 Found 96 hits of ki data for polymerid = 5112
Found 96 hits of ki data for polymerid = 5112 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039257
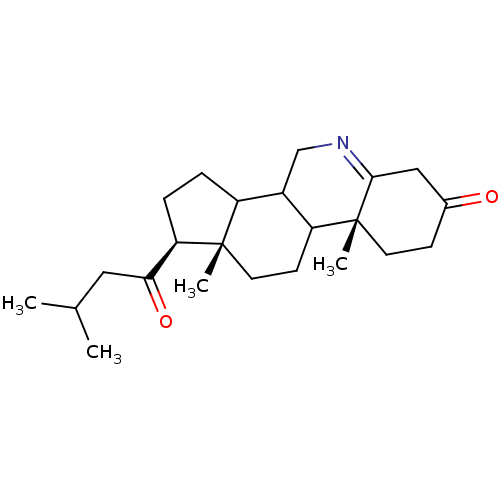
((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604
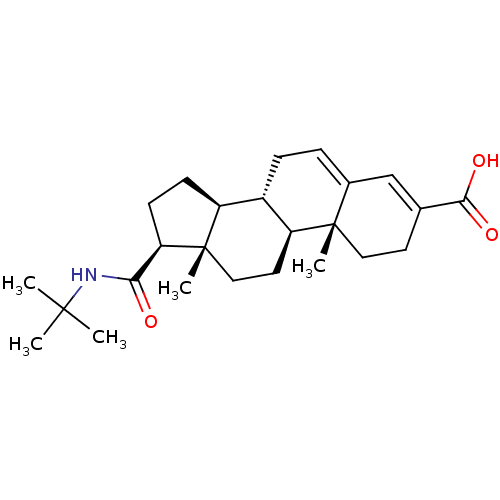
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403606
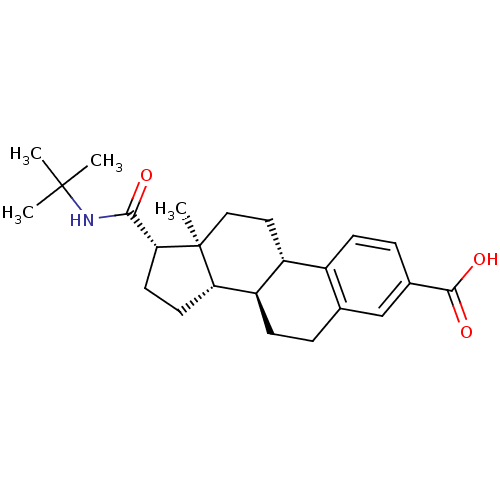
(CHEMBL1627951)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C24H33NO3/c1-23(2,3)25-21(26)20-10-9-19-18-8-5-14-13-15(22(27)28)6-7-16(14)17(18)11-12-24(19,20)4/h6-7,13,17-20H,5,8-12H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403324
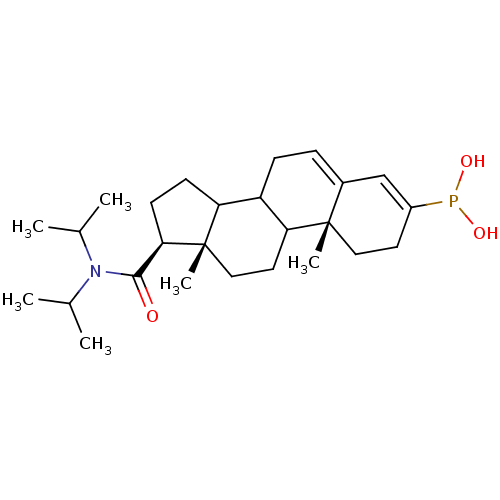
(CHEMBL78060)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)P(O)O |c:17,t:15| Show InChI InChI=1S/C26H42NO3P/c1-16(2)27(17(3)4)24(28)23-10-9-21-20-8-7-18-15-19(31(29)30)11-13-25(18,5)22(20)12-14-26(21,23)6/h7,15-17,20-23,29-30H,8-14H2,1-6H3/t20?,21?,22?,23-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039285
