 Found 17 hits of ki data for polymerid = 50001456
Found 17 hits of ki data for polymerid = 50001456 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Myeloblastin
(Homo sapiens (Human)) | BDBM50263166
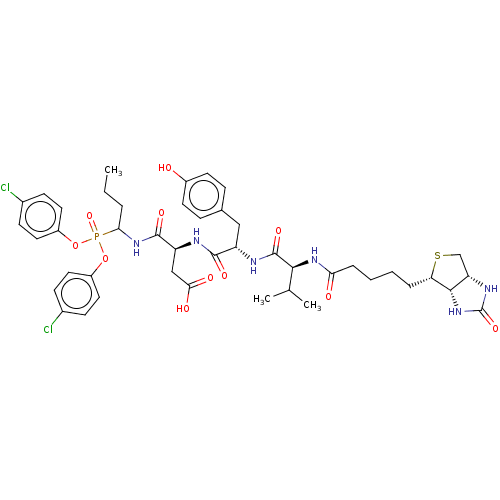
(CHEMBL4071346)Show SMILES [H][C@]12CS[C@@H](CCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CC(O)=O)C(=O)NC(CCC)P(=O)(Oc3ccc(Cl)cc3)Oc3ccc(Cl)cc3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C44H55Cl2N6O11PS/c1-4-7-37(64(61,62-30-18-12-27(45)13-19-30)63-31-20-14-28(46)15-21-31)51-42(58)33(23-38(55)56)47-41(57)32(22-26-10-16-29(53)17-11-26)48-43(59)39(25(2)3)50-36(54)9-6-5-8-35-40-34(24-65-35)49-44(60)52-40/h10-21,25,32-35,37,39-40,53H,4-9,22-24H2,1-3H3,(H,47,57)(H,48,59)(H,50,54)(H,51,58)(H,55,56)(H2,49,52,60)/t32-,33-,34-,35-,37?,39-,40-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM UMR1100,"Centre d'Etude des Pathologies Respiratoires" , Université de Tours , 37032 Tours , France.
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using ABZ-VADnVADYQ-EDDnp as substrate by FRET assay |
J Med Chem 61: 1858-1870 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01416
BindingDB Entry DOI: 10.7270/Q25B04ZT |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502655

(CHEMBL4560512)Show SMILES CCCC1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CO)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N2 |r| Show InChI InChI=1S/C78H100N16O19S2/c1-4-12-50-67(102)88-56(39-95)70(105)86-54(35-45-22-28-49(97)29-23-45)76(111)94-32-11-17-61(94)78(113)93-31-10-16-60(93)74(109)91-65(42(3)5-2)75(110)90-58-41-115-114-40-57(71(106)84-52(69(104)83-50)34-44-20-26-48(96)27-21-44)89-68(103)51(33-43-18-24-47(25-19-43)46-13-7-6-8-14-46)82-64(100)38-81-66(101)53(36-62(79)98)85-73(108)59-15-9-30-92(59)77(112)55(37-63(80)99)87-72(58)107/h6-8,13-14,18-29,42,50-61,65,95-97H,4-5,9-12,15-17,30-41H2,1-3H3,(H2,79,98)(H2,80,99)(H,81,101)(H,82,100)(H,83,104)(H,84,106)(H,85,108)(H,86,105)(H,87,107)(H,88,102)(H,89,103)(H,90,110)(H,91,109)/t42-,50?,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,65-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502658
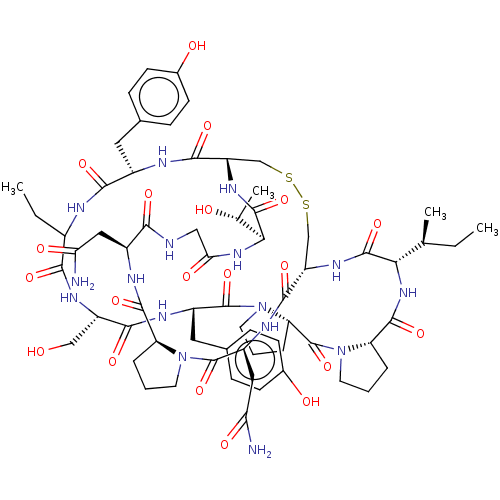
