 Found 15 hits of ki data for polymerid = 50001170
Found 15 hits of ki data for polymerid = 50001170 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-16 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50102608
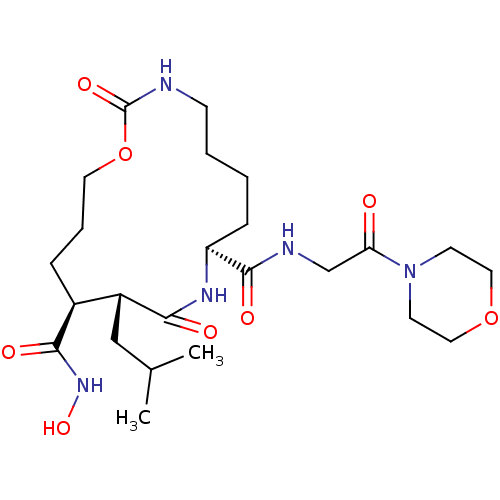
(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CC(C)C[C@@H]1[C@H](CCCOC(=O)NCCCC[C@H](NC1=O)C(=O)NCC(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C24H41N5O8/c1-16(2)14-18-17(22(32)28-35)6-5-11-37-24(34)25-8-4-3-7-19(27-21(18)31)23(33)26-15-20(30)29-9-12-36-13-10-29/h16-19,35H,3-15H2,1-2H3,(H,25,34)(H,26,33)(H,27,31)(H,28,32)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-16 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM26526
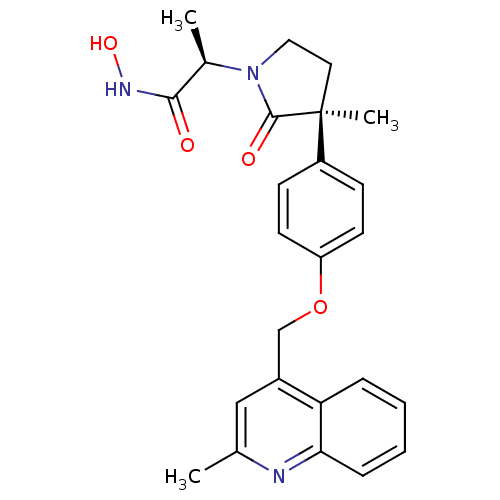
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP16 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM26526

((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Affinity for Matrix metalloprotease-16 (MMP-16) |
J Med Chem 45: 4954-7 (2002)
BindingDB Entry DOI: 10.7270/Q2XP7497 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50104973
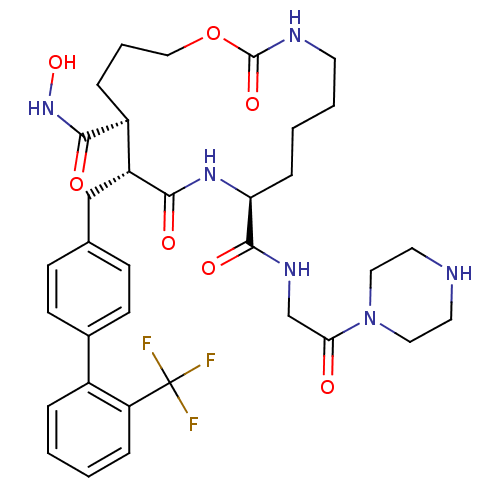
(2,10-Dioxo-11-(2'-trifluoromethyl-biphenyl-4-ylmet...)Show SMILES ONC(=O)[C@H]1CCCOC(=O)NCCCC[C@H](NC(=O)[C@@H]1Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)NCC(=O)N1CCNCC1 Show InChI InChI=1S/C34H43F3N6O7/c35-34(36,37)27-8-2-1-6-24(27)23-12-10-22(11-13-23)20-26-25(31(46)42-49)7-5-19-50-33(48)39-14-4-3-9-28(41-30(26)45)32(47)40-21-29(44)43-17-15-38-16-18-43/h1-2,6,8,10-13,25-26,28,38,49H,3-5,7,9,14-21H2,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t25-,26+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-16 |
J Med Chem 44: 3351-4 (2001)
BindingDB Entry DOI: 10.7270/Q2VD6XRT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50104976
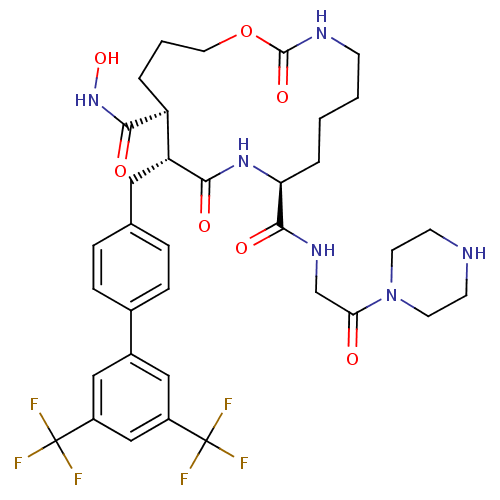
(11-(3',5'-Bis-trifluoromethyl-biphenyl-4-ylmethyl)...)Show SMILES ONC(=O)[C@H]1CCCOC(=O)NCCCC[C@H](NC(=O)[C@@H]1Cc1ccc(cc1)-c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)NCC(=O)N1CCNCC1 Show InChI InChI=1S/C35H42F6N6O7/c36-34(37,38)24-17-23(18-25(19-24)35(39,40)41)22-8-6-21(7-9-22)16-27-26(31(50)46-53)4-3-15-54-33(52)43-10-2-1-5-28(45-30(27)49)32(51)44-20-29(48)47-13-11-42-12-14-47/h6-9,17-19,26-28,42,53H,1-5,10-16,20H2,(H,43,52)(H,44,51)(H,45,49)(H,46,50)/t26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-16 |