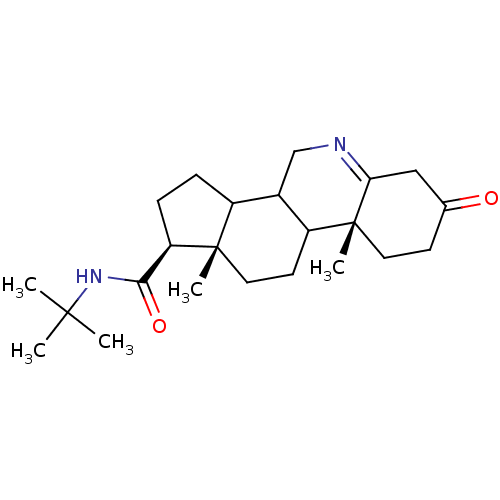
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)18-7-6-16-15-13-24-19-12-14(26)8-10-23(19,5)17(15)9-11-22(16,18)4/h15-18H,6-13H2,1-5H3,(H,25,27)/t15?,16?,17?,18-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403610
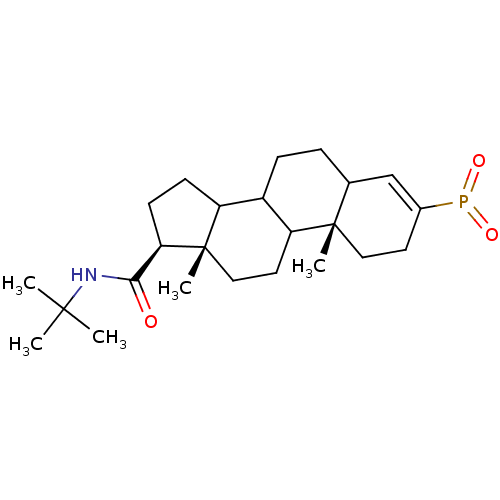
(CHEMBL143220)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CCC4C=C(CC[C@]4(C)C3CC[C@]12C)P(=O)=O |c:15| Show InChI InChI=1S/C24H38NO3P/c1-22(2,3)25-21(26)20-9-8-18-17-7-6-15-14-16(29(27)28)10-12-23(15,4)19(17)11-13-24(18,20)5/h14-15,17-20H,6-13H2,1-5H3,(H,25,26)/t15?,17?,18?,19?,20-,23+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50213061
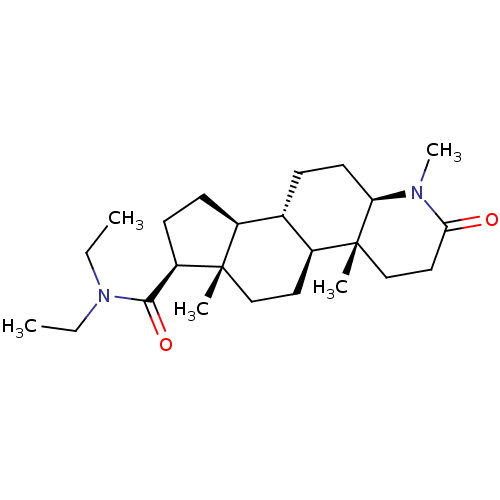
(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50180894
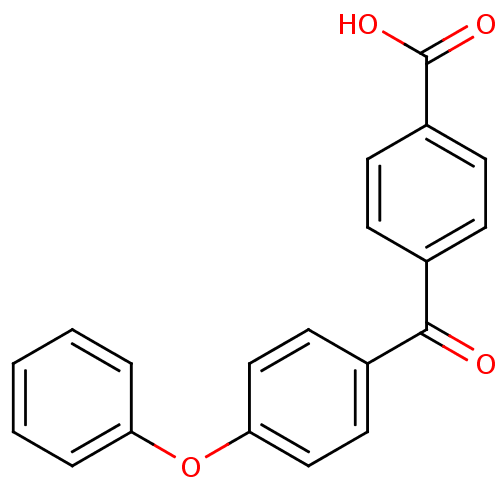
(4-(4-Phenoxy-benzoyl)-benzoic acid | 4-(4-phenoxyb...)Show InChI InChI=1S/C20H14O4/c21-19(14-6-8-16(9-7-14)20(22)23)15-10-12-18(13-11-15)24-17-4-2-1-3-5-17/h1-13H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057468

