

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Rattus norvegicus) | BDBM50266989 (CHEMBL4081142) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of rat mast cell chymase using Suc-AAPF-pNA as substrate after 30 mins | J Med Chem 60: 658-667 (2017) Article DOI: 10.1021/acs.jmedchem.6b01509 BindingDB Entry DOI: 10.7270/Q2FB55DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Rattus norvegicus) | BDBM50098853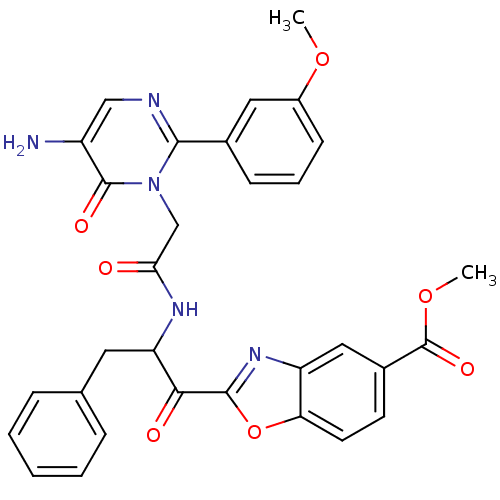 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 86.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against rat peritoneal chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Rattus norvegicus) | BDBM50098847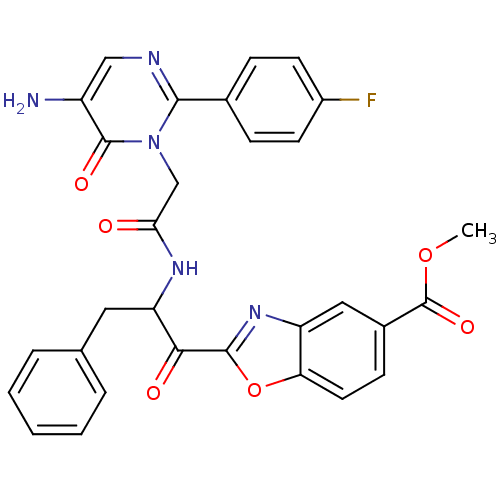 (2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against rat peritoneal chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Rattus norvegicus) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against rat peritoneal chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Rattus norvegicus) | BDBM50266984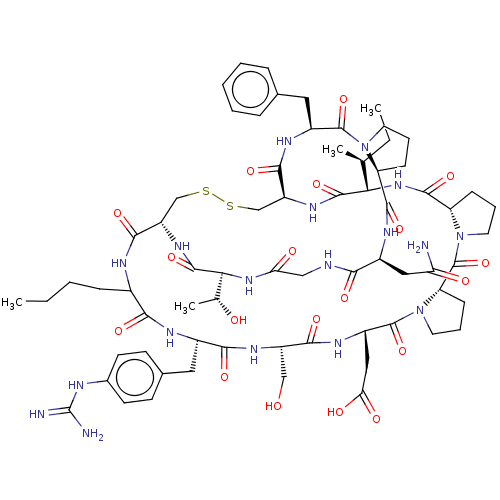 (CHEMBL4092637) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of rat mast cell chymase using Suc-AAPF-pNA as substrate after 30 mins | J Med Chem 60: 658-667 (2017) Article DOI: 10.1021/acs.jmedchem.6b01509 BindingDB Entry DOI: 10.7270/Q2FB55DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||