J Med Chem 44: 3351-4 (2001)
BindingDB Entry DOI: 10.7270/Q2VD6XRT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50104975
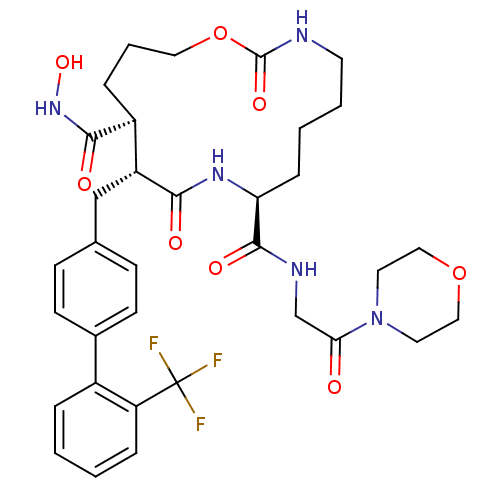
((8S,11R,12S)-2,10-Dioxo-11-(2'-trifluoromethyl-bip...)Show SMILES ONC(=O)[C@H]1CCCOC(=O)NCCCC[C@H](NC(=O)[C@@H]1Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)NCC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C34H42F3N5O8/c35-34(36,37)27-8-2-1-6-24(27)23-12-10-22(11-13-23)20-26-25(31(45)41-48)7-5-17-50-33(47)38-14-4-3-9-28(40-30(26)44)32(46)39-21-29(43)42-15-18-49-19-16-42/h1-2,6,8,10-13,25-26,28,48H,3-5,7,9,14-21H2,(H,38,47)(H,39,46)(H,40,44)(H,41,45)/t25-,26+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP16 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50104975

((8S,11R,12S)-2,10-Dioxo-11-(2'-trifluoromethyl-bip...)Show SMILES ONC(=O)[C@H]1CCCOC(=O)NCCCC[C@H](NC(=O)[C@@H]1Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)NCC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C34H42F3N5O8/c35-34(36,37)27-8-2-1-6-24(27)23-12-10-22(11-13-23)20-26-25(31(45)41-48)7-5-17-50-33(47)38-14-4-3-9-28(40-30(26)44)32(46)39-21-29(43)42-15-18-49-19-16-42/h1-2,6,8,10-13,25-26,28,48H,3-5,7,9,14-21H2,(H,38,47)(H,39,46)(H,40,44)(H,41,45)/t25-,26+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-16 |
J Med Chem 44: 3351-4 (2001)
BindingDB Entry DOI: 10.7270/Q2VD6XRT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50104974

(2,10-Dioxo-11-(2'-trifluoromethyl-biphenyl-4-ylmet...)Show SMILES NC(=O)CNC(=O)[C@@H]1CCCCNC(=O)OCCC[C@@H]([C@@H](Cc2ccc(cc2)-c2ccccc2C(F)(F)F)C(=O)N1)C(=O)NO Show InChI InChI=1S/C30H36F3N5O7/c31-30(32,33)23-8-2-1-6-20(23)19-12-10-18(11-13-19)16-22-21(27(41)38-44)7-5-15-45-29(43)35-14-4-3-9-24(37-26(22)40)28(42)36-17-25(34)39/h1-2,6,8,10-13,21-22,24,44H,3-5,7,9,14-17H2,(H2,34,39)(H,35,43)(H,36,42)(H,37,40)(H,38,41)/t21-,22+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-16 |
J Med Chem 44: 3351-4 (2001)
BindingDB Entry DOI: 10.7270/Q2VD6XRT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50183711