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3C=CC4=CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:14,18,t:16| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7-8,11,15-17,20-23H,9-10,12-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406356

(CHEMBL426217)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3C=C[C@]12C)C(O)=O |c:17,26,t:15| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,12,14-17,20-23H,8-11,13H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50009101
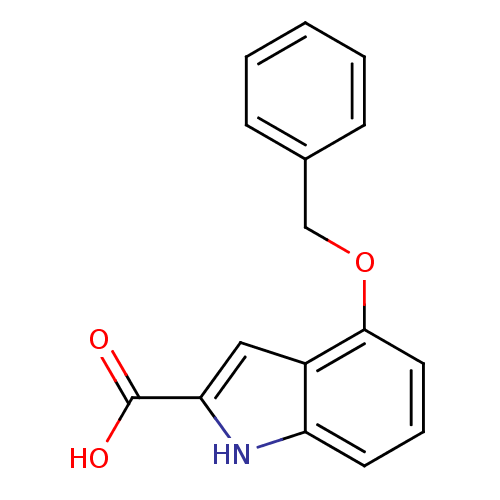
(4-Benzyloxy-1H-indole-2-carboxylic acid | CHEMBL23...)Show InChI InChI=1S/C16H13NO3/c18-16(19)14-9-12-13(17-14)7-4-8-15(12)20-10-11-5-2-1-3-6-11/h1-9,17H,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057467
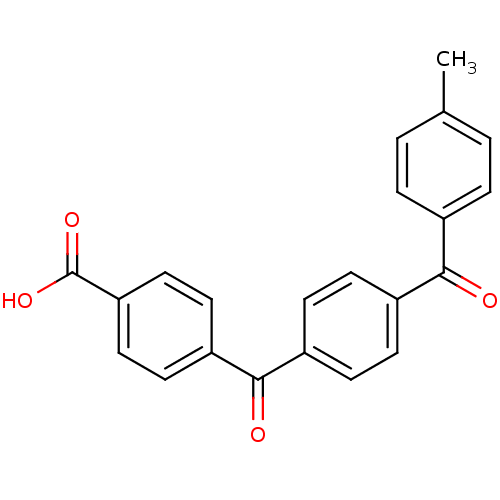
(4-[4-(4-Methyl-benzoyl)-benzoyl]-benzoic acid | CH...)Show SMILES Cc1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H16O4/c1-14-2-4-15(5-3-14)20(23)16-6-8-17(9-7-16)21(24)18-10-12-19(13-11-18)22(25)26/h2-13H,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration on human 5alpha reductase 2 isozyme |
J Med Chem 40: 1293-315 (1997)
Article DOI: 10.1021/jm960697s
BindingDB Entry DOI: 10.7270/Q2W096MN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057467