(CHEMBL4557877)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CO)NC(=O)C(CC)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C66H92N16O20S2/c1-5-32(3)52-62(98)76-45-31-104-103-30-44(77-63(99)53(33(4)84)78-51(89)28-69-54(90)40(26-49(67)87)72-60(96)46-10-7-21-80(46)65(101)42(27-50(68)88)74-59(45)95)58(94)71-39(24-34-13-17-36(85)18-14-34)56(92)70-38(6-2)55(91)75-43(29-83)57(93)73-41(25-35-15-19-37(86)20-16-35)64(100)82-23-9-12-48(82)66(102)81-22-8-11-47(81)61(97)79-52/h13-20,32-33,38-48,52-53,83-86H,5-12,21-31H2,1-4H3,(H2,67,87)(H2,68,88)(H,69,90)(H,70,92)(H,71,94)(H,72,96)(H,73,93)(H,74,95)(H,75,91)(H,76,98)(H,77,99)(H,78,89)(H,79,97)/t32-,33+,38?,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,52-,53-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502659
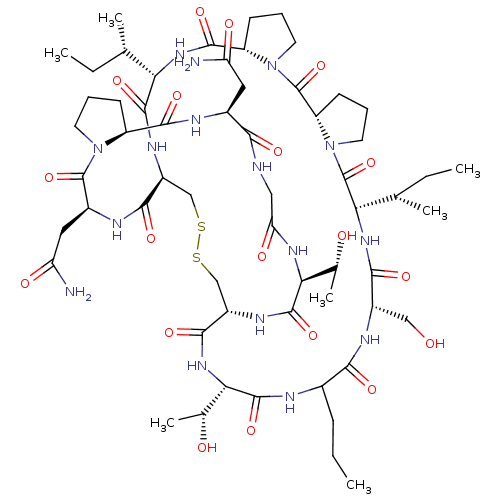
(CHEMBL4528256)Show SMILES CCCC1NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2)[C@@H](C)O |r| Show InChI InChI=1S/C59H94N16O19S2/c1-8-14-31-48(83)66-34(24-76)49(84)71-44(28(5)10-3)59(94)75-20-13-17-39(75)58(93)74-19-12-16-38(74)53(88)70-43(27(4)9-2)54(89)67-35-25-95-96-26-36(51(86)72-46(30(7)78)56(91)63-31)68-55(90)45(29(6)77)69-42(81)23-62-47(82)32(21-40(60)79)64-52(87)37-15-11-18-73(37)57(92)33(22-41(61)80)65-50(35)85/h27-39,43-46,76-78H,8-26H2,1-7H3,(H2,60,79)(H2,61,80)(H,62,82)(H,63,91)(H,64,87)(H,65,85)(H,66,83)(H,67,89)(H,68,90)(H,69,81)(H,70,88)(H,71,84)(H,72,86)/t27-,28-,29+,30+,31?,32-,33-,34-,35-,36-,37-,38-,39-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502656

(CHEMBL4455694)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CO)NC(=O)C(CC)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N2 |r| Show InChI InChI=1S/C77H98N16O19S2/c1-4-41(3)64-74(109)89-57-40-114-113-39-56(88-67(102)50(32-42-17-23-46(24-18-42)45-12-7-6-8-13-45)81-63(99)37-80-65(100)52(35-61(78)97)84-72(107)58-14-9-29-91(58)76(111)54(36-62(79)98)86-71(57)106)70(105)83-51(33-43-19-25-47(95)26-20-43)68(103)82-49(5-2)66(101)87-55(38-94)69(104)85-53(34-44-21-27-48(96)28-22-44)75(110)93-31-11-16-60(93)77(112)92-30-10-15-59(92)73(108)90-64/h6-8,12-13,17-28,41,49-60,64,94-96H,4-5,9-11,14-16,29-40H2,1-3H3,(H2,78,97)(H2,79,98)(H,80,100)(H,81,99)(H,82,103)(H,83,105)(H,84,107)(H,85,104)(H,86,106)(H,87,101)(H,88,102)(H,89,109)(H,90,108)/t41-,49?,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,64-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50263172
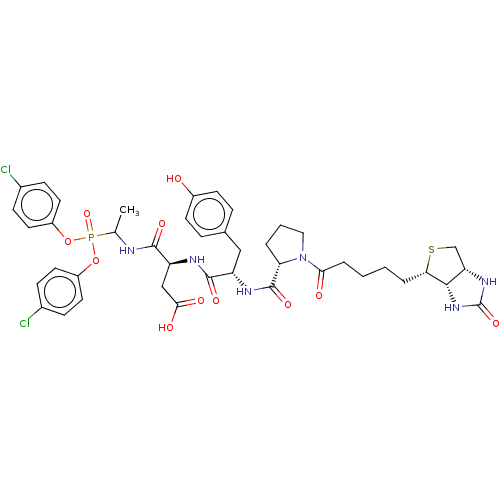