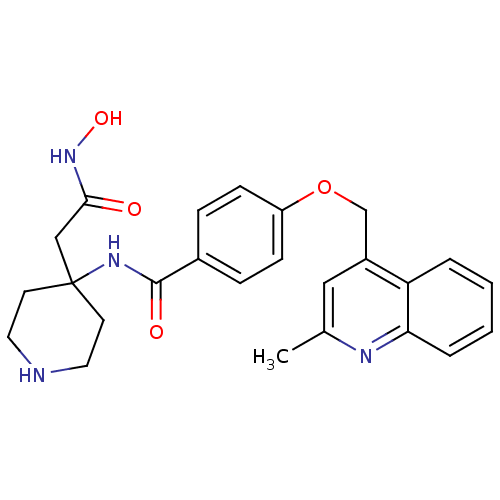
(CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCNCC2)c2ccccc2n1 Show InChI InChI=1S/C25H28N4O4/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-33-20-8-6-18(7-9-20)24(31)28-25(15-23(30)29-32)10-12-26-13-11-25/h2-9,14,26,32H,10-13,15-16H2,1H3,(H,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP16 |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50183715
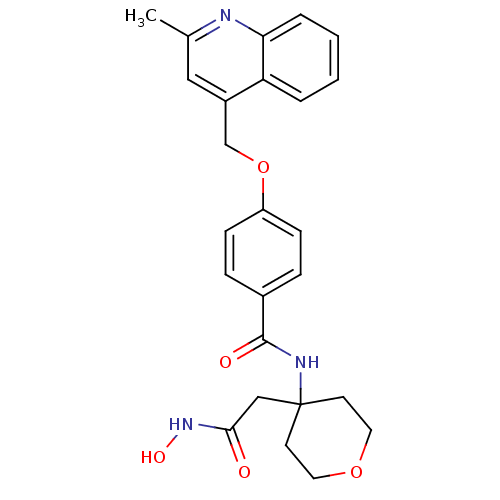
(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP16 |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50183715

(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP16 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP16 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM26806
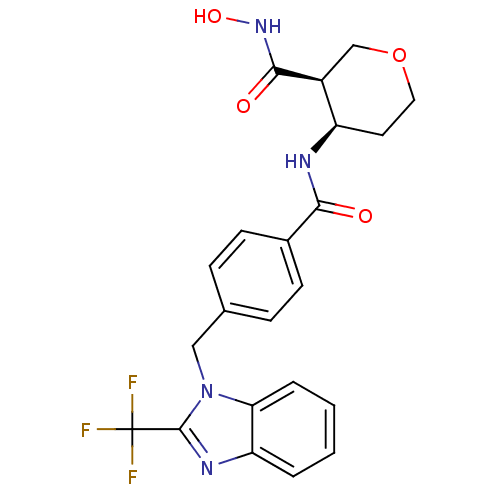
((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(trifluoromethyl)-1...)Show SMILES ONC(=O)[C@H]1COCC[C@H]1NC(=O)c1ccc(Cn2c(nc3ccccc23)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C22H21F3N4O4/c23-22(24,25)21-27-17-3-1-2-4-18(17)29(21)11-13-5-7-14(8-6-13)19(30)26-16-9-10-33-12-15(16)20(31)28-32/h1-8,15-16,32H,9-12H2,(H,26,30)(H,28,31)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP16 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50305842
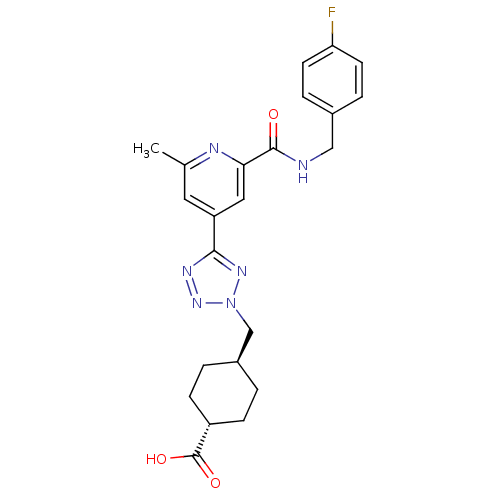
(CHEMBL603656 | trans-4-((5-(2-(4-fluorobenzylcarba...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)cc1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:26.31,wD:23.24,(30.11,-12.24,;29.32,-10.92,;30.07,-9.57,;29.28,-8.26,;27.74,-8.27,;26.98,-9.62,;27.77,-10.94,;25.44,-9.64,;24.69,-10.98,;24.66,-8.31,;23.12,-8.33,;22.33,-7.01,;23.08,-5.67,;22.3,-4.34,;20.75,-4.36,;19.97,-3.04,;20,-5.72,;20.79,-7.03,;30.03,-6.91,;29.39,-5.51,;30.52,-4.46,;31.87,-5.21,;33.27,-4.57,;34.61,-5.32,;34.63,-6.85,;35.98,-7.6,;37.3,-6.81,;37.27,-5.26,;35.93,-4.52,;38.65,-7.56,;38.68,-9.1,;39.97,-6.77,;31.57,-6.73,)| Show InChI InChI=1S/C23H25FN6O3/c1-14-10-18(11-20(26-14)22(31)25-12-15-4-8-19(24)9-5-15)21-27-29-30(28-21)13-16-2-6-17(7-3-16)23(32)33/h4-5,8-11,16-17H,2-3,6-7,12-13H2,1H3,(H,25,31)(H,32,33)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP16 |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data