(4-[4-(4-Methyl-benzoyl)-benzoyl]-benzoic acid | CH...)Show SMILES Cc1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H16O4/c1-14-2-4-15(5-3-14)20(23)16-6-8-17(9-7-16)21(24)18-10-12-19(13-11-18)22(25)26/h2-13H,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407305
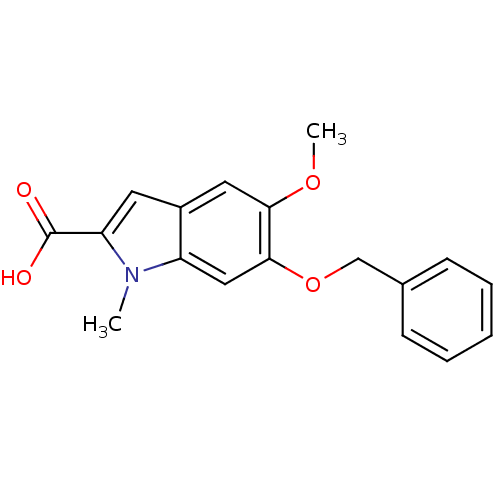
(CHEMBL36772)Show InChI InChI=1S/C18H17NO4/c1-19-14-10-17(23-11-12-6-4-3-5-7-12)16(22-2)9-13(14)8-15(19)18(20)21/h3-10H,11H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407304
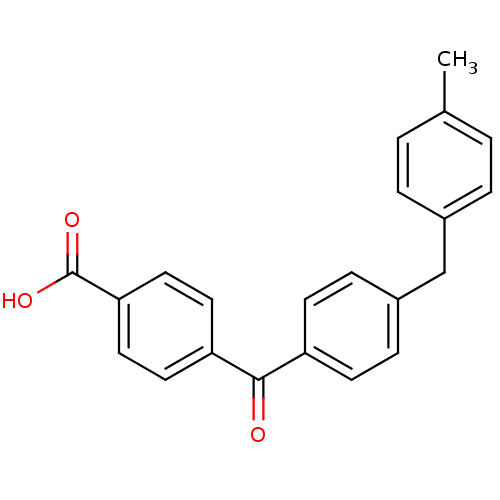
(CHEMBL289822)Show SMILES Cc1ccc(Cc2ccc(cc2)C(=O)c2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C22H18O3/c1-15-2-4-16(5-3-15)14-17-6-8-18(9-7-17)21(23)19-10-12-20(13-11-19)22(24)25/h2-13H,14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407307
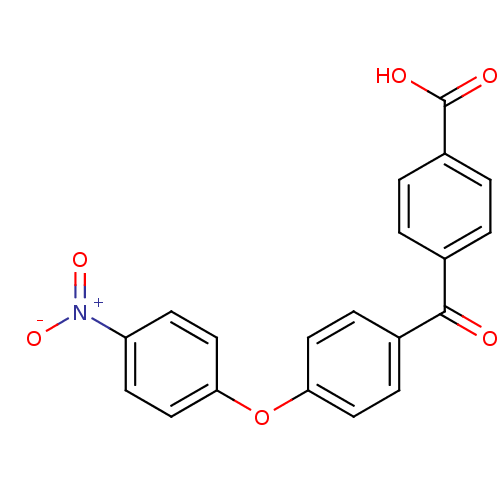
(CHEMBL285651)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(Oc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C20H13NO6/c22-19(13-1-3-15(4-2-13)20(23)24)14-5-9-17(10-6-14)27-18-11-7-16(8-12-18)21(25)26/h1-12H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50366682
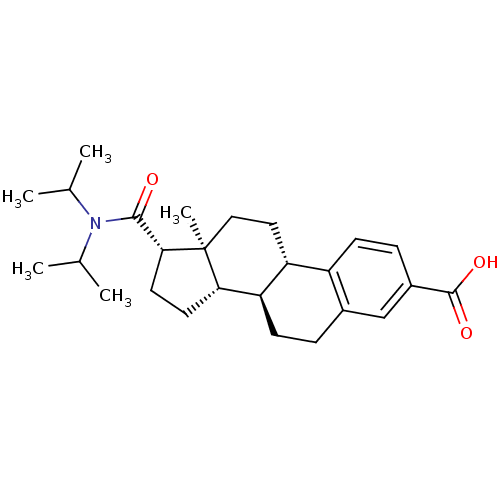
(CHEMBL1627395)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C26H37NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h7-8,14-16,20-23H,6,9-13H2,1-5H3,(H,29,30)/t20-,21-,22+,23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407316
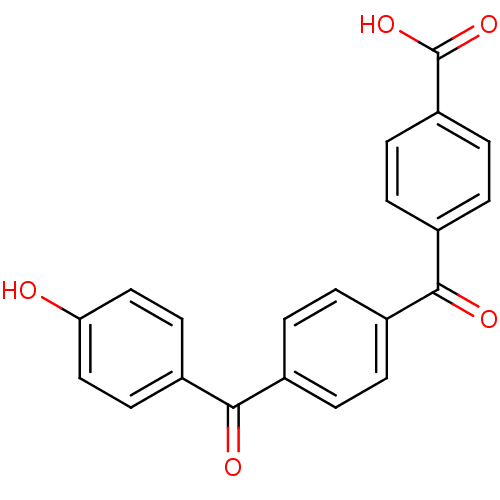
(CHEMBL286372)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(O)cc1 Show InChI InChI=1S/C21H14O5/c22-18-11-9-16(10-12-18)20(24)14-3-1-13(2-4-14)19(23)15-5-7-17(8-6-15)21(25)26/h1-12,22H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50452770

