

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243396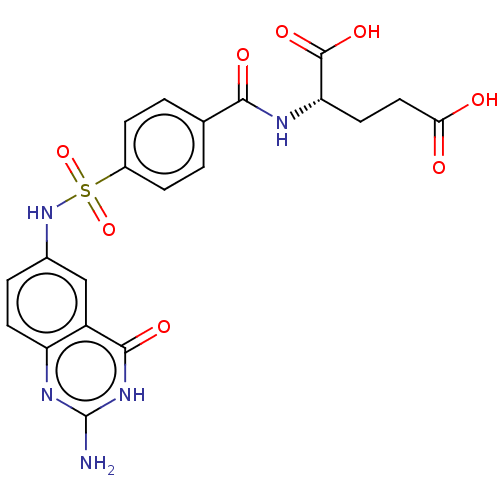 (CHEMBL1231520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human AICARFT | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50277356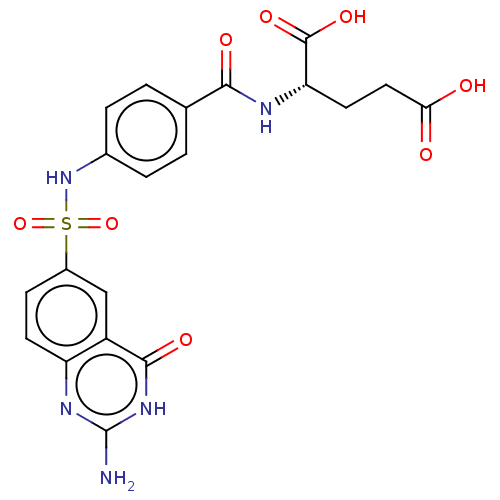 (CHEMBL4168306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AICARFTase using AICAR as substrate incubated at 37 degC for 30 mins measured after overnight incubation at 4 degC | Eur J Med Chem 139: 531-541 (2017) Article DOI: 10.1016/j.ejmech.2017.08.032 BindingDB Entry DOI: 10.7270/Q2WW7M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22585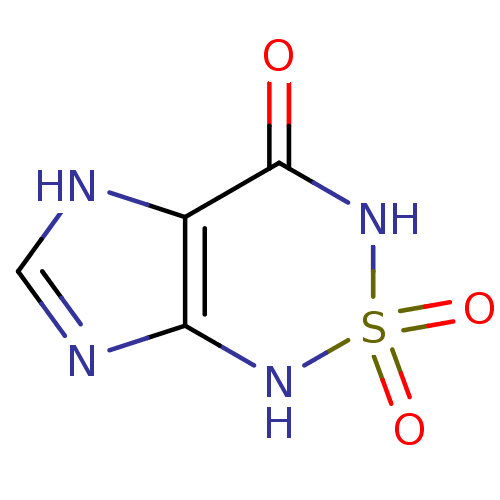 (1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 130 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay using the spectrophotometric method monitoring the appearance of IMP by measuring absorbance ... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22588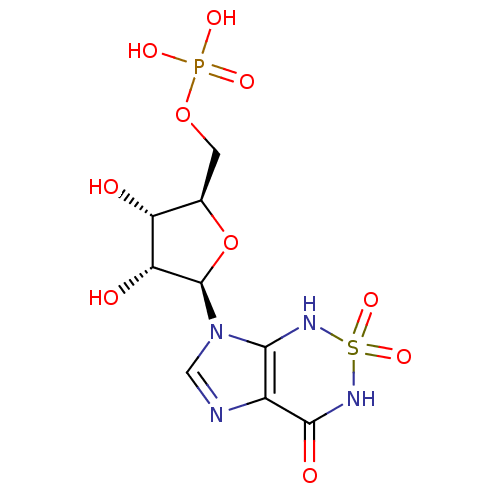 (Nucleotide, 3 | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay using the spectrophotometric method monitoring the appearance of IMP by measuring absorbance ... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158378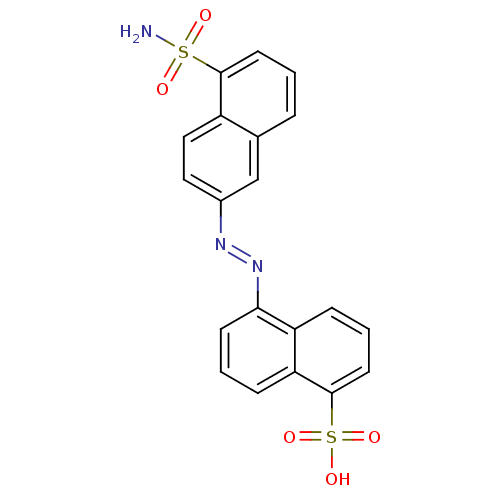 (5-(5-sulfamoyl-naphthalen-2-ylazo)-naphthalene-1-s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22587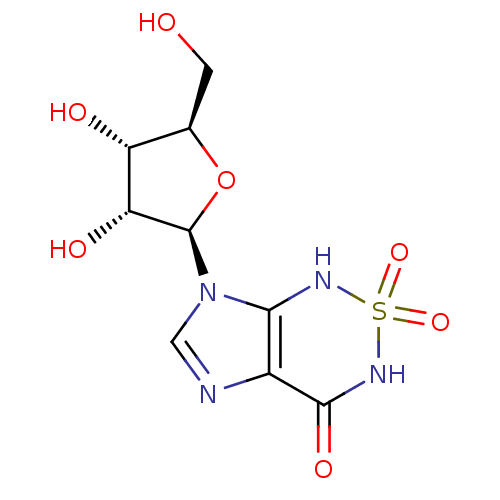 (7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 230 | -37.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay using the spectrophotometric method monitoring the appearance of IMP by measuring absorbance ... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92374 (RY Analogue, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 685 | -35.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557564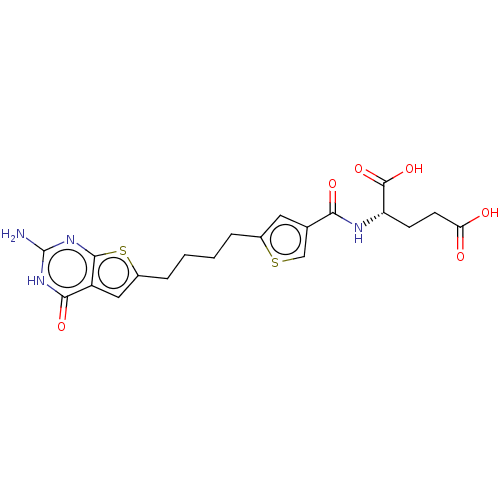 (CHEMBL4783397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92376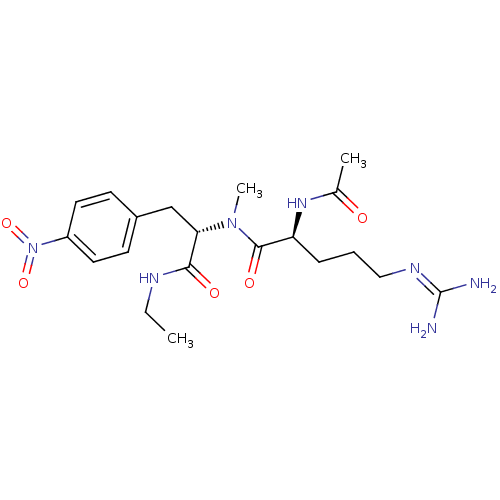 (RY Analogue, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50167703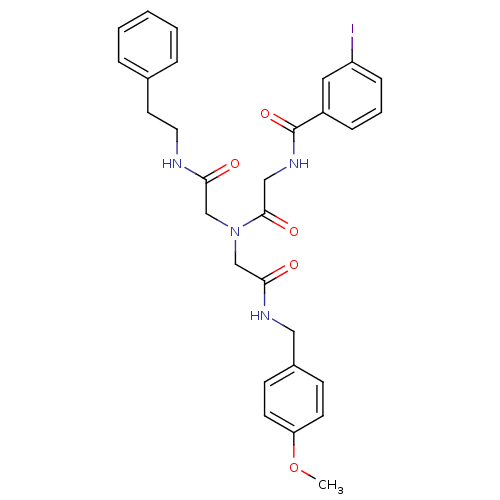 (3-Iodo-N-{[[(4-methoxy-benzylcarbamoyl)-methyl]-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant against AICAR