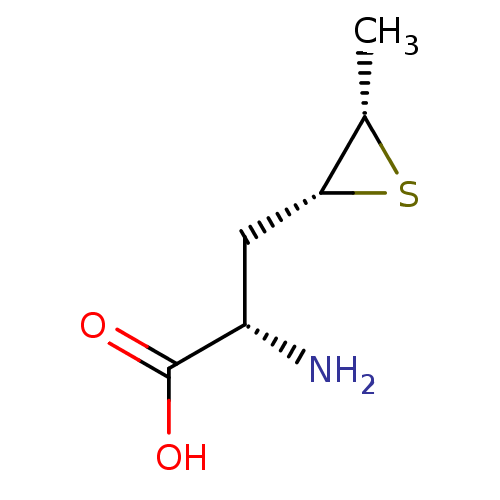 (Epithioamino acid analogue, II(c)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | -22.9 | 4.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Laboratoire de chimie bioorganique | Assay Description AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi... | J Enzym Inhib 13: 361-7 (1998) Article DOI: 10.3109/14756369809021481 BindingDB Entry DOI: 10.7270/Q2668BQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367331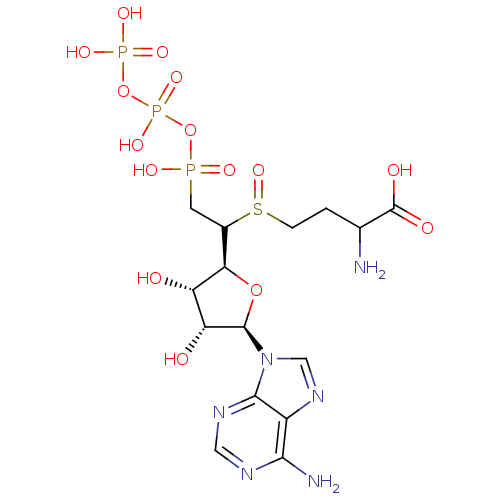 (CHEMBL1791417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50026197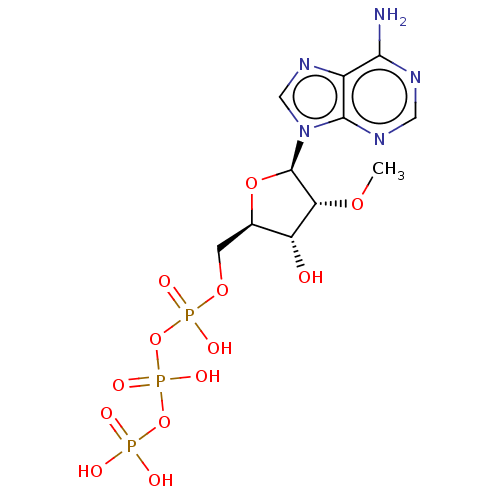 (Adenosine 5'-triphosphate derivative | CHEMBL31428...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50026193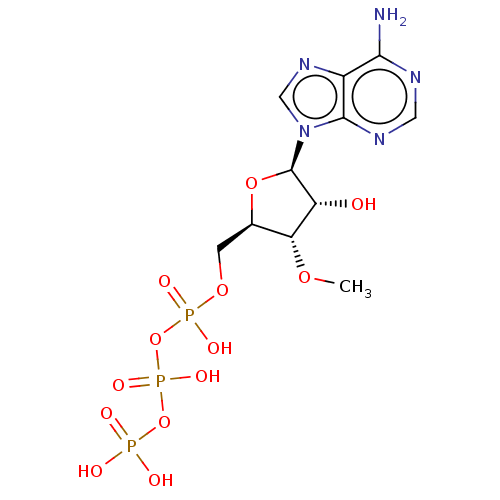 (Adenosine 5'-triphosphate derivative | CHEMBL31428...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367305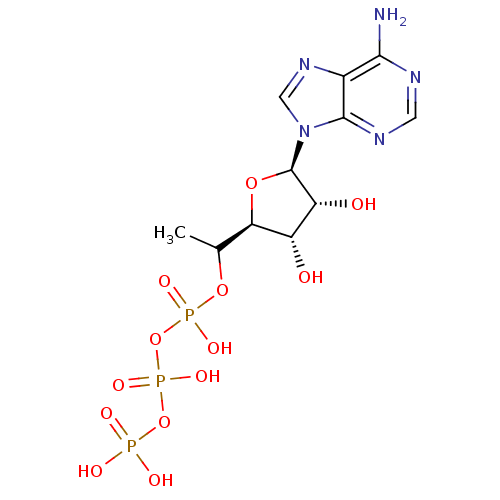 (CHEMBL1791430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367307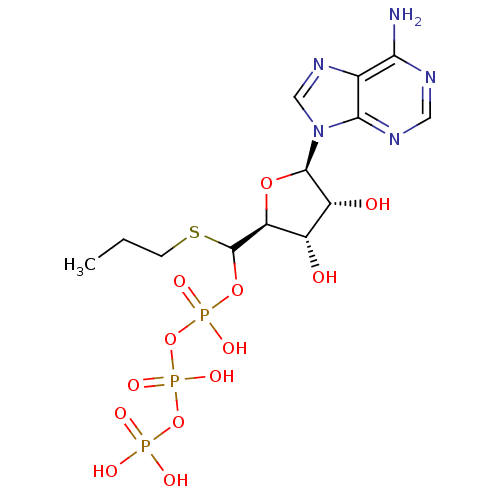 (CHEMBL1791431) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50118221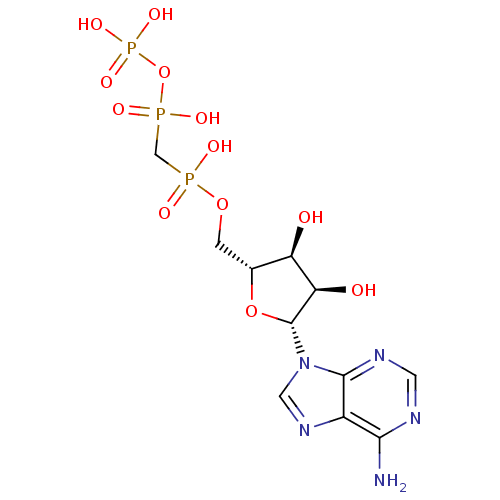 (9H-purine derivative | CHEMBL132722 | DIPHOSPHOMET...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367300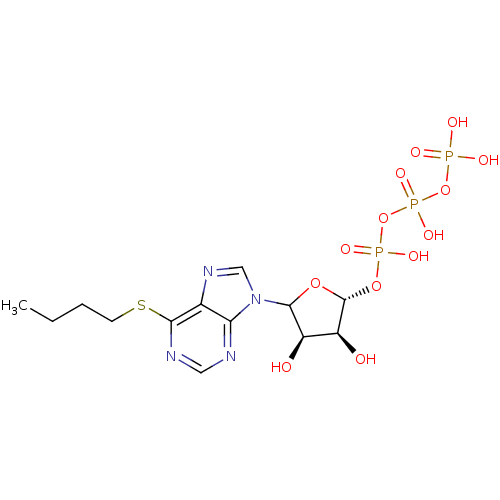 (CHEMBL610977) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367303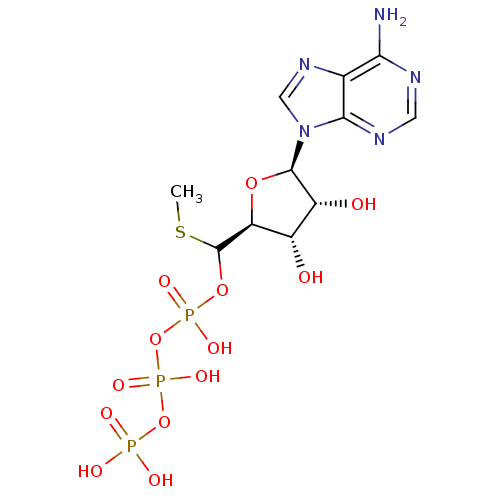 (CHEMBL1791433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50025158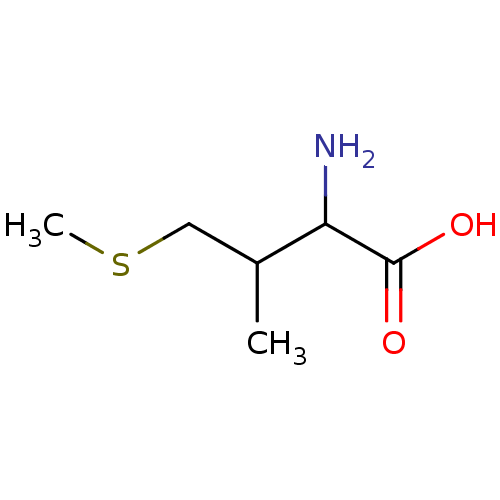 (2-Amino-3-methyl-4-methylsulfanyl-butyric acid | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibitory activity against Methionine adenosyltransferase II | J Med Chem 29: 1743-8 (1986) BindingDB Entry DOI: 10.7270/Q24748V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367306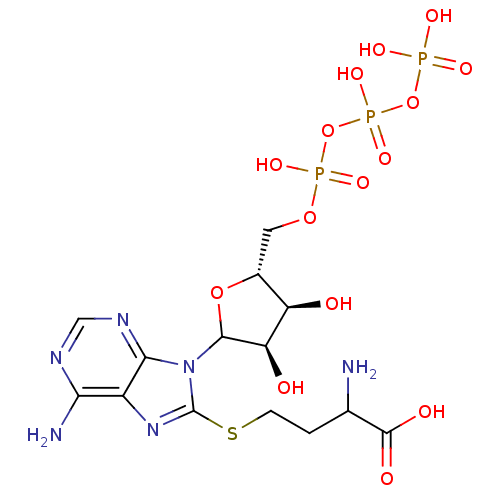 (CHEMBL608929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367304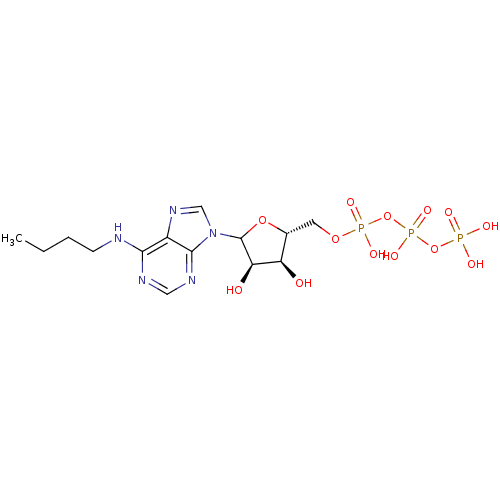 (CHEMBL610404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50118232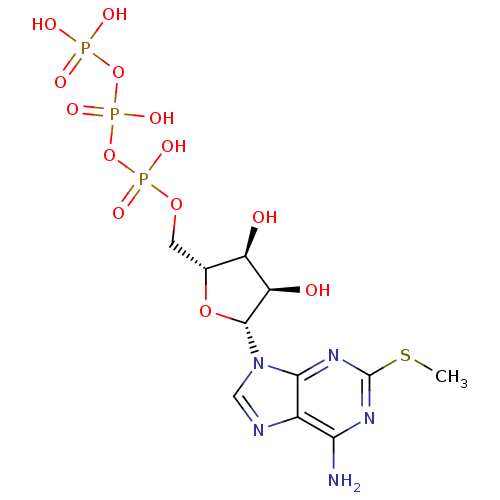 (2-MeSATP | ATP, 2-meS | CHEMBL336208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat kidney Methionine adenosyltransferase II | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50025157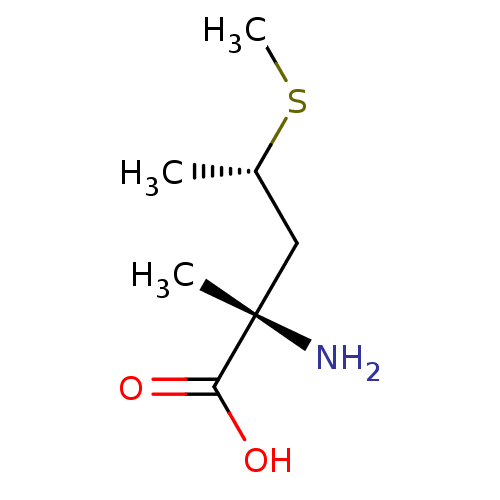 (2-Amino-2-methyl-4-methylsulfanyl-pentanoic acid |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibitory activity against M-2 Methionine adenosyltransferase II | J Med Chem 29: 1743-8 (1986) BindingDB Entry DOI: 10.7270/Q24748V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50025156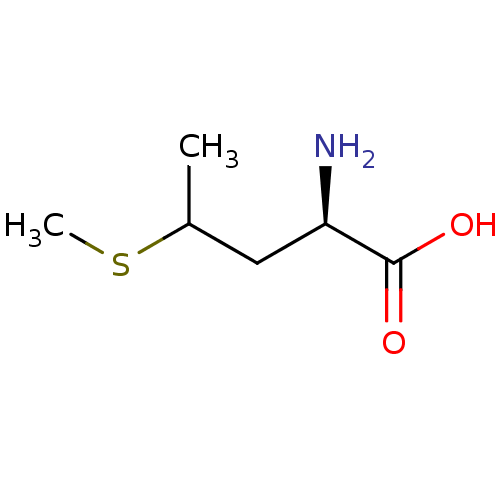 (2-Amino-4-methylsulfanyl-pentanoic acid | CHEMBL45...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibitory activity against Methionine adenosyltransferase II | J Med Chem 29: 1743-8 (1986) BindingDB Entry DOI: 10.7270/Q24748V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||