(CHEMBL2311127)Show SMILES [H][C@@]12CC[C@H](C(=O)N(C(C)C)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(F)=C(CC[C@]12C)C(O)=O |c:29| Show InChI InChI=1S/C27H42FNO3/c1-15(2)29(16(3)4)24(30)22-10-9-19-17-7-8-21-23(28)18(25(31)32)11-13-26(21,5)20(17)12-14-27(19,22)6/h15-17,19-22H,7-14H2,1-6H3,(H,31,32)/t17-,19-,20-,21-,22+,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407303
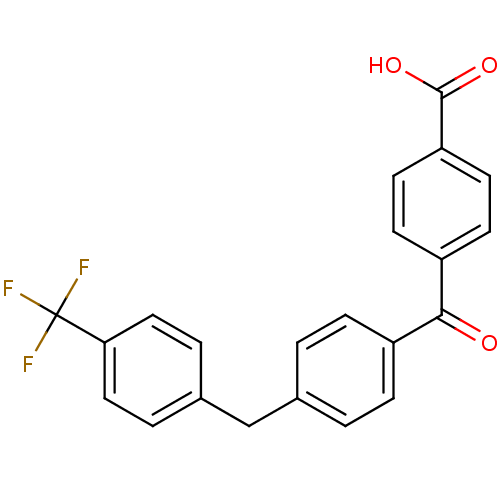
(CHEMBL36688)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(Cc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H15F3O3/c23-22(24,25)19-11-3-15(4-12-19)13-14-1-5-16(6-2-14)20(26)17-7-9-18(10-8-17)21(27)28/h1-12H,13H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391268

(CHEMBL110001)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)[N+]([O-])=O |c:17| Show InChI InChI=1S/C26H42N2O3/c1-16(2)27(17(3)4)24(29)23-10-9-21-20-8-7-18-15-19(28(30)31)11-13-25(18,5)22(20)12-14-26(21,23)6/h15-18,20-23H,7-14H2,1-6H3/t18-,20?,21?,22?,23+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391269

(CHEMBL48467)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h15-17,19-23H,7-14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against 5-alpha reductase was determined in human prostatic tissue expressed as apparent inhibition constant |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391269

(CHEMBL48467)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h15-17,19-23H,7-14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407311
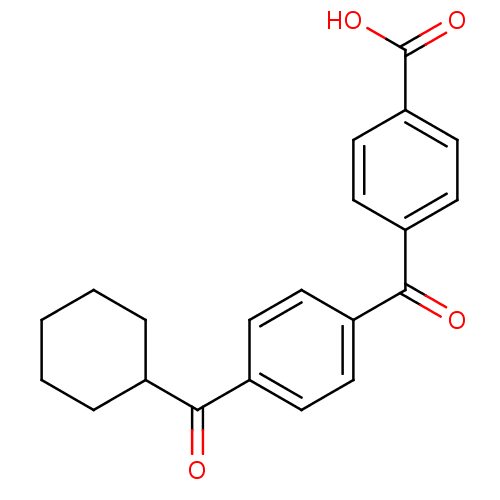
(CHEMBL34847)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)C1CCCCC1 Show InChI InChI=1S/C21H20O4/c22-19(14-4-2-1-3-5-14)15-6-8-16(9-7-15)20(23)17-10-12-18(13-11-17)21(24)25/h6-14H,1-5H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407310
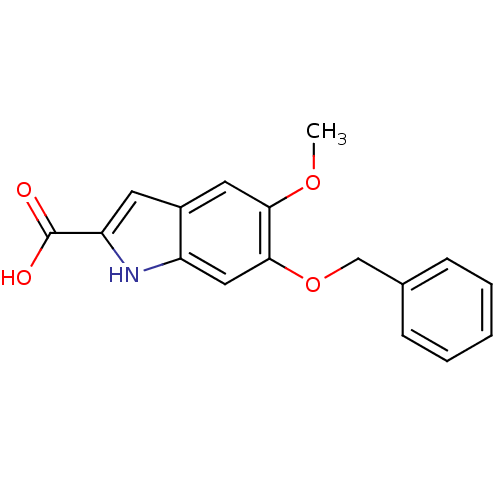
(CHEMBL37880)Show InChI InChI=1S/C17H15NO4/c1-21-15-8-12-7-14(17(19)20)18-13(12)9-16(15)22-10-11-5-3-2-4-6-11/h2-9,18H,10H2,1H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407308

(CHEMBL35176)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H13F3O4/c23-22(24,25)18-11-9-16(10-12-18)20(27)14-3-1-13(2-4-14)19(26)15-5-7-17(8-6-15)21(28)29/h1-12H,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406357