formyltransferase | Bioorg Med Chem Lett 15: 2840-4 (2005) Article DOI: 10.1016/j.bmcl.2005.03.094 BindingDB Entry DOI: 10.7270/Q22V2FNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557566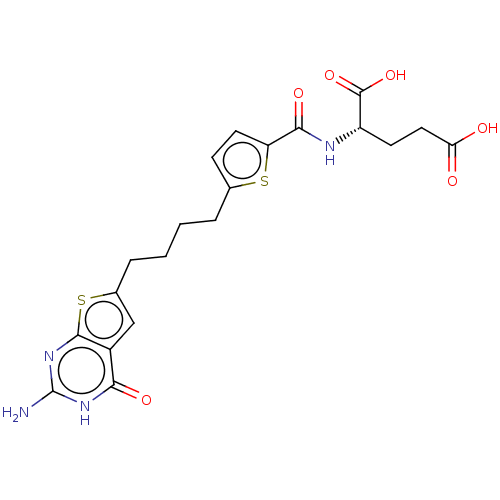 (CHEMBL4741259) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22578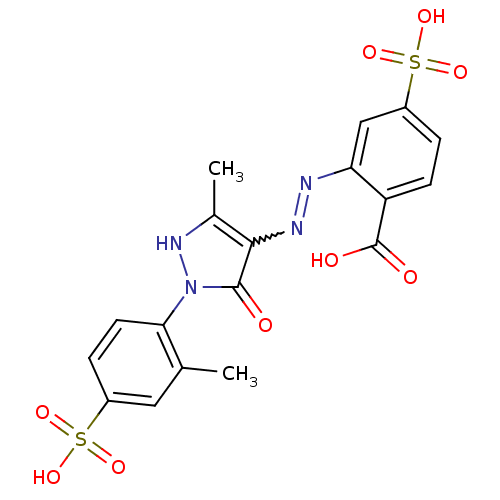 (2-[(E)-2-[5-hydroxy-3-methyl-1-(2-methyl-4-sulfoph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | Article PubMed | 7.10E+3 | -29.1 | 1.16E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... | J Biol Chem 279: 50555-65 (2004) Article DOI: 10.1074/jbc.M406801200 BindingDB Entry DOI: 10.7270/Q2MC8X97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50167702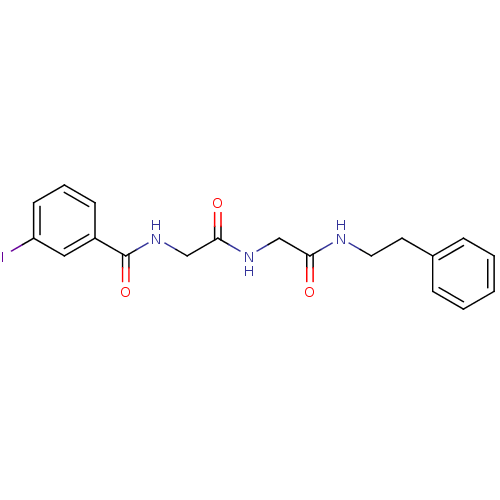 (3-Iodo-N-{[(phenethylcarbamoyl-methyl)-carbamoyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant against AICAR formyltransferase | Bioorg Med Chem Lett 15: 2840-4 (2005) Article DOI: 10.1016/j.bmcl.2005.03.094 BindingDB Entry DOI: 10.7270/Q22V2FNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557565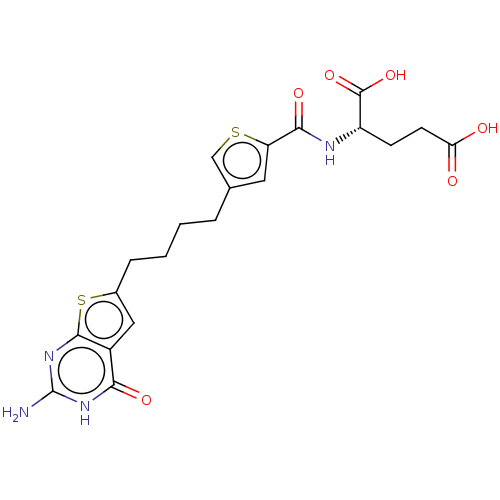 (CHEMBL4755197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557570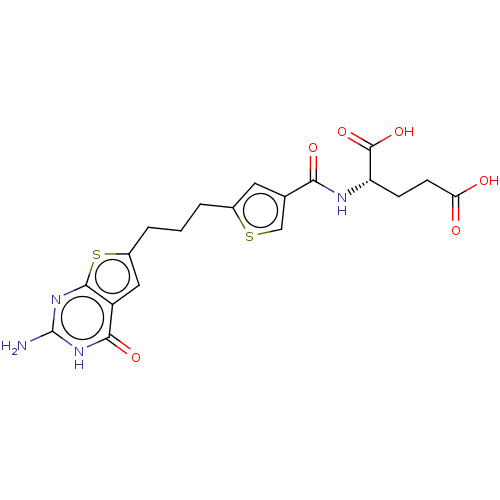 (CHEMBL4761741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50249877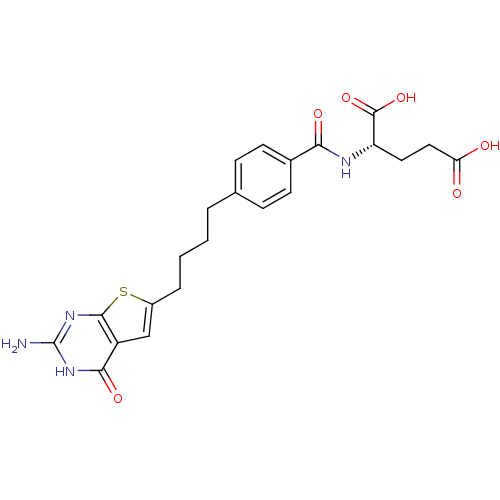 ((S)-2-(4-(4-(2-amino-4-oxo-3,4-dihydrothieno[2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557568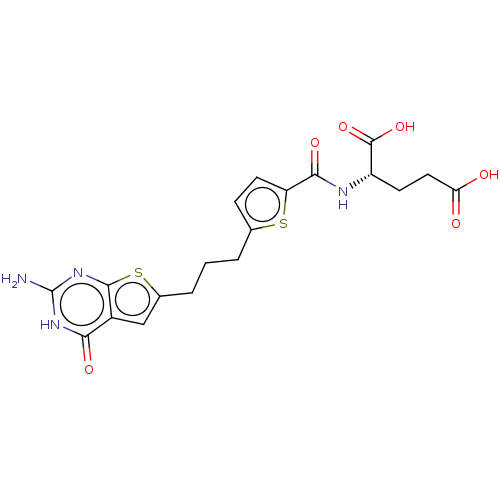 (CHEMBL4790375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50089572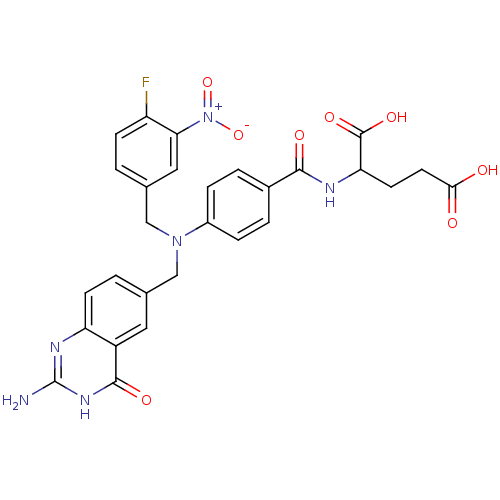 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylase | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557569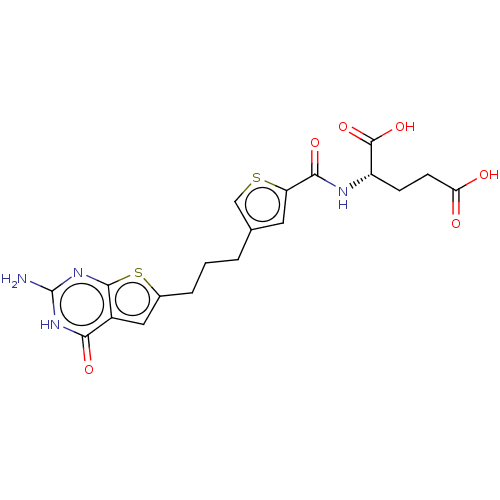 (CHEMBL4759798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24692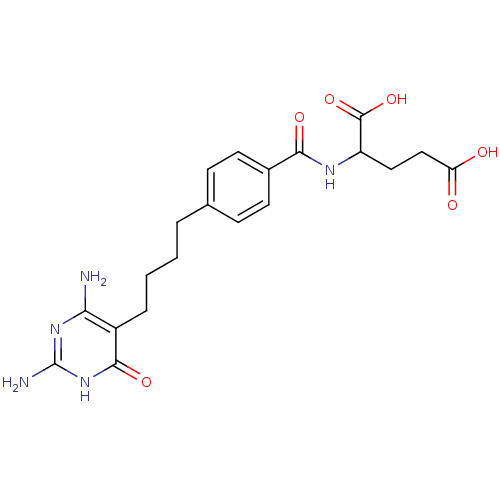 (2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -26.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24691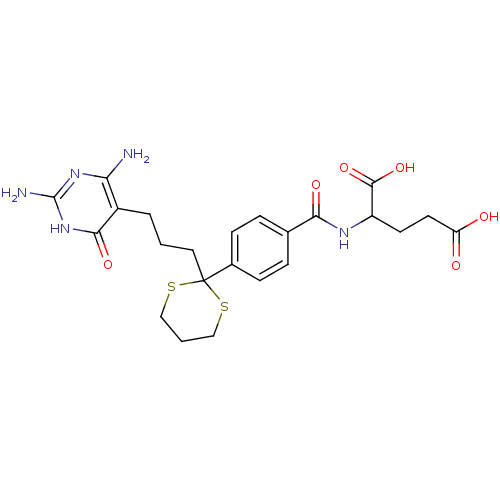 (2-[(4-{2-[3-(2,4-diamino-6-oxo-1,6-dihydropyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -26.