 Found 187 hits of ki data for polymerid = 50005810,5360
Found 187 hits of ki data for polymerid = 50005810,5360 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207116

(CHEMBL3905247 | US9550741, I-4)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2[nH]1 |r,wU:6.6,wD:3.2,(25.87,-26.54,;26.59,-25.21,;25.82,-23.88,;24.29,-23.88,;23.52,-22.54,;21.97,-22.54,;21.2,-23.88,;19.67,-23.88,;18.9,-22.54,;17.36,-22.54,;16.59,-23.88,;15.06,-23.88,;14.29,-22.54,;15.06,-21.21,;16.59,-21.21,;12.75,-22.54,;11.83,-23.78,;10.4,-23.31,;10.4,-21.77,;9.27,-20.75,;9.58,-19.21,;11.07,-18.75,;12.13,-19.78,;11.83,-21.26,;21.97,-25.21,;23.52,-25.21,;28.13,-25.11,;28.95,-23.83,;30.43,-24.24,;31.72,-23.36,;33.1,-24.08,;33.15,-25.62,;31.87,-26.44,;30.48,-25.77,;29.05,-26.29,)| Show InChI InChI=1S/C28H33N5OS/c34-28(25-19-21-5-1-3-7-24(21)30-25)29-22-11-9-20(10-12-22)13-14-32-15-17-33(18-16-32)27-23-6-2-4-8-26(23)35-31-27/h1-8,19-20,22,30H,9-18H2,(H,29,34)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207116

(CHEMBL3905247 | US9550741, I-4)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2[nH]1 |r,wU:6.6,wD:3.2,(25.87,-26.54,;26.59,-25.21,;25.82,-23.88,;24.29,-23.88,;23.52,-22.54,;21.97,-22.54,;21.2,-23.88,;19.67,-23.88,;18.9,-22.54,;17.36,-22.54,;16.59,-23.88,;15.06,-23.88,;14.29,-22.54,;15.06,-21.21,;16.59,-21.21,;12.75,-22.54,;11.83,-23.78,;10.4,-23.31,;10.4,-21.77,;9.27,-20.75,;9.58,-19.21,;11.07,-18.75,;12.13,-19.78,;11.83,-21.26,;21.97,-25.21,;23.52,-25.21,;28.13,-25.11,;28.95,-23.83,;30.43,-24.24,;31.72,-23.36,;33.1,-24.08,;33.15,-25.62,;31.87,-26.44,;30.48,-25.77,;29.05,-26.29,)| Show InChI InChI=1S/C28H33N5OS/c34-28(25-19-21-5-1-3-7-24(21)30-25)29-22-11-9-20(10-12-22)13-14-32-15-17-33(18-16-32)27-23-6-2-4-8-26(23)35-31-27/h1-8,19-20,22,30H,9-18H2,(H,29,34)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263372
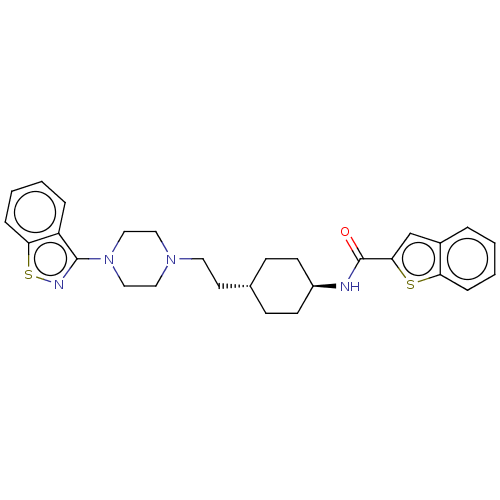
(US9550741, I-6)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2s1 |r,wU:3.2,wD:6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| Show InChI InChI=1S/C28H32N4OS2/c33-28(26-19-21-5-1-3-7-24(21)34-26)29-22-11-9-20(10-12-22)13-14-31-15-17-32(18-16-31)27-23-6-2-4-8-25(23)35-30-27/h1-8,19-20,22H,9-18H2,(H,29,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263372

(US9550741, I-6)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2s1 |r,wU:3.2,wD:6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| Show InChI InChI=1S/C28H32N4OS2/c33-28(26-19-21-5-1-3-7-24(21)34-26)29-22-11-9-20(10-12-22)13-14-31-15-17-32(18-16-31)27-23-6-2-4-8-25(23)35-30-27/h1-8,19-20,22H,9-18H2,(H,29,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207143
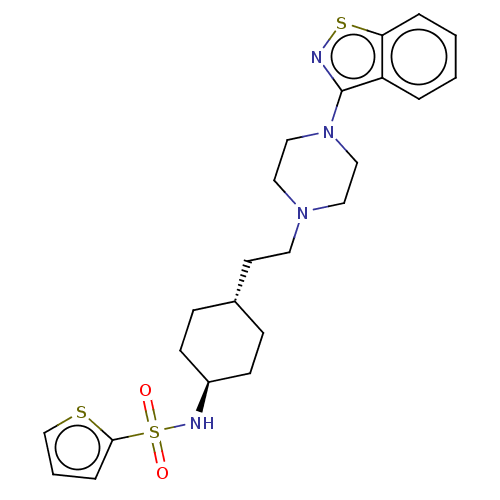
(CHEMBL3966842 | US9550741, II-1)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:4.3,wD:7.7,(11.63,-23.22,;13.07,-23.76,;11.88,-24.73,;13.9,-22.46,;15.43,-22.53,;16.27,-21.24,;17.8,-21.31,;18.51,-22.68,;20.04,-22.75,;20.87,-21.45,;22.41,-21.53,;23.25,-20.23,;24.78,-20.3,;25.49,-21.67,;24.66,-22.97,;23.12,-22.9,;27.03,-21.75,;27.88,-23.03,;29.36,-22.62,;29.43,-21.08,;30.62,-20.11,;30.37,-18.59,;28.93,-18.05,;27.74,-19.02,;27.99,-20.54,;17.68,-23.98,;16.14,-23.9,;13.77,-25.13,;15.29,-25.35,;15.54,-26.87,;14.18,-27.58,;13.08,-26.5,)| Show InChI InChI=1S/C23H30N4O2S3/c28-32(29,22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-26-13-15-27(16-14-26)23-20-4-1-2-5-21(20)31-24-23/h1-6,17-19,25H,7-16H2/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207143