(CHEMBL172595)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC(F)=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C27H40FNO3/c1-15(2)29(16(3)4)24(30)21-8-7-19-18-14-23(28)22-13-17(25(31)32)9-11-27(22,6)20(18)10-12-26(19,21)5/h13,15-16,18-21H,7-12,14H2,1-6H3,(H,31,32)/t18?,19?,20?,21-,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407323
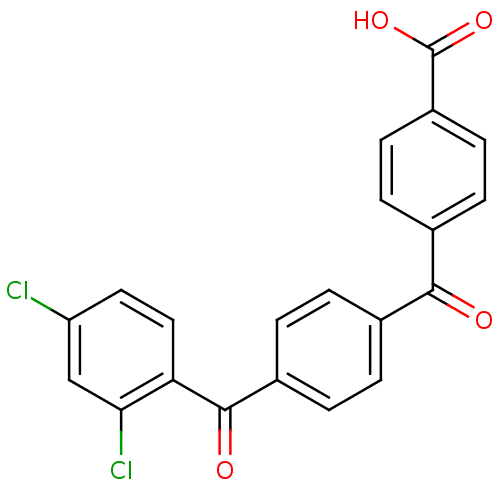
(CHEMBL37091)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C21H12Cl2O4/c22-16-9-10-17(18(23)11-16)20(25)14-3-1-12(2-4-14)19(24)13-5-7-15(8-6-13)21(26)27/h1-11H,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407320
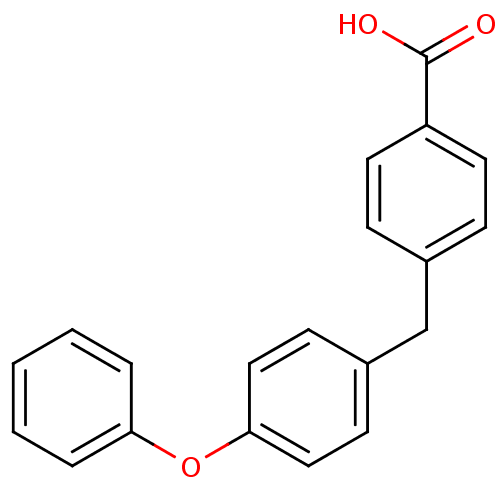
(CHEMBL35884)Show InChI InChI=1S/C20H16O3/c21-20(22)17-10-6-15(7-11-17)14-16-8-12-19(13-9-16)23-18-4-2-1-3-5-18/h1-13H,14H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407313
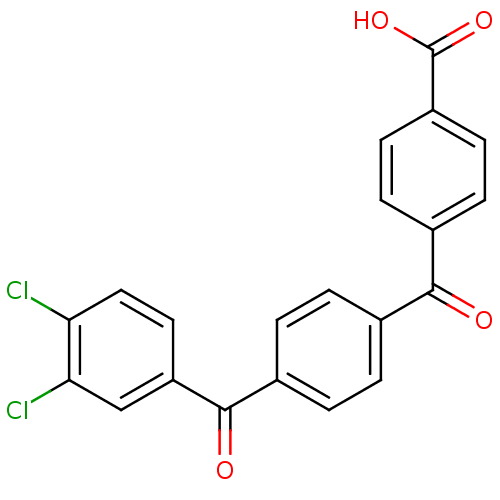
(CHEMBL34924)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H12Cl2O4/c22-17-10-9-16(11-18(17)23)20(25)14-3-1-12(2-4-14)19(24)13-5-7-15(8-6-13)21(26)27/h1-11H,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057469

((10R,13S,17S)-17-Diisopropylcarbamoyl-4,10,13-trim...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C(C)=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:15| Show InChI InChI=1S/C28H43NO3/c1-16(2)29(17(3)4)25(30)24-11-10-22-20-8-9-21-18(5)19(26(31)32)12-14-27(21,6)23(20)13-15-28(22,24)7/h9,16-17,20,22-24H,8,10-15H2,1-7H3,(H,31,32)/t20?,22?,23?,24-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407324
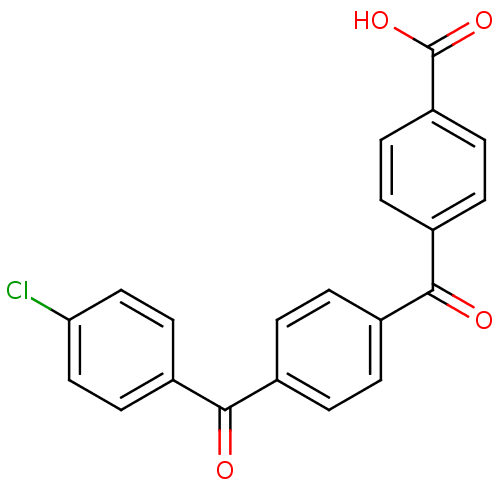
(CHEMBL287996)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H13ClO4/c22-18-11-9-16(10-12-18)20(24)14-3-1-13(2-4-14)19(23)15-5-7-17(8-6-15)21(25)26/h1-12H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407317
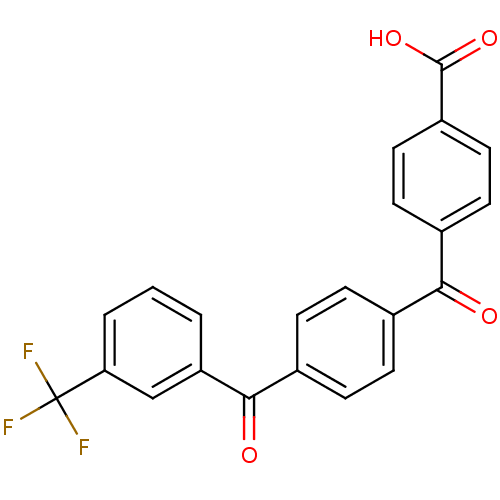
(CHEMBL36022)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H13F3O4/c23-22(24,25)18-3-1-2-17(12-18)20(27)15-6-4-13(5-7-15)19(26)14-8-10-16(11-9-14)21(28)29/h1-12H,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407315
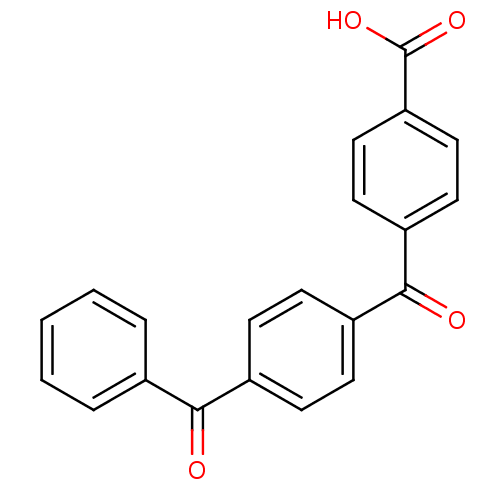
(CHEMBL37660)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C21H14O4/c22-19(14-4-2-1-3-5-14)15-6-8-16(9-7-15)20(23)17-10-12-18(13-11-17)21(24)25/h1-13H,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057498
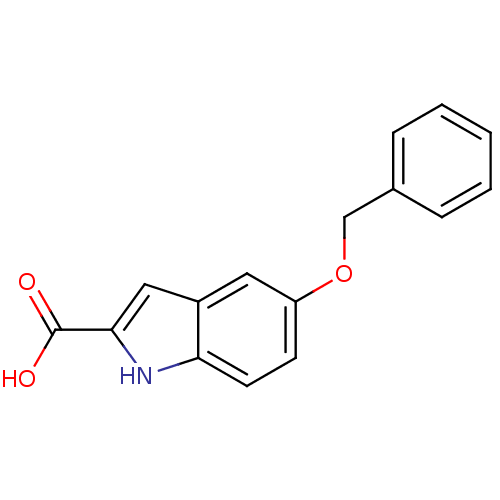
(5-Benzyloxy-1H-indole-2-carboxylic acid | CHEMBL24...)Show InChI InChI=1S/C16H13NO3/c18-16(19)15-9-12-8-13(6-7-14(12)17-15)20-10-11-4-2-1-3-5-11/h1-9,17H,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057498

(5-Benzyloxy-1H-indole-2-carboxylic acid | CHEMBL24...)Show InChI InChI=1S/C16H13NO3/c18-16(19)15-9-12-8-13(6-7-14(12)17-15)20-10-11-4-2-1-3-5-11/h1-9,17H,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration on human 5alpha reductase 2 isozyme |
J Med Chem 40: 1293-315 (1997)
Article DOI: 10.1021/jm960697s
BindingDB Entry DOI: 10.7270/Q2W096MN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407321
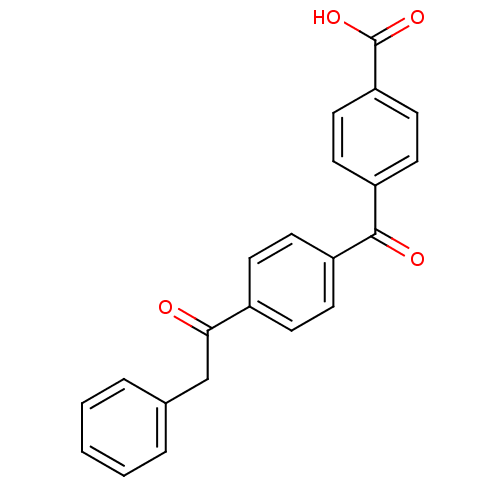
(CHEMBL264757)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H16O4/c23-20(14-15-4-2-1-3-5-15)16-6-8-17(9-7-16)21(24)18-10-12-19(13-11-18)22(25)26/h1-13H,14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391268