(CHEMBL4085013)Show SMILES [H][C@]12CS[C@@H](CCCCC(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CC(O)=O)C(=O)NC(C)P(=O)(Oc3ccc(Cl)cc3)Oc3ccc(Cl)cc3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C42H49Cl2N6O11PS/c1-24(62(59,60-29-16-10-26(43)11-17-29)61-30-18-12-27(44)13-19-30)45-39(55)32(22-37(53)54)46-40(56)31(21-25-8-14-28(51)15-9-25)47-41(57)34-5-4-20-50(34)36(52)7-3-2-6-35-38-33(23-63-35)48-42(58)49-38/h8-19,24,31-35,38,51H,2-7,20-23H2,1H3,(H,45,55)(H,46,56)(H,47,57)(H,53,54)(H2,48,49,58)/t24?,31-,32-,33-,34-,35-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM UMR1100,"Centre d'Etude des Pathologies Respiratoires" , Université de Tours , 37032 Tours , France.
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using ABZ-VADnVADYQ-EDDnp as substrate by FRET assay |
J Med Chem 61: 1858-1870 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01416
BindingDB Entry DOI: 10.7270/Q25B04ZT |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502657
(CHEMBL4589744)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)C(CC)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C58H92N16O19S2/c1-8-26(4)42-53(88)66-34-24-94-95-25-35(67-54(89)44(28(6)76)68-41(80)22-61-46(81)31(20-39(59)78)63-51(86)36-14-11-17-72(36)56(91)32(21-40(60)79)64-49(34)84)50(85)71-45(29(7)77)55(90)62-30(10-3)47(82)65-33(23-75)48(83)70-43(27(5)9-2)58(93)74-19-13-16-38(74)57(92)73-18-12-15-37(73)52(87)69-42/h26-38,42-45,75-77H,8-25H2,1-7H3,(H2,59,78)(H2,60,79)(H,61,81)(H,62,90)(H,63,86)(H,64,84)(H,65,82)(H,66,88)(H,67,89)(H,68,80)(H,69,87)(H,70,83)(H,71,85)/t26-,27-,28+,29+,30?,31-,32-,33-,34-,35-,36-,37-,38-,42-,43-,44-,45-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502654
(CHEMBL4470455)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C57H90N16O19S2/c1-8-25(3)41-52(87)65-33-23-93-94-24-34(66-54(89)43(28(6)75)67-40(79)21-60-46(81)30(19-38(58)77)62-50(85)35-13-10-16-71(35)55(90)31(20-39(59)78)63-48(33)83)49(84)70-44(29(7)76)53(88)61-27(5)45(80)64-32(22-74)47(82)69-42(26(4)9-2)57(92)73-18-12-15-37(73)56(91)72-17-11-14-36(72)51(86)68-41/h25-37,41-44,74-76H,8-24H2,1-7H3,(H2,58,77)(H2,59,78)(H,60,81)(H,61,88)(H,62,85)(H,63,83)(H,64,80)(H,65,87)(H,66,89)(H,67,79)(H,68,86)(H,69,82)(H,70,84)/t25-,26-,27-,28+,29+,30-,31-,32-,33-,34-,35-,36-,37-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50502660