(CHEMBL3966842 | US9550741, II-1)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:4.3,wD:7.7,(11.63,-23.22,;13.07,-23.76,;11.88,-24.73,;13.9,-22.46,;15.43,-22.53,;16.27,-21.24,;17.8,-21.31,;18.51,-22.68,;20.04,-22.75,;20.87,-21.45,;22.41,-21.53,;23.25,-20.23,;24.78,-20.3,;25.49,-21.67,;24.66,-22.97,;23.12,-22.9,;27.03,-21.75,;27.88,-23.03,;29.36,-22.62,;29.43,-21.08,;30.62,-20.11,;30.37,-18.59,;28.93,-18.05,;27.74,-19.02,;27.99,-20.54,;17.68,-23.98,;16.14,-23.9,;13.77,-25.13,;15.29,-25.35,;15.54,-26.87,;14.18,-27.58,;13.08,-26.5,)| Show InChI InChI=1S/C23H30N4O2S3/c28-32(29,22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-26-13-15-27(16-14-26)23-20-4-1-2-5-21(20)31-24-23/h1-6,17-19,25H,7-16H2/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207094
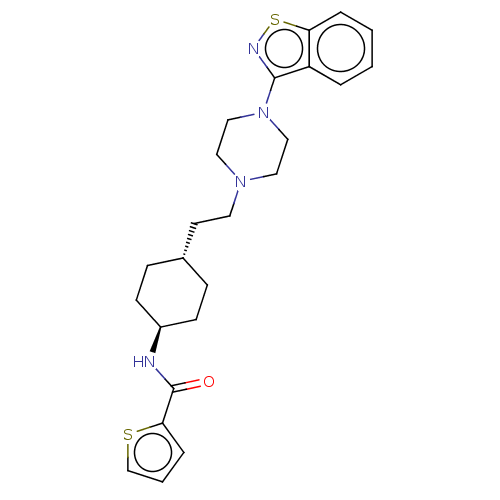
(CHEMBL3976282 | US9550741, I-2)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:3.2,wD:6.6,(15.49,-22.15,;14.77,-20.77,;15.54,-19.44,;17.08,-19.44,;17.84,-18.11,;19.39,-18.11,;20.15,-19.44,;21.69,-19.44,;22.46,-18.11,;23.99,-18.11,;24.76,-19.44,;26.3,-19.44,;27.06,-18.11,;26.3,-16.77,;24.76,-16.77,;28.61,-18.11,;29.53,-19.33,;31.01,-18.87,;31.01,-17.34,;32.14,-16.31,;31.83,-14.78,;30.34,-14.31,;29.22,-15.34,;29.53,-16.88,;19.39,-20.77,;17.84,-20.77,;13.24,-20.67,;12.42,-19.38,;10.93,-19.79,;10.88,-21.34,;12.32,-21.9,)| Show InChI InChI=1S/C24H30N4OS2/c29-24(22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-5-21(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207094

(CHEMBL3976282 | US9550741, I-2)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:3.2,wD:6.6,(15.49,-22.15,;14.77,-20.77,;15.54,-19.44,;17.08,-19.44,;17.84,-18.11,;19.39,-18.11,;20.15,-19.44,;21.69,-19.44,;22.46,-18.11,;23.99,-18.11,;24.76,-19.44,;26.3,-19.44,;27.06,-18.11,;26.3,-16.77,;24.76,-16.77,;28.61,-18.11,;29.53,-19.33,;31.01,-18.87,;31.01,-17.34,;32.14,-16.31,;31.83,-14.78,;30.34,-14.31,;29.22,-15.34,;29.53,-16.88,;19.39,-20.77,;17.84,-20.77,;13.24,-20.67,;12.42,-19.38,;10.93,-19.79,;10.88,-21.34,;12.32,-21.9,)| Show InChI InChI=1S/C24H30N4OS2/c29-24(22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-5-21(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207158
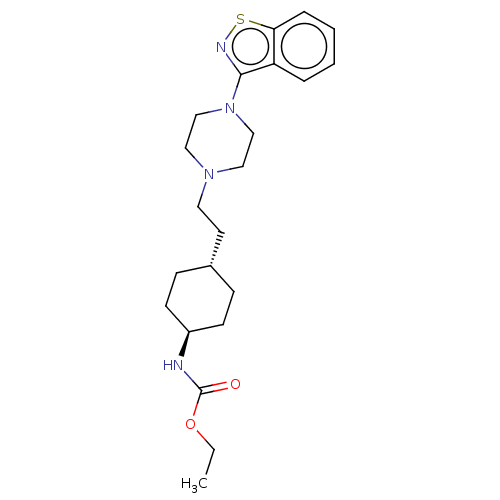
(CHEMBL3982486 | US9550741, III-2)Show SMILES CCOC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:6.5,wD:9.9,(23.48,-37.39,;25.01,-37.44,;25.84,-36.17,;27.39,-36.27,;28.1,-37.6,;28.15,-34.94,;29.7,-34.94,;30.48,-33.61,;32.01,-33.61,;32.78,-34.95,;34.32,-34.96,;35.09,-33.62,;36.64,-33.62,;37.41,-34.96,;38.95,-34.97,;39.72,-33.63,;38.95,-32.29,;37.42,-32.29,;41.26,-33.64,;42.19,-34.87,;43.56,-34.41,;43.57,-32.88,;44.7,-31.85,;44.39,-30.31,;43.02,-29.84,;41.84,-30.87,;42.19,-32.36,;32.01,-36.28,;30.47,-36.28,)| Show InChI InChI=1S/C22H32N4O2S/c1-2-28-22(27)23-18-9-7-17(8-10-18)11-12-25-13-15-26(16-14-25)21-19-5-3-4-6-20(19)29-24-21/h3-6,17-18H,2,7-16H2,1H3,(H,23,27)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207158

(CHEMBL3982486 | US9550741, III-2)Show SMILES CCOC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:6.5,wD:9.9,(23.48,-37.39,;25.01,-37.44,;25.84,-36.17,;27.39,-36.27,;28.1,-37.6,;28.15,-34.94,;29.7,-34.94,;30.48,-33.61,;32.01,-33.61,;32.78,-34.95,;34.32,-34.96,;35.09,-33.62,;36.64,-33.62,;37.41,-34.96,;38.95,-34.97,;39.72,-33.63,;38.95,-32.29,;37.42,-32.29,;41.26,-33.64,;42.19,-34.87,;43.56,-34.41,;43.57,-32.88,;44.7,-31.85,;44.39,-30.31,;43.02,-29.84,;41.84,-30.87,;42.19,-32.36,;32.01,-36.28,;30.47,-36.28,)| Show InChI InChI=1S/C22H32N4O2S/c1-2-28-22(27)23-18-9-7-17(8-10-18)11-12-25-13-15-26(16-14-25)21-19-5-3-4-6-20(19)29-24-21/h3-6,17-18H,2,7-16H2,1H3,(H,23,27)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263429

(US9550741, III-11)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)OCc1ccccc1 |r,wU:3.2,wD:6.6,(-5.6,.24,;-5.6,1.78,;-4.26,2.55,;-2.93,1.78,;-1.6,2.55,;-.26,1.78,;-.26,.24,;1.07,-.53,;2.4,.24,;3.74,-.53,;3.74,-2.07,;5.07,-2.84,;6.41,-2.07,;6.41,-.53,;5.07,.24,;7.74,-2.84,;7.74,-4.38,;9.2,-4.86,;10.11,-3.61,;11.64,-3.45,;12.27,-2.04,;11.36,-.8,;9.83,-.96,;9.2,-2.37,;-1.6,-.53,;-2.93,.24,;-6.93,2.55,;-8.27,1.78,;-9.6,2.55,;-9.6,4.09,;-10.93,4.86,;-12.27,4.09,;-12.27,2.55,;-10.93,1.78,)| Show InChI InChI=1S/C27H34N4O2S/c32-27(33-20-22-6-2-1-3-7-22)28-23-12-10-21(11-13-23)14-15-30-16-18-31(19-17-30)26-24-8-4-5-9-25(24)34-29-26/h1-9,21,23H,10-20H2,(H,28,32)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263429

(US9550741, III-11)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)OCc1ccccc1 |r,wU:3.2,wD:6.6,(-5.6,.24,;-5.6,1.78,;-4.26,2.55,;-2.93,1.78,;-1.6,2.55,;-.26,1.78,;-.26,.24,;1.07,-.53,;2.4,.24,;3.74,-.53,;3.74,-2.07,;5.07,-2.84,;6.41,-2.07,;6.41,-.53,;5.07,.24,;7.74,-2.84,;7.74,-4.38,;9.2,-4.86,;10.11,-3.61,;11.64,-3.45,;12.27,-2.04,;11.36,-.8,;9.83,-.96,;9.2,-2.37,;-1.6,-.53,;-2.93,.24,;-6.93,2.55,;-8.27,1.78,;-9.6,2.55,;-9.6,4.09,;-10.93,4.86,;-12.27,4.09,;-12.27,2.55,;-10.93,1.78,)| Show InChI InChI=1S/C27H34N4O2S/c32-27(33-20-22-6-2-1-3-7-22)28-23-12-10-21(11-13-23)14-15-30-16-18-31(19-17-30)26-24-8-4-5-9-25(24)34-29-26/h1-9,21,23H,10-20H2,(H,28,32)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263393
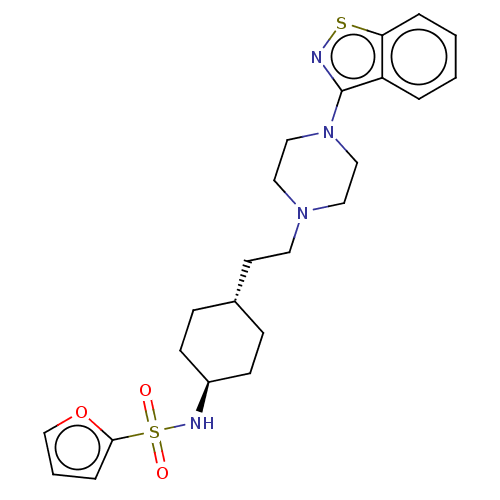
(US9550741, II-3)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1 |r,wU:4.3,wD:7.7,(-7.08,.38,;-7.08,1.92,;-7.08,3.46,;-5.75,2.69,;-4.41,1.92,;-3.08,2.7,;-1.75,1.92,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-3.08,-.39,;-4.41,.38,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| Show InChI InChI=1S/C23H30N4O3S2/c28-32(29,22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-26-13-15-27(16-14-26)23-20-4-1-2-5-21(20)31-24-23/h1-6,17-19,25H,7-16H2/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263393

(US9550741, II-3)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1 |r,wU:4.3,wD:7.7,(-7.08,.38,;-7.08,1.92,;-7.08,3.46,;-5.75,2.69,;-4.41,1.92,;-3.08,2.7,;-1.75,1.92,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-3.08,-.39,;-4.41,.38,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| Show InChI InChI=1S/C23H30N4O3S2/c28-32(29,22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-26-13-15-27(16-14-26)23-20-4-1-2-5-21(20)31-24-23/h1-6,17-19,25H,7-16H2/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207113
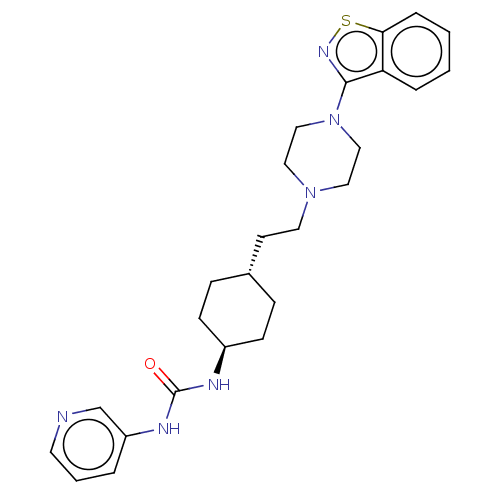
(CHEMBL3896937 | US9550741, IV-3)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)Nc1cccnc1 |r,wU:3.2,wD:6.6,(15.23,-21.5,;14.88,-22.99,;15.9,-24.12,;17.39,-23.82,;17.85,-22.32,;19.4,-22.02,;20.42,-23.15,;21.91,-22.84,;22.94,-23.97,;24.43,-23.66,;24.95,-22.17,;26.43,-21.87,;27.46,-22.99,;27,-24.48,;25.45,-24.79,;28.95,-22.68,;29.62,-21.3,;31.1,-21.45,;31.47,-22.94,;32.8,-23.71,;32.8,-25.25,;31.47,-26.02,;30.13,-25.25,;30.13,-23.71,;19.91,-24.63,;18.42,-24.95,;13.38,-23.35,;12.25,-22.27,;10.76,-22.68,;9.75,-21.6,;10.16,-20.12,;11.58,-19.7,;12.67,-20.78,)| Show InChI InChI=1S/C25H32N6OS/c32-25(28-21-4-3-12-26-18-21)27-20-9-7-19(8-10-20)11-13-30-14-16-31(17-15-30)24-22-5-1-2-6-23(22)33-29-24/h1-6,12,18-20H,7-11,13-17H2,(H2,27,28,32)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207113

(CHEMBL3896937 | US9550741, IV-3)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)Nc1cccnc1 |r,wU:3.2,wD:6.6,(15.23,-21.5,;14.88,-22.99,;15.9,-24.12,;17.39,-23.82,;17.85,-22.32,;19.4,-22.02,;20.42,-23.15,;21.91,-22.84,;22.94,-23.97,;24.43,-23.66,;24.95,-22.17,;26.43,-21.87,;27.46,-22.99,;27,-24.48,;25.45,-24.79,;28.95,-22.68,;29.62,-21.3,;31.1,-21.45,;31.47,-22.94,;32.8,-23.71,;32.8,-25.25,;31.47,-26.02,;30.13,-25.25,;30.13,-23.71,;19.91,-24.63,;18.42,-24.95,;13.38,-23.35,;12.25,-22.27,;10.76,-22.68,;9.75,-21.6,;10.16,-20.12,;11.58,-19.7,;12.67,-20.78,)| Show InChI InChI=1S/C25H32N6OS/c32-25(28-21-4-3-12-26-18-21)27-20-9-7-19(8-10-20)11-13-30-14-16-31(17-15-30)24-22-5-1-2-6-23(22)33-29-24/h1-6,12,18-20H,7-11,13-17H2,(H2,27,28,32)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263388

(US9550741, I-22)Show SMILES O=C(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2[nH]1 |r,wU:3.2,6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-3.22,-.2,;-1.89,-.97,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-.56,1.34,;-1.89,2.11,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263388

(US9550741, I-22)Show SMILES O=C(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2[nH]1 |r,wU:3.2,6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-3.22,-.2,;-1.89,-.97,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-.56,1.34,;-1.89,2.11,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263371
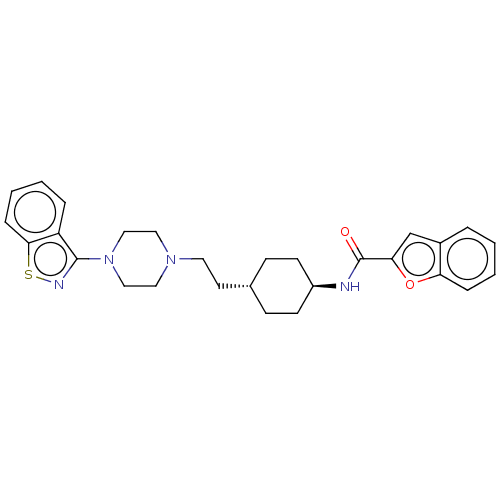
(US9550741, I-5)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2o1 |r,wU:3.2,wD:6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| Show InChI InChI=1S/C28H32N4O2S/c33-28(25-19-21-5-1-3-7-24(21)34-25)29-22-11-9-20(10-12-22)13-14-31-15-17-32(18-16-31)27-23-6-2-4-8-26(23)35-30-27/h1-8,19-20,22H,9-18H2,(H,29,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263371