(CHEMBL110001)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)[N+]([O-])=O |c:17| Show InChI InChI=1S/C26H42N2O3/c1-16(2)27(17(3)4)24(29)23-10-9-21-20-8-7-18-15-19(28(30)31)11-13-25(18,5)22(20)12-14-26(21,23)6/h15-18,20-23H,7-14H2,1-6H3/t18-,20?,21?,22?,23+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406358

(CHEMBL366660)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@@H]4C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C26H39NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h6,14-16,19-23H,7-13H2,1-5H3,(H,29,30)/t19-,20?,21?,22?,23+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406353

(CHEMBL172416)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CCC4=CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,15-17,20-23H,7-10,12-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407322
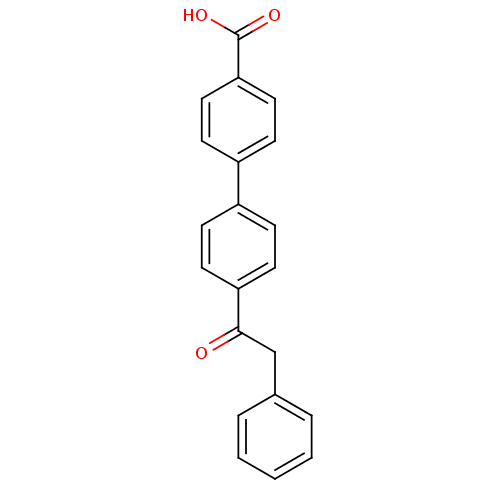
(CHEMBL37254)Show InChI InChI=1S/C21H16O3/c22-20(14-15-4-2-1-3-5-15)18-10-6-16(7-11-18)17-8-12-19(13-9-17)21(23)24/h1-13H,14H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407312
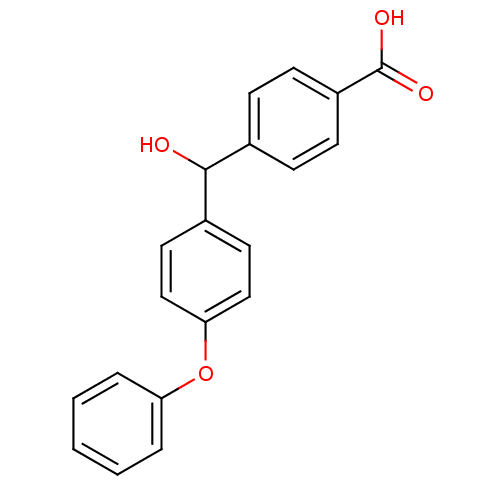
(CHEMBL286363)Show InChI InChI=1S/C20H16O4/c21-19(14-6-8-16(9-7-14)20(22)23)15-10-12-18(13-11-15)24-17-4-2-1-3-5-17/h1-13,19,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406351
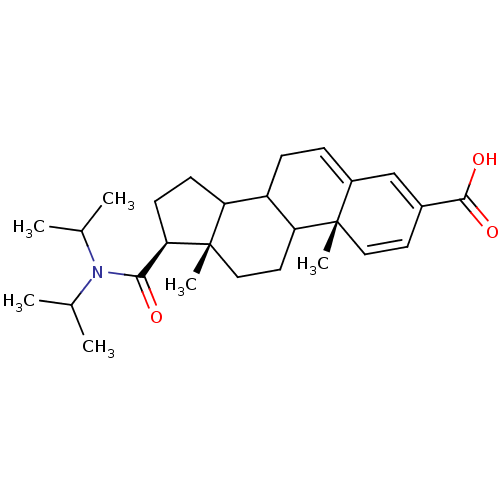
(CHEMBL427113)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(C=C[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,19,t:15| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,11,13,15-17,20-23H,8-10,12,14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406359

(CHEMBL172025)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(C=C[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,19| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,13,15-17,19-23H,7-10,12,14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406361

(CHEMBL172951)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,16-17,19-23H,7-10,12-15H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data