(CHEMBL4469546)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2)[C@@H](C)O)C(C)C)[C@@H](C)CC |r| Show InChI InChI=1S/C59H94N16O19S2/c1-9-27(5)43-54(89)66-34-24-95-96-25-35(67-55(90)45(29(7)77)68-41(81)22-62-47(82)31(20-39(60)79)63-51(86)36-14-11-17-73(36)57(92)32(21-40(61)80)64-49(34)84)50(85)72-46(30(8)78)56(91)69-42(26(3)4)53(88)65-33(23-76)48(83)71-44(28(6)10-2)59(94)75-19-13-16-38(75)58(93)74-18-12-15-37(74)52(87)70-43/h26-38,42-46,76-78H,9-25H2,1-8H3,(H2,60,79)(H2,61,80)(H,62,82)(H,63,86)(H,64,84)(H,65,88)(H,66,89)(H,67,90)(H,68,81)(H,69,91)(H,70,87)(H,71,83)(H,72,85)/t27-,28-,29+,30+,31-,32-,33-,34-,35-,36-,37-,38-,42-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of purified human proteinase 3 using MeOSuc-AAPV-MCA as substrate by fluorescence based assay |
ACS Med Chem Lett 10: 1234-1239 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00253
BindingDB Entry DOI: 10.7270/Q2N58QNG |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50031630
(CHEMBL3359765)Show SMILES CCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1N)C(C)C)C(=O)C[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)NCCNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O |r| Show InChI InChI=1S/C54H71N13O19/c1-6-9-36(60-52(80)40(26-45(73)74)63-48(76)29(5)59-54(82)46(27(2)3)65-49(77)33-10-7-8-11-34(33)55)42(69)22-28(4)47(75)62-39(25-44(71)72)53(81)64-38(23-30-12-15-32(68)16-13-30)51(79)61-37(18-19-43(56)70)50(78)58-21-20-57-35-17-14-31(66(83)84)24-41(35)67(85)86/h7-8,10-17,24,27-29,36-40,46,57,68H,6,9,18-23,25-26,55H2,1-5H3,(H2,56,70)(H,58,78)(H,59,82)(H,60,80)(H,61,79)(H,62,75)(H,63,76)(H,64,81)(H,65,77)(H,71,72)(H,73,74)/t28-,29+,36+,37+,38+,39+,40+,46+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bergen
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using keto-D-DY-FRET as substrate |
J Med Chem 57: 9396-408 (2014)
Article DOI: 10.1021/jm500782s
BindingDB Entry DOI: 10.7270/Q2VQ349F |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50263165
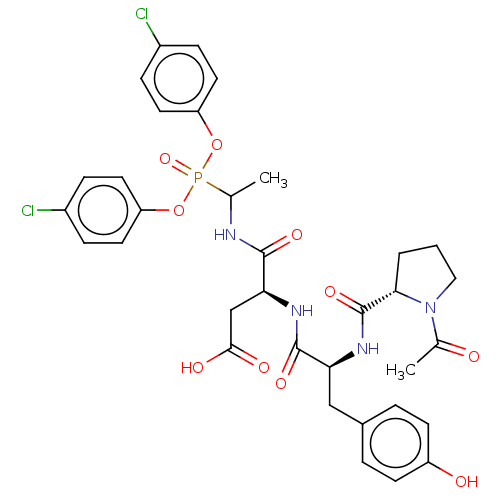
(CHEMBL4102959)Show SMILES CC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(C)=O)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C34H37Cl2N4O10P/c1-20(51(48,49-26-13-7-23(35)8-14-26)50-27-15-9-24(36)10-16-27)37-32(45)29(19-31(43)44)38-33(46)28(18-22-5-11-25(42)12-6-22)39-34(47)30-4-3-17-40(30)21(2)41/h5-16,20,28-30,42H,3-4,17-19H2,1-2H3,(H,37,45)(H,38,46)(H,39,47)(H,43,44)/t20?,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM UMR1100,"Centre d'Etude des Pathologies Respiratoires" , Université de Tours , 37032 Tours , France.
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using ABZ-VADnVADYQ-EDDnp as substrate by FRET assay |
J Med Chem 61: 1858-1870 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01416
BindingDB Entry DOI: 10.7270/Q25B04ZT |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50266984
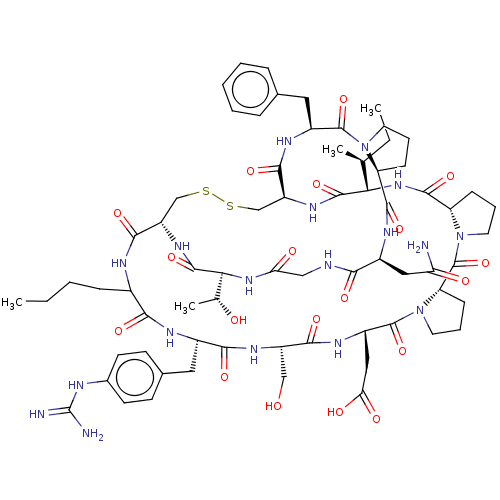
(CHEMBL4092637)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(NC(N)=N)cc3)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C69H98N18O19S2/c1-5-7-16-40-57(95)76-41(27-38-20-22-39(23-21-38)74-69(71)72)58(96)80-45(32-88)59(97)79-44(30-53(92)93)67(105)87-26-13-19-50(87)68(106)86-25-12-18-49(86)63(101)84-54(35(3)6-2)64(102)81-47-34-108-107-33-46(60(98)75-40)82-65(103)55(36(4)89)83-52(91)31-73-56(94)42(29-51(70)90)77-62(100)48-17-11-24-85(48)66(104)43(78-61(47)99)28-37-14-9-8-10-15-37/h8-10,14-15,20-23,35-36,40-50,54-55,88-89H,5-7,11-13,16-19,24-34H2,1-4H3,(H2,70,90)(H,73,94)(H,75,98)(H,76,95)(H,77,100)(H,78,99)(H,79,97)(H,80,96)(H,81,102)(H,82,103)(H,83,91)(H,84,101)(H,92,93)(H4,71,72,74)/t35-,36+,40?,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using Suc(OMe)-AAPV-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50266979
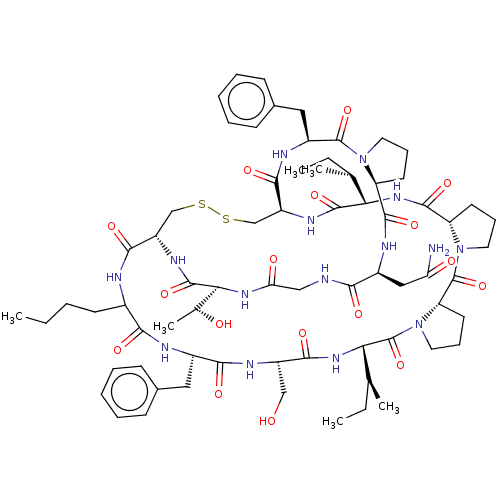
(CHEMBL4061897)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C70H101N15O17S2/c1-7-10-24-43-59(91)74-44(31-41-20-13-11-14-21-41)60(92)77-47(35-86)61(93)82-56(39(5)9-3)70(102)85-30-19-27-52(85)69(101)84-29-18-26-51(84)65(97)81-55(38(4)8-2)66(98)78-49-37-104-103-36-48(62(94)73-43)79-67(99)57(40(6)87)80-54(89)34-72-58(90)45(33-53(71)88)75-64(96)50-25-17-28-83(50)68(100)46(76-63(49)95)32-42-22-15-12-16-23-42/h11-16,20-23,38-40,43-52,55-57,86-87H,7-10,17-19,24-37H2,1-6H3,(H2,71,88)(H,72,90)(H,73,94)(H,74,91)(H,75,96)(H,76,95)(H,77,92)(H,78,98)(H,79,99)(H,80,89)(H,81,97)(H,82,93)/t38-,39-,40+,43?,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using Suc(OMe)-AAPV-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50266989

(CHEMBL4081142)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C68H95N15O19S2/c1-5-7-21-40-57(91)72-41(28-38-17-10-8-11-18-38)58(92)76-45(33-84)59(93)75-44(31-53(88)89)67(101)83-27-16-24-50(83)68(102)82-26-15-23-49(82)63(97)80-54(36(3)6-2)64(98)77-47-35-104-103-34-46(60(94)71-40)78-65(99)55(37(4)85)79-52(87)32-70-56(90)42(30-51(69)86)73-62(96)48-22-14-25-81(48)66(100)43(74-61(47)95)29-39-19-12-9-13-20-39/h8-13,17-20,36-37,40-50,54-55,84-85H,5-7,14-16,21-35H2,1-4H3,(H2,69,86)(H,70,90)(H,71,94)(H,72,91)(H,73,96)(H,74,95)(H,75,93)(H,76,92)(H,77,98)(H,78,99)(H,79,87)(H,80,97)(H,88,89)/t36-,37+,40?,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human PR3 using Suc(OMe)-AAPV-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM247411
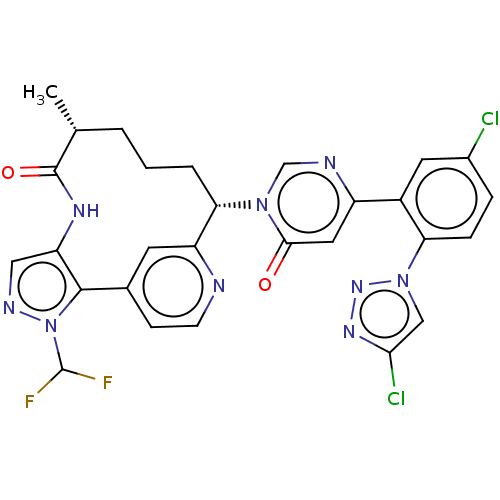
(US10336754, Example 353 | US11053247, Example 353 ...)Show SMILES C[C@@H]1CCC[C@@H](c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F)n1cnc(cc1=O)-c1cc(Cl)ccc1-n1cc(Cl)nn1 |r| Show InChI InChI=1S/C28H23Cl2F2N9O2/c1-15-3-2-4-23(20-9-16(7-8-33-20)26-21(36-27(15)43)12-35-41(26)28(31)32)39-14-34-19(11-25(39)42)18-10-17(29)5-6-22(18)40-13-24(30)37-38-40/h5-15,23,28H,2-4H2,1H3,(H,36,43)/t15-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human neutrophil proteinase 3 assessed as inhibition constant using Methoxysuccinyl-L-alanyl-L-alanyl-L-prolylL-valine p-Nitroani... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50544646

(CHEMBL4635621)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H26NO4P/c1-19(2)23(25-24(26)28-18-20-12-6-3-7-13-20)30(27,22-16-10-5-11-17-22)29-21-14-8-4-9-15-21/h3-17,19,23H,18H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human proteinase 3 using flurogenic substrate as elastase substrate V by fluorescence assay |
ACS Med Chem Lett 11: 1739-1744 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00284
BindingDB Entry DOI: 10.7270/Q29P357N |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50544648

(CHEMBL4636971)Show SMILES CCCP(=O)(Oc1ccccc1)C(NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C21H28NO4P/c1-4-15-27(24,26-19-13-9-6-10-14-19)20(17(2)3)22-21(23)25-16-18-11-7-5-8-12-18/h5-14,17,20H,4,15-16H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human proteinase 3 using flurogenic substrate as elastase substrate V by fluorescence assay |
ACS Med Chem Lett 11: 1739-1744 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00284
BindingDB Entry DOI: 10.7270/Q29P357N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
