(US9550741, I-5)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2o1 |r,wU:3.2,wD:6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| Show InChI InChI=1S/C28H32N4O2S/c33-28(25-19-21-5-1-3-7-24(21)34-25)29-22-11-9-20(10-12-22)13-14-31-15-17-32(18-16-31)27-23-6-2-4-8-26(23)35-30-27/h1-8,19-20,22H,9-18H2,(H,29,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263395
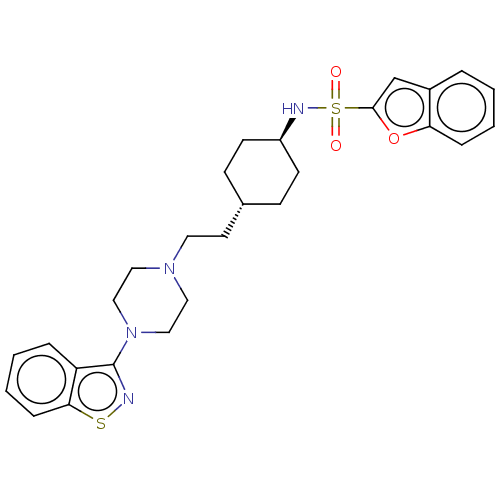
(US9550741, II-5)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2o1 |r,wU:4.3,wD:7.7,(-5.89,-.2,;-5.89,1.34,;-5.89,2.88,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| Show InChI InChI=1S/C27H32N4O3S2/c32-36(33,26-19-21-5-1-3-7-24(21)34-26)29-22-11-9-20(10-12-22)13-14-30-15-17-31(18-16-30)27-23-6-2-4-8-25(23)35-28-27/h1-8,19-20,22,29H,9-18H2/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263395

(US9550741, II-5)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2o1 |r,wU:4.3,wD:7.7,(-5.89,-.2,;-5.89,1.34,;-5.89,2.88,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)| Show InChI InChI=1S/C27H32N4O3S2/c32-36(33,26-19-21-5-1-3-7-24(21)34-26)29-22-11-9-20(10-12-22)13-14-30-15-17-31(18-16-30)27-23-6-2-4-8-25(23)35-28-27/h1-8,19-20,22,29H,9-18H2/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263432

(US9550741, III-14)Show SMILES COC(=O)N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:5.4,8.8,(-10.27,2.93,;-8.93,3.7,;-7.6,2.93,;-7.6,1.39,;-6.26,3.7,;-4.93,2.93,;-4.93,1.39,;-3.6,.62,;-2.26,1.39,;-.93,.62,;.4,1.39,;1.74,.62,;1.74,-.92,;3.07,-1.69,;4.4,-.92,;4.4,.62,;3.07,1.39,;5.74,-1.69,;5.74,-3.23,;7.2,-3.7,;8.11,-2.46,;9.64,-2.3,;10.27,-.89,;9.36,.36,;7.83,.2,;7.2,-1.21,;-2.26,2.93,;-3.6,3.7,)| Show InChI InChI=1S/C21H30N4O2S/c1-27-21(26)22-17-8-6-16(7-9-17)10-11-24-12-14-25(15-13-24)20-18-4-2-3-5-19(18)28-23-20/h2-5,16-17H,6-15H2,1H3,(H,22,26)/t16-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263432

(US9550741, III-14)Show SMILES COC(=O)N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:5.4,8.8,(-10.27,2.93,;-8.93,3.7,;-7.6,2.93,;-7.6,1.39,;-6.26,3.7,;-4.93,2.93,;-4.93,1.39,;-3.6,.62,;-2.26,1.39,;-.93,.62,;.4,1.39,;1.74,.62,;1.74,-.92,;3.07,-1.69,;4.4,-.92,;4.4,.62,;3.07,1.39,;5.74,-1.69,;5.74,-3.23,;7.2,-3.7,;8.11,-2.46,;9.64,-2.3,;10.27,-.89,;9.36,.36,;7.83,.2,;7.2,-1.21,;-2.26,2.93,;-3.6,3.7,)| Show InChI InChI=1S/C21H30N4O2S/c1-27-21(26)22-17-8-6-16(7-9-17)10-11-24-12-14-25(15-13-24)20-18-4-2-3-5-19(18)28-23-20/h2-5,16-17H,6-15H2,1H3,(H,22,26)/t16-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263449

(US9550741, RGH-188)Show SMILES CN(C)C(=O)NC1CCC(CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |(,-4.62,;1.33,-5.39,;2.67,-4.62,;1.33,-6.93,;,-7.7,;2.67,-7.7,;2.67,-9.24,;4,-10.01,;4,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-14.63,;4,-16.17,;5.33,-16.94,;5.33,-18.48,;4,-19.25,;2.67,-18.48,;2.67,-16.94,;4,-20.79,;2.67,-21.56,;2.67,-23.1,;4,-23.87,;5.33,-23.1,;6.67,-23.87,;5.33,-21.56,;6.67,-20.79,;1.33,-11.55,;1.33,-10.01,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263412
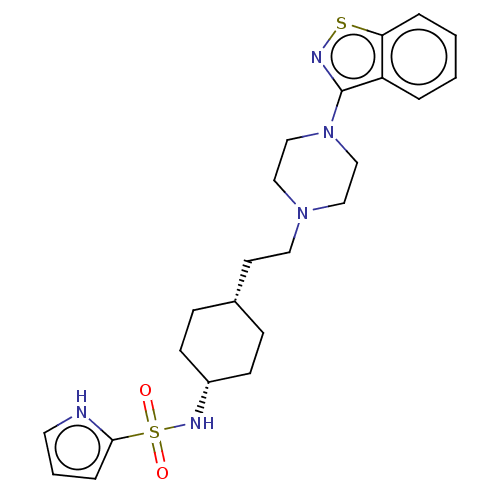
(US9550741, II-17)Show SMILES O=S(=O)(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccc[nH]1 |r,wU:4.3,7.7,(-7.08,.38,;-7.08,1.92,;-7.08,3.46,;-5.75,2.69,;-4.41,1.92,;-4.41,.38,;-3.08,-.39,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-1.75,1.92,;-3.08,2.7,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263412

(US9550741, II-17)Show SMILES O=S(=O)(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccc[nH]1 |r,wU:4.3,7.7,(-7.08,.38,;-7.08,1.92,;-7.08,3.46,;-5.75,2.69,;-4.41,1.92,;-4.41,.38,;-3.08,-.39,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-1.75,1.92,;-3.08,2.7,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263421
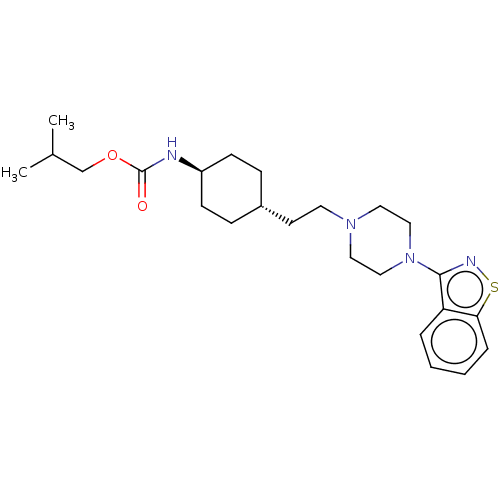
(US9550741, III-3)Show SMILES CC(C)COC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:8.7,wD:11.11,(-11.6,2.16,;-10.27,2.93,;-10.27,4.47,;-8.93,2.16,;-7.6,2.93,;-6.26,2.16,;-6.26,.62,;-4.93,2.93,;-3.6,2.16,;-2.26,2.93,;-.93,2.16,;-.93,.62,;.4,-.15,;1.74,.62,;3.07,-.15,;3.07,-1.69,;4.4,-2.46,;5.74,-1.69,;5.74,-.15,;4.4,.62,;7.07,-2.46,;7.07,-4,;8.54,-4.47,;9.44,-3.23,;10.97,-3.07,;11.6,-1.66,;10.69,-.41,;9.16,-.57,;8.54,-1.98,;-2.26,-.15,;-3.6,.62,)| Show InChI InChI=1S/C24H36N4O2S/c1-18(2)17-30-24(29)25-20-9-7-19(8-10-20)11-12-27-13-15-28(16-14-27)23-21-5-3-4-6-22(21)31-26-23/h3-6,18-20H,7-17H2,1-2H3,(H,25,29)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263421

(US9550741, III-3)Show SMILES CC(C)COC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:8.7,wD:11.11,(-11.6,2.16,;-10.27,2.93,;-10.27,4.47,;-8.93,2.16,;-7.6,2.93,;-6.26,2.16,;-6.26,.62,;-4.93,2.93,;-3.6,2.16,;-2.26,2.93,;-.93,2.16,;-.93,.62,;.4,-.15,;1.74,.62,;3.07,-.15,;3.07,-1.69,;4.4,-2.46,;5.74,-1.69,;5.74,-.15,;4.4,.62,;7.07,-2.46,;7.07,-4,;8.54,-4.47,;9.44,-3.23,;10.97,-3.07,;11.6,-1.66,;10.69,-.41,;9.16,-.57,;8.54,-1.98,;-2.26,-.15,;-3.6,.62,)| Show InChI InChI=1S/C24H36N4O2S/c1-18(2)17-30-24(29)25-20-9-7-19(8-10-20)11-12-27-13-15-28(16-14-27)23-21-5-3-4-6-22(21)31-26-23/h3-6,18-20H,7-17H2,1-2H3,(H,25,29)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
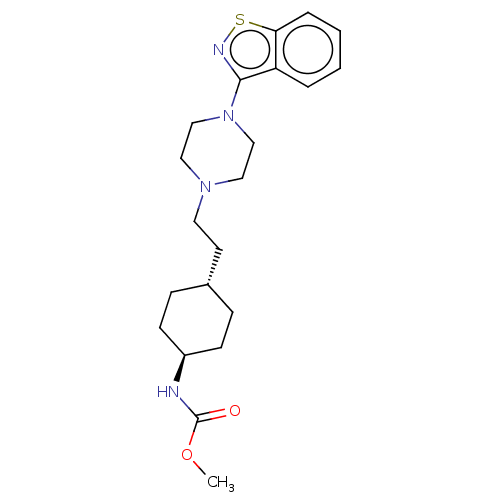
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263419
(US9550741, III-1)Show SMILES COC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:5.4,wD:8.8,(-10.27,2.93,;-8.93,3.7,;-7.6,2.93,;-7.6,1.39,;-6.26,3.7,;-4.93,2.93,;-3.6,3.7,;-2.26,2.93,;-2.26,1.39,;-.93,.62,;.4,1.39,;1.74,.62,;1.74,-.92,;3.07,-1.69,;4.4,-.92,;4.4,.62,;3.07,1.39,;5.74,-1.69,;5.74,-3.23,;7.2,-3.7,;8.11,-2.46,;9.64,-2.3,;10.27,-.89,;9.36,.36,;7.83,.2,;7.2,-1.21,;-3.6,.62,;-4.93,1.39,)| Show InChI InChI=1S/C21H30N4O2S/c1-27-21(26)22-17-8-6-16(7-9-17)10-11-24-12-14-25(15-13-24)20-18-4-2-3-5-19(18)28-23-20/h2-5,16-17H,6-15H2,1H3,(H,22,26)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263383
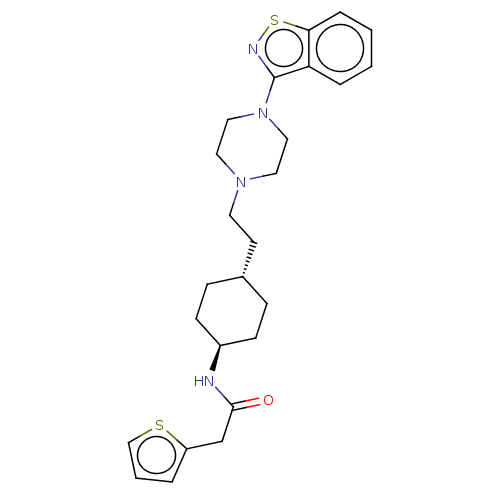
(US9550741, I-17)Show SMILES O=C(Cc1cccs1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:9.9,wD:12.13,(-6.35,1.05,;-6.35,2.59,;-7.69,3.36,;-9.02,2.59,;-9.5,4.05,;-11.04,4.05,;-11.51,2.59,;-10.27,1.68,;-5.02,3.36,;-3.69,2.59,;-2.35,3.36,;-1.02,2.59,;-1.02,1.05,;.32,.28,;1.65,1.05,;2.98,.28,;2.98,-1.26,;4.32,-2.03,;5.65,-1.26,;5.65,.28,;4.32,1.05,;6.98,-2.03,;6.98,-3.57,;8.45,-4.05,;9.35,-2.8,;10.89,-2.64,;11.51,-1.24,;10.61,.01,;9.08,-.15,;8.45,-1.56,;-2.35,.28,;-3.69,1.05,)| Show InChI InChI=1S/C25H32N4OS2/c30-24(18-21-4-3-17-31-21)26-20-9-7-19(8-10-20)11-12-28-13-15-29(16-14-28)25-22-5-1-2-6-23(22)32-27-25/h1-6,17,19-20H,7-16,18H2,(H,26,30)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM93062

(Vitamin D analog, 11 | Vitamin D analog, 15)Show SMILES C[C@@H](CCCCO)C1CCC2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:23.25,25.27,15.17,1.0,wD:21.23,@@:23,(-.69,7.62,;.08,6.29,;1.59,6.61,;2.62,5.46,;4.13,5.78,;5.16,4.64,;6.66,4.96,;-.39,4.82,;.51,3.58,;-.39,2.33,;-1.86,2.81,;-3.19,2.04,;-4.52,2.81,;-4.52,4.35,;-3.19,5.12,;-1.86,4.35,;-1.86,5.89,;-3.19,.5,;-4.52,-.27,;-4.52,-1.81,;-5.86,-2.58,;-5.86,-4.13,;-7.19,-4.9,;-4.52,-4.9,;-4.52,-6.44,;-3.19,-4.13,;-1.86,-4.9,;-3.19,-2.58,)| Show InChI InChI=1S/C25H42O3/c1-17(7-4-5-14-26)21-11-12-22-20(8-6-13-25(21,22)3)10-9-19-15-23(27)18(2)24(28)16-19/h9-10,17-18,21-24,26-28H,4-8,11-16H2,1-3H3/b19-9-,20-10+/t17-,18?,21?,22?,23+,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
| Assay Description
In vitro study, VDR binding assay were performed as previously described. |
Bioorg Chem 47: 9-16 (2013)
Article DOI: 10.1016/j.bioorg.2013.01.001
BindingDB Entry DOI: 10.7270/Q2FX782T |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263383

(US9550741, I-17)Show SMILES O=C(Cc1cccs1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:9.9,wD:12.13,(-6.35,1.05,;-6.35,2.59,;-7.69,3.36,;-9.02,2.59,;-9.5,4.05,;-11.04,4.05,;-11.51,2.59,;-10.27,1.68,;-5.02,3.36,;-3.69,2.59,;-2.35,3.36,;-1.02,2.59,;-1.02,1.05,;.32,.28,;1.65,1.05,;2.98,.28,;2.98,-1.26,;4.32,-2.03,;5.65,-1.26,;5.65,.28,;4.32,1.05,;6.98,-2.03,;6.98,-3.57,;8.45,-4.05,;9.35,-2.8,;10.89,-2.64,;11.51,-1.24,;10.61,.01,;9.08,-.15,;8.45,-1.56,;-2.35,.28,;-3.69,1.05,)| Show InChI InChI=1S/C25H32N4OS2/c30-24(18-21-4-3-17-31-21)26-20-9-7-19(8-10-20)11-12-28-13-15-29(16-14-28)25-22-5-1-2-6-23(22)32-27-25/h1-6,17,19-20H,7-16,18H2,(H,26,30)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263419

(US9550741, III-1)Show SMILES COC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:5.4,wD:8.8,(-10.27,2.93,;-8.93,3.7,;-7.6,2.93,;-7.6,1.39,;-6.26,3.7,;-4.93,2.93,;-3.6,3.7,;-2.26,2.93,;-2.26,1.39,;-.93,.62,;.4,1.39,;1.74,.62,;1.74,-.92,;3.07,-1.69,;4.4,-.92,;4.4,.62,;3.07,1.39,;5.74,-1.69,;5.74,-3.23,;7.2,-3.7,;8.11,-2.46,;9.64,-2.3,;10.27,-.89,;9.36,.36,;7.83,.2,;7.2,-1.21,;-3.6,.62,;-4.93,1.39,)| Show InChI InChI=1S/C21H30N4O2S/c1-27-21(26)22-17-8-6-16(7-9-17)10-11-24-12-14-25(15-13-24)20-18-4-2-3-5-19(18)28-23-20/h2-5,16-17H,6-15H2,1H3,(H,22,26)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207091

(CHEMBL3953773 | US9550741, IV-6)Show SMILES CN(C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccccc1 |r,wU:5.4,wD:8.8,(19.37,-25.45,;20.19,-24.17,;21.74,-24.22,;22.46,-25.6,;22.51,-22.88,;24.05,-22.88,;24.83,-21.54,;26.37,-21.54,;27.14,-22.88,;28.69,-22.88,;29.46,-21.54,;31,-21.54,;31.77,-22.88,;33.32,-22.88,;34.09,-21.54,;33.32,-20.2,;31.77,-20.2,;35.63,-21.54,;36.56,-22.83,;38,-22.31,;38,-20.77,;39.12,-19.74,;38.76,-18.25,;37.38,-17.78,;36.25,-18.82,;36.56,-20.3,;26.37,-24.22,;24.83,-24.22,;19.53,-22.78,;20.35,-21.49,;19.63,-20.1,;18.09,-20.05,;17.26,-21.34,;17.98,-22.67,)| Show InChI InChI=1S/C27H35N5OS/c1-30(23-7-3-2-4-8-23)27(33)28-22-13-11-21(12-14-22)15-16-31-17-19-32(20-18-31)26-24-9-5-6-10-25(24)34-29-26/h2-10,21-22H,11-20H2,1H3,(H,28,33)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207145

(CHEMBL3946995 | US9550741, I-1)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1 |r,wU:3.2,wD:6.6,(13.36,-22.55,;12.59,-21.22,;13.36,-19.89,;14.89,-19.89,;15.67,-18.56,;17.21,-18.56,;17.97,-19.89,;19.51,-19.89,;20.29,-18.56,;21.83,-18.56,;22.59,-17.22,;24.13,-17.22,;24.9,-18.56,;24.13,-19.89,;22.59,-19.89,;26.44,-18.56,;27.34,-19.8,;28.81,-19.32,;28.81,-17.78,;29.95,-16.75,;29.63,-15.25,;28.16,-14.78,;27.02,-15.81,;27.34,-17.31,;17.21,-21.22,;15.67,-21.22,;11.05,-21.22,;10.15,-19.97,;8.68,-20.45,;8.68,-21.98,;10.15,-22.46,)| Show InChI InChI=1S/C24H30N4O2S/c29-24(21-5-3-17-30-21)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-6-22(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207091

(CHEMBL3953773 | US9550741, IV-6)Show SMILES CN(C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccccc1 |r,wU:5.4,wD:8.8,(19.37,-25.45,;20.19,-24.17,;21.74,-24.22,;22.46,-25.6,;22.51,-22.88,;24.05,-22.88,;24.83,-21.54,;26.37,-21.54,;27.14,-22.88,;28.69,-22.88,;29.46,-21.54,;31,-21.54,;31.77,-22.88,;33.32,-22.88,;34.09,-21.54,;33.32,-20.2,;31.77,-20.2,;35.63,-21.54,;36.56,-22.83,;38,-22.31,;38,-20.77,;39.12,-19.74,;38.76,-18.25,;37.38,-17.78,;36.25,-18.82,;36.56,-20.3,;26.37,-24.22,;24.83,-24.22,;19.53,-22.78,;20.35,-21.49,;19.63,-20.1,;18.09,-20.05,;17.26,-21.34,;17.98,-22.67,)| Show InChI InChI=1S/C27H35N5OS/c1-30(23-7-3-2-4-8-23)27(33)28-22-13-11-21(12-14-22)15-16-31-17-19-32(20-18-31)26-24-9-5-6-10-25(24)34-29-26/h2-10,21-22H,11-20H2,1H3,(H,28,33)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207145

(CHEMBL3946995 | US9550741, I-1)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1 |r,wU:3.2,wD:6.6,(13.36,-22.55,;12.59,-21.22,;13.36,-19.89,;14.89,-19.89,;15.67,-18.56,;17.21,-18.56,;17.97,-19.89,;19.51,-19.89,;20.29,-18.56,;21.83,-18.56,;22.59,-17.22,;24.13,-17.22,;24.9,-18.56,;24.13,-19.89,;22.59,-19.89,;26.44,-18.56,;27.34,-19.8,;28.81,-19.32,;28.81,-17.78,;29.95,-16.75,;29.63,-15.25,;28.16,-14.78,;27.02,-15.81,;27.34,-17.31,;17.21,-21.22,;15.67,-21.22,;11.05,-21.22,;10.15,-19.97,;8.68,-20.45,;8.68,-21.98,;10.15,-22.46,)| Show InChI InChI=1S/C24H30N4O2S/c29-24(21-5-3-17-30-21)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-6-22(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263384
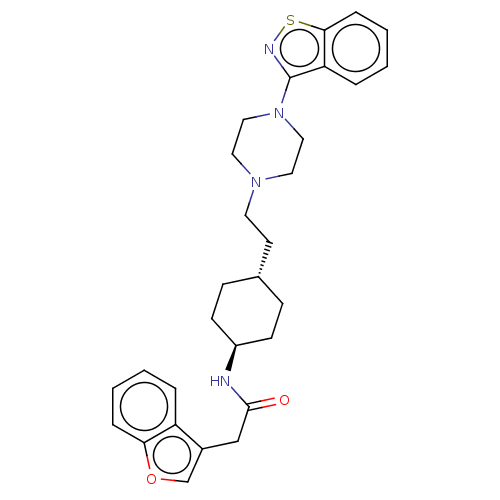
(US9550741, I-18)Show SMILES O=C(Cc1coc2ccccc12)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:13.14,wD:16.18,(-5.33,.76,;-5.33,2.3,;-6.67,3.07,;-8,2.3,;-8,.76,;-9.47,.28,;-10.37,1.53,;-11.9,1.69,;-12.53,3.09,;-11.62,4.34,;-10.09,4.18,;-9.47,2.77,;-4,3.07,;-2.67,2.3,;-1.33,3.07,;,2.3,;,.76,;1.33,-.01,;2.67,.76,;4,-.01,;4,-1.55,;5.33,-2.32,;6.67,-1.55,;6.67,-.01,;5.33,.76,;8,-2.32,;8,-3.86,;9.47,-4.34,;10.37,-3.09,;11.9,-2.93,;12.53,-1.53,;11.62,-.28,;10.09,-.44,;9.47,-1.85,;-1.33,-.01,;-2.67,.76,)| Show InChI InChI=1S/C29H34N4O2S/c34-28(19-22-20-35-26-7-3-1-5-24(22)26)30-23-11-9-21(10-12-23)13-14-32-15-17-33(18-16-32)29-25-6-2-4-8-27(25)36-31-29/h1-8,20-21,23H,9-19H2,(H,30,34)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207144

(CHEMBL3940712 | US9550741, IV-11)Show SMILES O=C(NCc1ccccc1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:11.11,wD:14.15,(26.22,-29.78,;25.55,-28.45,;24.01,-28.34,;23.14,-29.63,;21.6,-29.58,;20.94,-28.19,;19.4,-28.09,;18.58,-29.42,;19.25,-30.75,;20.78,-30.86,;26.32,-27.12,;27.86,-27.12,;28.63,-25.78,;30.16,-25.78,;30.93,-27.12,;32.47,-27.12,;33.24,-25.78,;34.77,-25.78,;35.54,-27.12,;37.08,-27.12,;37.85,-25.78,;37.08,-24.45,;35.54,-24.45,;39.39,-25.78,;40.26,-27.02,;41.68,-26.55,;41.68,-25.01,;42.81,-23.99,;42.5,-22.45,;41.01,-21.99,;39.95,-23.02,;40.26,-24.5,;30.16,-28.45,;28.63,-28.45,)| Show InChI InChI=1S/C27H35N5OS/c33-27(28-20-22-6-2-1-3-7-22)29-23-12-10-21(11-13-23)14-15-31-16-18-32(19-17-31)26-24-8-4-5-9-25(24)34-30-26/h1-9,21,23H,10-20H2,(H2,28,29,33)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263384

(US9550741, I-18)Show SMILES O=C(Cc1coc2ccccc12)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:13.14,wD:16.18,(-5.33,.76,;-5.33,2.3,;-6.67,3.07,;-8,2.3,;-8,.76,;-9.47,.28,;-10.37,1.53,;-11.9,1.69,;-12.53,3.09,;-11.62,4.34,;-10.09,4.18,;-9.47,2.77,;-4,3.07,;-2.67,2.3,;-1.33,3.07,;,2.3,;,.76,;1.33,-.01,;2.67,.76,;4,-.01,;4,-1.55,;5.33,-2.32,;6.67,-1.55,;6.67,-.01,;5.33,.76,;8,-2.32,;8,-3.86,;9.47,-4.34,;10.37,-3.09,;11.9,-2.93,;12.53,-1.53,;11.62,-.28,;10.09,-.44,;9.47,-1.85,;-1.33,-.01,;-2.67,.76,)| Show InChI InChI=1S/C29H34N4O2S/c34-28(19-22-20-35-26-7-3-1-5-24(22)26)30-23-11-9-21(10-12-23)13-14-32-15-17-33(18-16-32)29-25-6-2-4-8-27(25)36-31-29/h1-8,20-21,23H,9-19H2,(H,30,34)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207144

(CHEMBL3940712 | US9550741, IV-11)Show SMILES O=C(NCc1ccccc1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:11.11,wD:14.15,(26.22,-29.78,;25.55,-28.45,;24.01,-28.34,;23.14,-29.63,;21.6,-29.58,;20.94,-28.19,;19.4,-28.09,;18.58,-29.42,;19.25,-30.75,;20.78,-30.86,;26.32,-27.12,;27.86,-27.12,;28.63,-25.78,;30.16,-25.78,;30.93,-27.12,;32.47,-27.12,;33.24,-25.78,;34.77,-25.78,;35.54,-27.12,;37.08,-27.12,;37.85,-25.78,;37.08,-24.45,;35.54,-24.45,;39.39,-25.78,;40.26,-27.02,;41.68,-26.55,;41.68,-25.01,;42.81,-23.99,;42.5,-22.45,;41.01,-21.99,;39.95,-23.02,;40.26,-24.5,;30.16,-28.45,;28.63,-28.45,)| Show InChI InChI=1S/C27H35N5OS/c33-27(28-20-22-6-2-1-3-7-22)29-23-12-10-21(11-13-23)14-15-31-16-18-32(19-17-31)26-24-8-4-5-9-25(24)34-30-26/h1-9,21,23H,10-20H2,(H2,28,29,33)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263386

(US9550741, I-20)Show SMILES O=C(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:3.2,6.6,(-7.08,.38,;-7.08,1.92,;-5.75,2.69,;-4.41,1.92,;-4.41,.38,;-3.08,-.39,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-1.75,1.92,;-3.08,2.7,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| Show InChI InChI=1S/C24H30N4OS2/c29-24(22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-5-21(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263386

(US9550741, I-20)Show SMILES O=C(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:3.2,6.6,(-7.08,.38,;-7.08,1.92,;-5.75,2.69,;-4.41,1.92,;-4.41,.38,;-3.08,-.39,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-1.75,1.92,;-3.08,2.7,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| Show InChI InChI=1S/C24H30N4OS2/c29-24(22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-5-21(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM93061
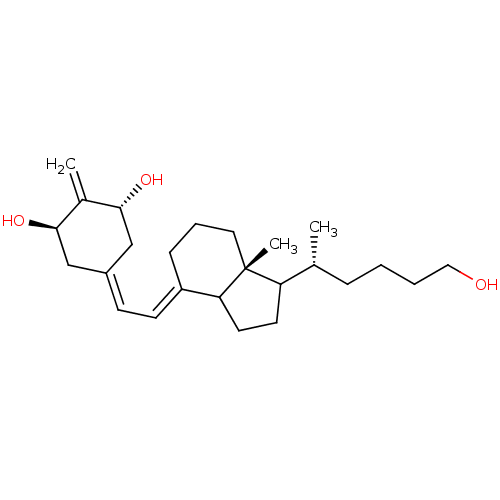
(Vitamin D analog, 6)Show SMILES [#6]-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#8])-[#6]1-[#6]-[#6]-[#6]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C25H40O3/c1-17(7-4-5-14-26)21-11-12-22-20(8-6-13-25(21,22)3)10-9-19-15-23(27)18(2)24(28)16-19/h9-10,17,21-24,26-28H,2,4-8,11-16H2,1,3H3/b20-10+/t17-,21?,22?,23-,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
| Assay Description
In vitro study, VDR binding assay were performed as previously described. |
Bioorg Chem 47: 9-16 (2013)
Article DOI: 10.1016/j.bioorg.2013.01.001
BindingDB Entry DOI: 10.7270/Q2FX782T |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM93067

(Vitamin D analog, 13 | Vitamin D analog, 17)Show SMILES CCCC[C@H](C)C1CCC2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,24.26,14.16,4.4,wD:20.22,@@:22,(5.16,4.64,;4.13,5.78,;2.62,5.46,;1.59,6.61,;.08,6.29,;-.69,7.62,;-.39,4.82,;.51,3.58,;-.39,2.33,;-1.86,2.81,;-3.19,2.04,;-4.52,2.81,;-4.52,4.35,;-3.19,5.12,;-1.86,4.35,;-1.86,5.89,;-3.19,.5,;-4.52,-.27,;-4.52,-1.81,;-5.86,-2.58,;-5.86,-4.13,;-7.19,-4.9,;-4.52,-4.9,;-4.52,-6.44,;-3.19,-4.13,;-1.86,-4.9,;-3.19,-2.58,)| Show InChI InChI=1S/C25H42O2/c1-5-6-8-17(2)21-12-13-22-20(9-7-14-25(21,22)4)11-10-19-15-23(26)18(3)24(27)16-19/h10-11,17-18,21-24,26-27H,5-9,12-16H2,1-4H3/b19-10-,20-11+/t17-,18?,21?,22?,23+,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
| Assay Description
In vitro study, VDR binding assay were performed as previously described. |
Bioorg Chem 47: 9-16 (2013)
Article DOI: 10.1016/j.bioorg.2013.01.001
BindingDB Entry DOI: 10.7270/Q2FX782T |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263411
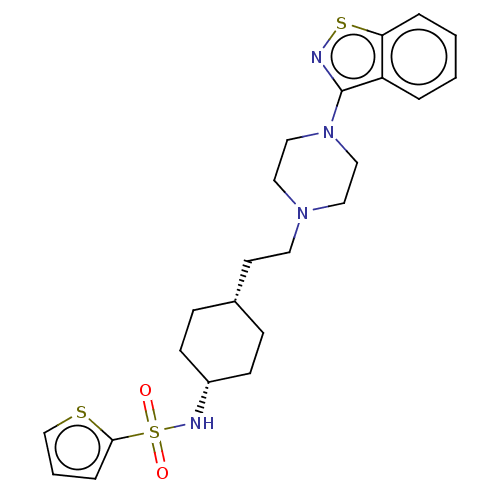
(US9550741, II-16)Show SMILES O=S(=O)(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:4.3,7.7,(-7.08,.38,;-7.08,1.92,;-7.08,3.46,;-5.75,2.69,;-4.41,1.92,;-4.41,.38,;-3.08,-.39,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-1.75,1.92,;-3.08,2.7,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| Show InChI InChI=1S/C23H30N4O2S3/c28-32(29,22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-26-13-15-27(16-14-26)23-20-4-1-2-5-21(20)31-24-23/h1-6,17-19,25H,7-16H2/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM263411

(US9550741, II-16)Show SMILES O=S(=O)(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cccs1 |r,wU:4.3,7.7,(-7.08,.38,;-7.08,1.92,;-7.08,3.46,;-5.75,2.69,;-4.41,1.92,;-4.41,.38,;-3.08,-.39,;-1.75,.38,;-.41,-.39,;.92,.38,;2.26,-.39,;2.26,-1.93,;3.59,-2.7,;4.92,-1.93,;4.92,-.39,;3.59,.38,;6.26,-2.7,;6.26,-4.24,;7.72,-4.71,;8.63,-3.47,;10.16,-3.3,;10.78,-1.9,;9.88,-.65,;8.35,-.81,;7.72,-2.22,;-1.75,1.92,;-3.08,2.7,;-8.41,2.69,;-8.41,4.23,;-9.88,4.71,;-10.78,3.46,;-9.88,2.22,)| Show InChI InChI=1S/C23H30N4O2S3/c28-32(29,22-6-3-17-30-22)25-19-9-7-18(8-10-19)11-12-26-13-15-27(16-14-26)23-20-4-1-2-5-21(20)31-24-23/h1-6,17-19,25H,7-16H2/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207161
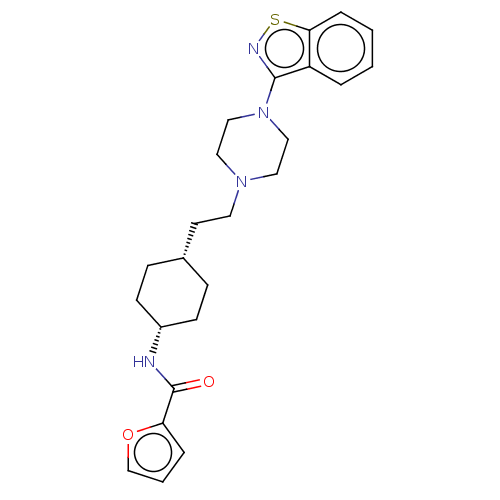
(CHEMBL3950977 | US9550741, I-19)Show SMILES O=C(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1 |r,wU:3.2,6.6,(25.23,-30.94,;25.94,-29.6,;25.17,-28.27,;23.64,-28.27,;22.87,-26.94,;21.34,-26.94,;20.57,-28.27,;19.03,-28.27,;18.26,-26.94,;16.72,-26.94,;15.95,-28.27,;14.41,-28.27,;13.64,-26.94,;14.41,-25.61,;15.95,-25.61,;12.17,-26.94,;11.25,-28.17,;9.81,-27.71,;9.81,-26.17,;8.64,-25.15,;8.99,-23.61,;10.43,-23.15,;11.55,-24.17,;11.25,-25.66,;21.34,-29.6,;22.87,-29.6,;27.48,-29.5,;28.3,-28.22,;29.79,-28.63,;29.89,-30.17,;28.45,-30.68,)| Show InChI InChI=1S/C24H30N4O2S/c29-24(21-5-3-17-30-21)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-6-22(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50207161

(CHEMBL3950977 | US9550741, I-19)Show SMILES O=C(N[C@@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1 |r,wU:3.2,6.6,(25.23,-30.94,;25.94,-29.6,;25.17,-28.27,;23.64,-28.27,;22.87,-26.94,;21.34,-26.94,;20.57,-28.27,;19.03,-28.27,;18.26,-26.94,;16.72,-26.94,;15.95,-28.27,;14.41,-28.27,;13.64,-26.94,;14.41,-25.61,;15.95,-25.61,;12.17,-26.94,;11.25,-28.17,;9.81,-27.71,;9.81,-26.17,;8.64,-25.15,;8.99,-23.61,;10.43,-23.15,;11.55,-24.17,;11.25,-25.66,;21.34,-29.6,;22.87,-29.6,;27.48,-29.5,;28.3,-28.22,;29.79,-28.63,;29.89,-30.17,;28.45,-30.68,)| Show InChI InChI=1S/C24H30N4O2S/c29-24(21-5-3-17-30-21)25-19-9-7-18(8-10-19)11-12-27-13-15-28(16-14-27)23-20-4-1-2-6-22(20)31-26-23/h1-6,17-19H,7-16H2,(H,25,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry
US Patent
| Assay Description
The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... |
US Patent US9550741 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4726 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