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24692 (2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50103596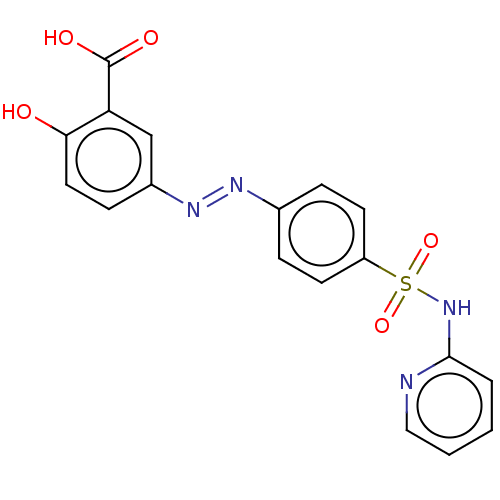 ((E)-2-hydroxy-5-((4-(N-pyridin-2-ylsulfamoyl)-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Curated by ChEMBL | Assay Description Inhibition of AICAR transformylase (unknown origin) | J Med Chem 63: 8314-8324 (2020) Article DOI: 10.1021/acs.jmedchem.0c00546 BindingDB Entry DOI: 10.7270/Q2T72N1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50557567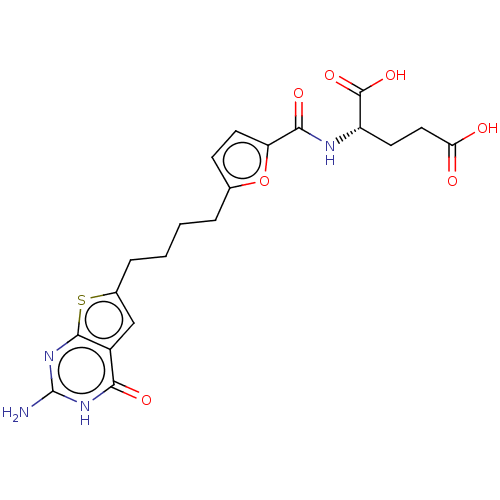 (CHEMBL4789686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length N-terminal His-tagged ATIC expressed in Chinese Hamster MTXRII-OuaR2-4 R2 cells assessed as reduction in THF formatio... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116093 BindingDB Entry DOI: 10.7270/Q2RF5ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50186739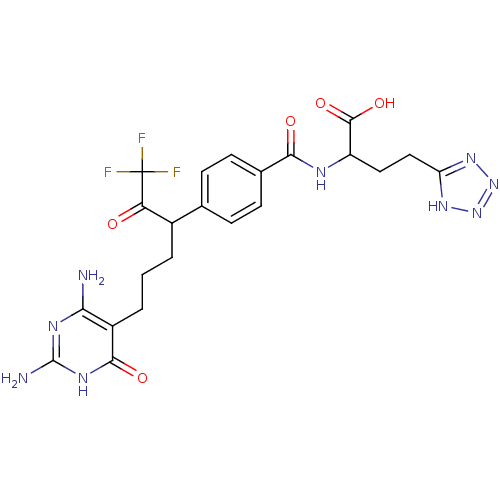 ((2S)-2-(4-(6-(2,4-diamino-6-oxo-1,6-dihydropyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant AICAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50089594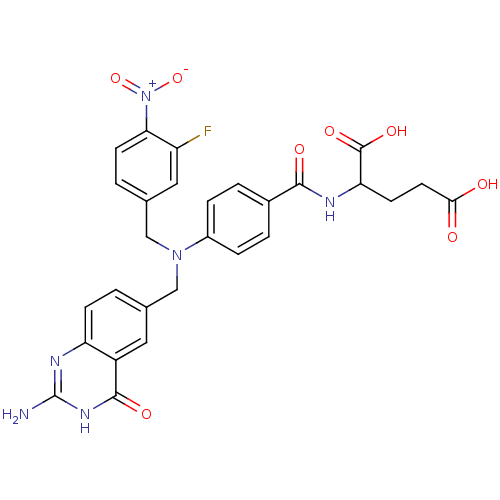 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylase | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92372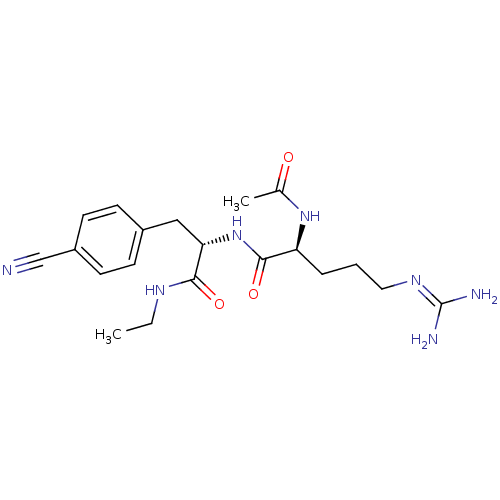 (RY Analogue, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | -24.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92373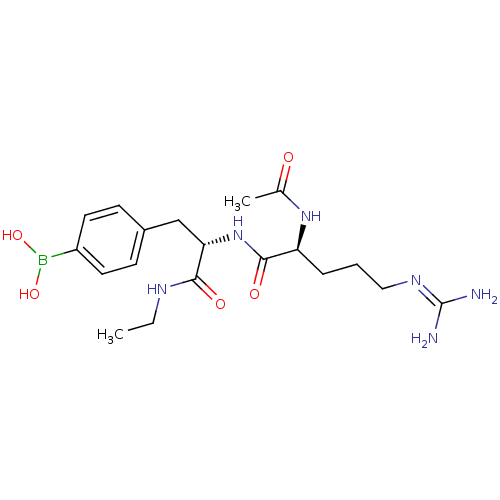 (RY Analogue, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92368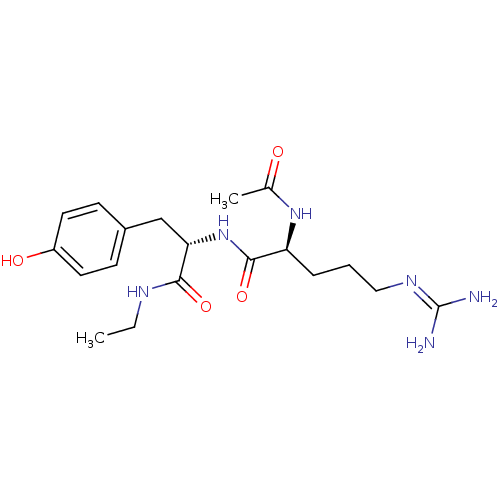 (RY Analogue, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.40E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92371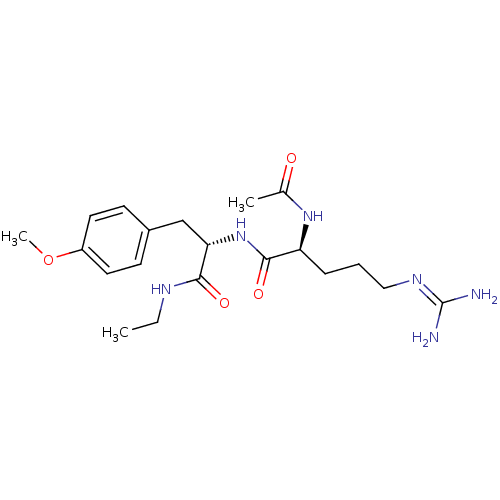 (RY Analogue, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70E+4 | -23.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50374377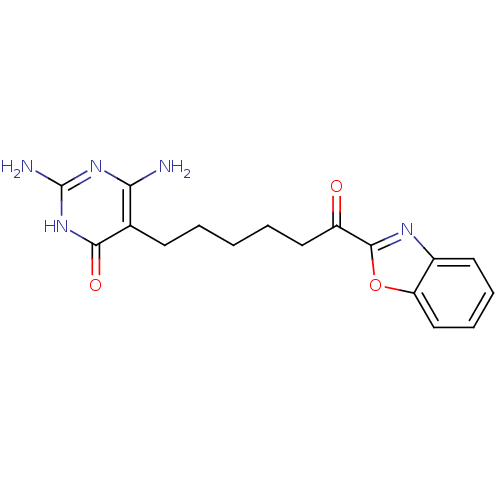 (CHEMBL273174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human aminoimidazole carboxamide transformylase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50186740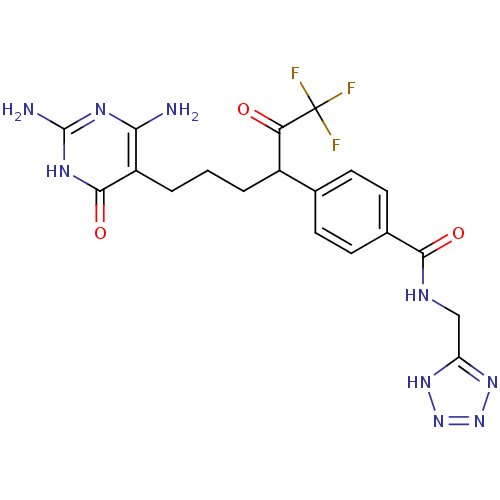 (CHEMBL381706 | N-((1H-tetrazol-5-yl)methyl)-4-(6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant AICAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50186741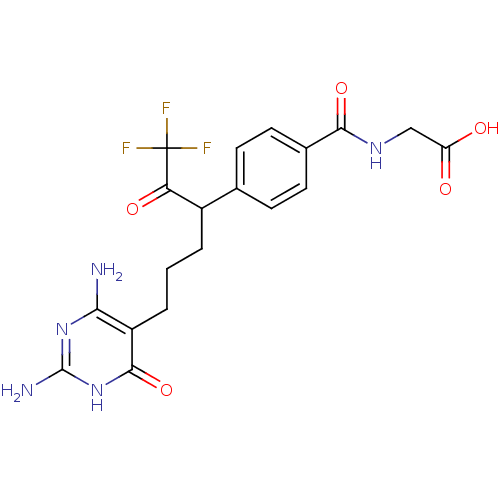 (2-(4-(6-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant AICAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24693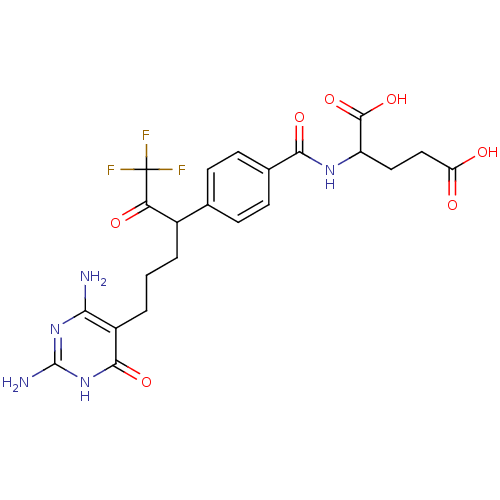 (10-CF3CO-DDACTHF (5) | 10-CF3CO-DDACTHF, 2 | 2-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant AICAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005518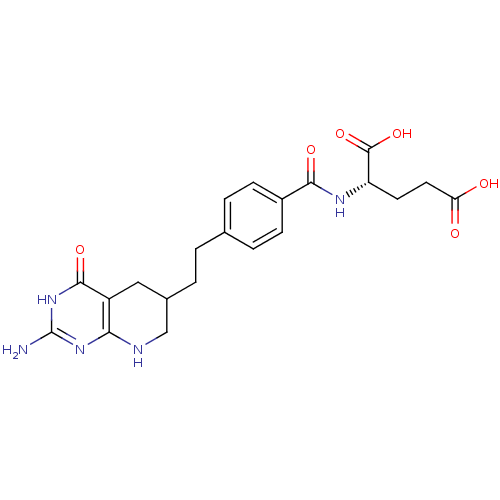 ((S)-2-(4-(2-((R)-2-amino-4-oxo-1,4,5,6,7,8-hexahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant AICAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM119131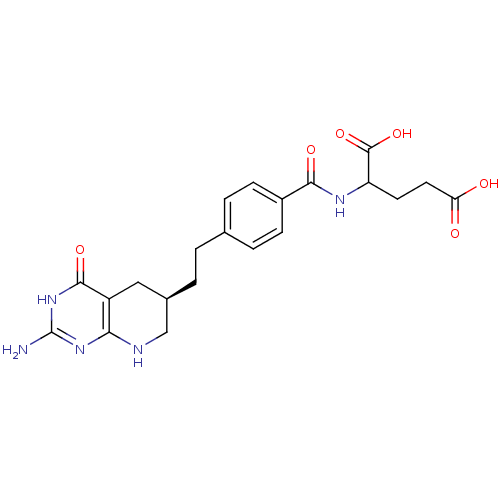 (Lometrexol (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24693 (10-CF3CO-DDACTHF (5) | 10-CF3CO-DDACTHF, 2 | 2-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24686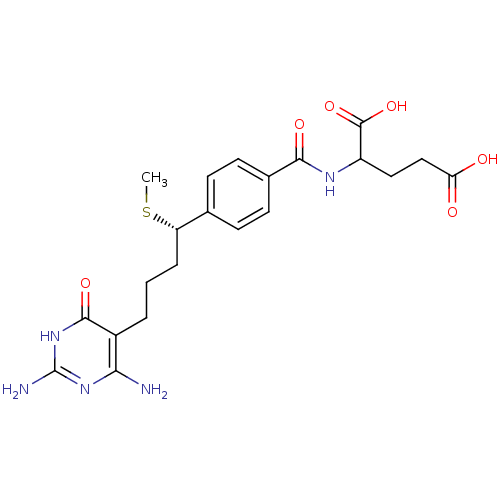 (10-thiomethyl-DDACTHF, 10S-3 | 10S (7) | 2-({4-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22590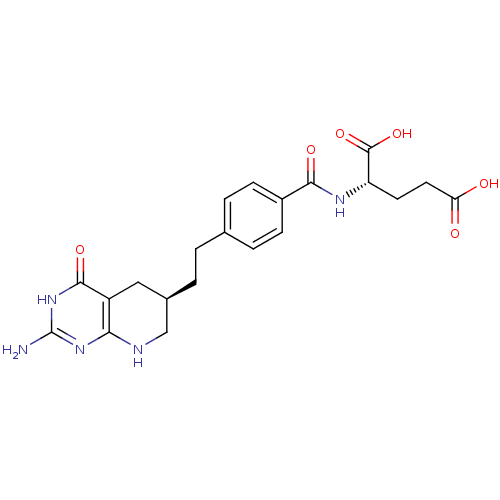 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24693 (10-CF3CO-DDACTHF (5) | 10-CF3CO-DDACTHF, 2 | 2-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24690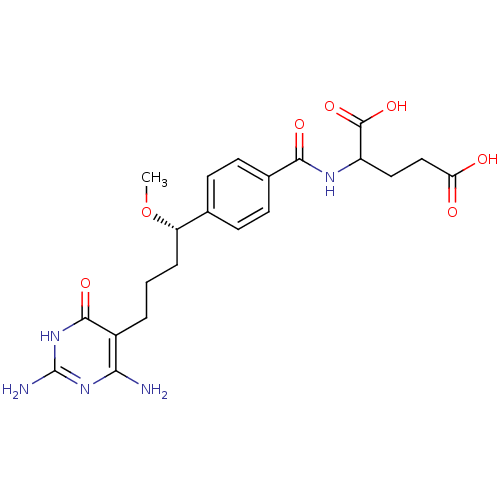 (10-methoxy-DDACTHF, 10S-8 | 2-({4-[(1S)-4-(2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24689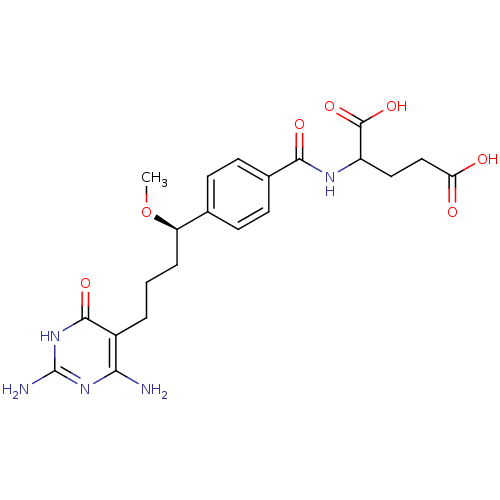 (10-methoxy-DDACTHF, 10R-8 | 2-({4-[(1R)-4-(2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24688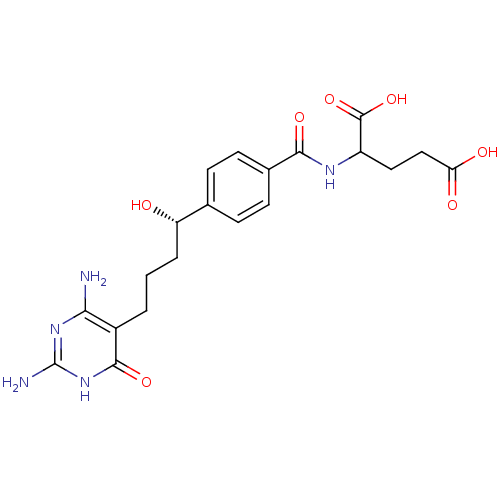 (10-hydroxy-DDACTHF, 10S-7 | 2-({4-[(1S)-4-(2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24687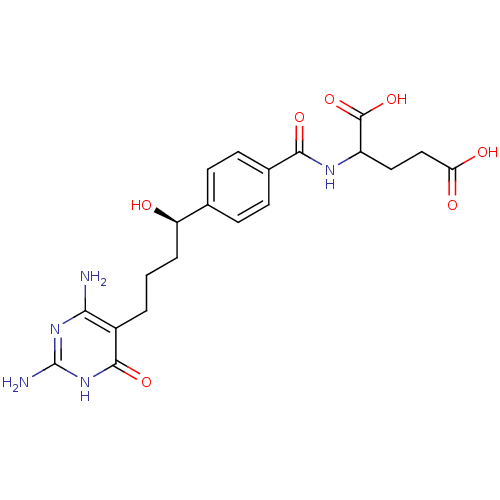 (10-hydroxy-DDACTHF, 10R-7 | 2-({4-[(1R)-4-(2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24686 (10-thiomethyl-DDACTHF, 10S-3 | 10S (7) | 2-({4-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM24685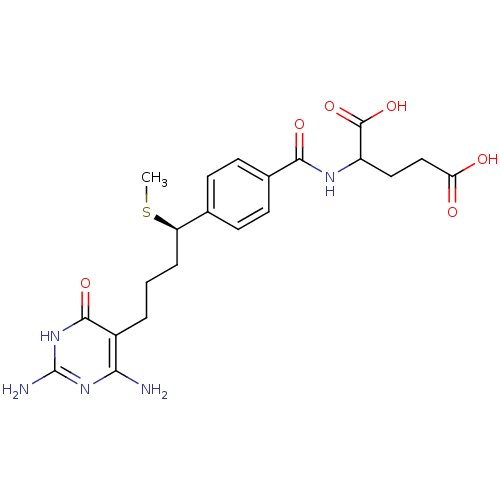 (10-thiomethyl-DDACTHF, 10R-3 | 10R (8) | 2-({4-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22588 (Nucleotide, 3 | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22587 (7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22585 (1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |