 Found 16 hits of kon data for polymerid = 104,1110,127,50001624,50001758
Found 16 hits of kon data for polymerid = 104,1110,127,50001624,50001758 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50284739

(3-[2-(2-Benzyloxycarbonylamino-3-methyl-butyrylami...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 |r| Show InChI InChI=1S/C27H33N3O8/c1-17(2)24(30-27(36)38-15-19-10-6-4-7-11-19)26(35)28-18(3)25(34)29-21(14-23(32)33)22(31)16-37-20-12-8-5-9-13-20/h4-13,17-18,21,24H,14-16H2,1-3H3,(H,28,35)(H,29,34)(H,30,36)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50213208
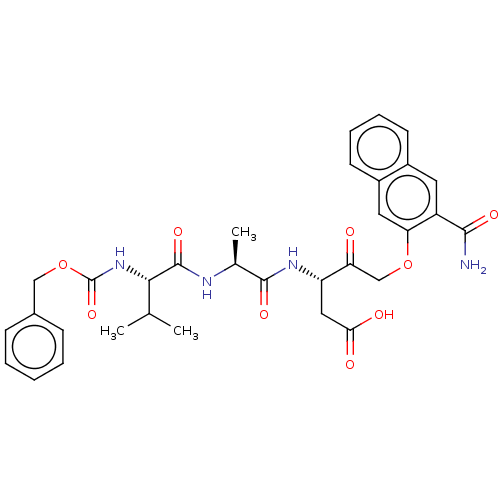
(CHEMBL3143890)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1cc2ccccc2cc1C(N)=O |r| Show InChI InChI=1S/C32H36N4O9/c1-18(2)28(36-32(43)45-16-20-9-5-4-6-10-20)31(42)34-19(3)30(41)35-24(15-27(38)39)25(37)17-44-26-14-22-12-8-7-11-21(22)13-23(26)29(33)40/h4-14,18-19,24,28H,15-17H2,1-3H3,(H2,33,40)(H,34,42)(H,35,41)(H,36,43)(H,38,39)/t19-,24-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 4.30 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50450287
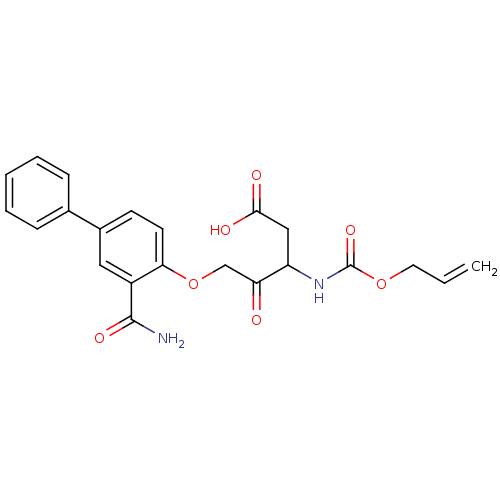
(CHEMBL31061)Show SMILES NC(=O)c1cc(ccc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C)-c1ccccc1 Show InChI InChI=1S/C22H22N2O7/c1-2-10-30-22(29)24-17(12-20(26)27)18(25)13-31-19-9-8-15(11-16(19)21(23)28)14-6-4-3-5-7-14/h2-9,11,17H,1,10,12-13H2,(H2,23,28)(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284734
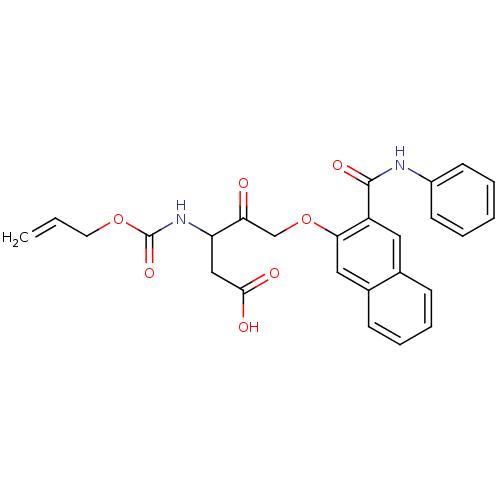
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenylcarbamoyl...)Show SMILES OC(=O)CC(NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1C(=O)Nc1ccccc1 Show InChI InChI=1S/C26H24N2O7/c1-2-12-34-26(33)28-21(15-24(30)31)22(29)16-35-23-14-18-9-7-6-8-17(18)13-20(23)25(32)27-19-10-4-3-5-11-19/h2-11,13-14,21H,1,12,15-16H2,(H,27,32)(H,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284733
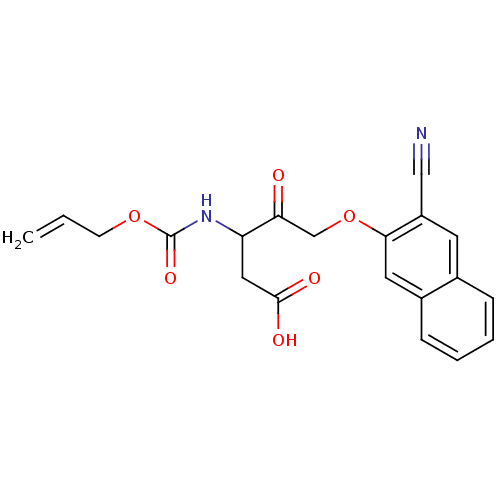
(3-Allyloxycarbonylamino-5-(3-cyano-naphthalen-2-yl...)Show SMILES OC(=O)CC(NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1C#N Show InChI InChI=1S/C20H18N2O6/c1-2-7-27-20(26)22-16(10-19(24)25)17(23)12-28-18-9-14-6-4-3-5-13(14)8-15(18)11-21/h2-6,8-9,16H,1,7,10,12H2,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284735

(3-Allyloxycarbonylamino-5-(3-carbamoyl-naphthalen-...)Show SMILES NC(=O)c1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C20H20N2O7/c1-2-7-28-20(27)22-15(10-18(24)25)16(23)11-29-17-9-13-6-4-3-5-12(13)8-14(17)19(21)26/h2-6,8-9,15H,1,7,10-11H2,(H2,21,26)(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 5.60E+3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284744

(3-Allyloxycarbonylamino-5-[3-(1H-imidazol-2-yl)-na...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1-c1ncc[nH]1 Show InChI InChI=1S/C22H21N3O6/c1-2-9-30-22(29)25-17(12-20(27)28)18(26)13-31-19-11-15-6-4-3-5-14(15)10-16(19)21-23-7-8-24-21/h2-8,10-11,17H,1,9,12-13H2,(H,23,24)(H,25,29)(H,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50422308

(CHEMBL71295)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COP(=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C33H38N3O9P/c1-22(2)30(36-33(42)44-20-24-13-7-4-8-14-24)32(41)34-23(3)31(40)35-27(19-29(38)39)28(37)21-45-46(43,25-15-9-5-10-16-25)26-17-11-6-12-18-26/h4-18,22-23,27,30H,19-21H2,1-3H3,(H,34,41)(H,35,40)(H,36,42)(H,38,39)/t23-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.17E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Evaluated for kinetic dissociation constant (kon) for the inhibition of caspase-1. |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091570
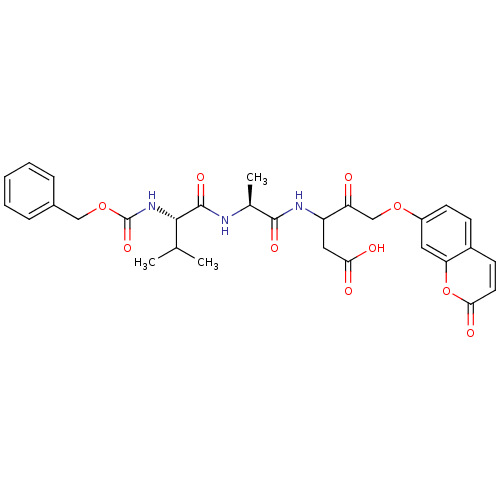
(3-[2-(2-Benzyloxycarbonylamino-3-methyl-butyrylami...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COc1ccc2ccc(=O)oc2c1 Show InChI InChI=1S/C30H33N3O10/c1-17(2)27(33-30(40)42-15-19-7-5-4-6-8-19)29(39)31-18(3)28(38)32-22(14-25(35)36)23(34)16-41-21-11-9-20-10-12-26(37)43-24(20)13-21/h4-13,17-18,22,27H,14-16H2,1-3H3,(H,31,39)(H,32,38)(H,33,40)(H,35,36)/t18-,22?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.60E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Evaluated for kinetic dissociation constant (kon) for the inhibition of caspase-1. |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50291983

(5-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxy)-3-[(S...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COC1=C(Cc2ccccc2)C(=O)OC1 |c:33| Show InChI InChI=1S/C32H37N3O10/c1-19(2)28(35-32(42)45-16-22-12-8-5-9-13-22)30(40)33-20(3)29(39)34-24(15-27(37)38)25(36)17-43-26-18-44-31(41)23(26)14-21-10-6-4-7-11-21/h4-13,19-20,24,28H,14-18H2,1-3H3,(H,33,40)(H,34,39)(H,35,42)(H,37,38)/t20-,24?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 2.52E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Evaluated for kinetic dissociation constant (kon) for the inhibition of caspase-1. |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50291985
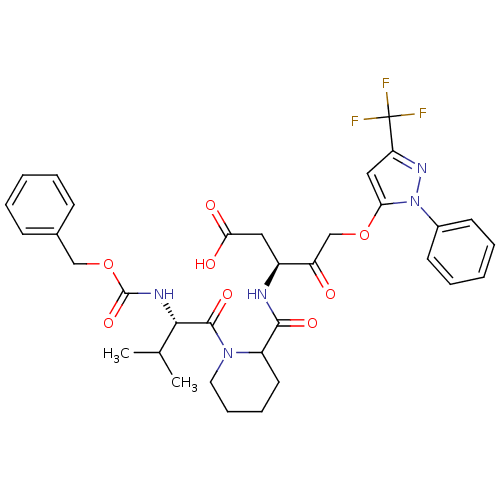
(3-{[1-(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCCCC1C(=O)N[C@@H](CC(O)=O)C(=O)COc1cc(nn1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C34H38F3N5O8/c1-21(2)30(39-33(48)50-19-22-11-5-3-6-12-22)32(47)41-16-10-9-15-25(41)31(46)38-24(17-29(44)45)26(43)20-49-28-18-27(34(35,36)37)40-42(28)23-13-7-4-8-14-23/h3-8,11-14,18,21,24-25,30H,9-10,15-17,19-20H2,1-2H3,(H,38,46)(H,39,48)(H,44,45)/t24-,25?,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 2.71E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against caspase-1 |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50291984

(3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-bu...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COc1cc(nn1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C31H34F3N5O8/c1-18(2)27(37-30(45)47-16-20-10-6-4-7-11-20)29(44)35-19(3)28(43)36-22(14-26(41)42)23(40)17-46-25-15-24(31(32,33)34)38-39(25)21-12-8-5-9-13-21/h4-13,15,18-19,22,27H,14,16-17H2,1-3H3,(H,35,44)(H,36,43)(H,37,45)(H,41,42)/t19-,22?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 2.80E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against caspase-1 |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091566

(3-(2-{2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propio...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C=O Show InChI InChI=1S/C23H32N4O8/c1-12(2)20(23(35)24-13(3)21(33)26-16(11-28)10-19(31)32)27-22(34)18(25-14(4)29)9-15-5-7-17(30)8-6-15/h5-8,11-13,16,18,20,30H,9-10H2,1-4H3,(H,24,35)(H,25,29)(H,26,33)(H,27,34)(H,31,32)/t13-,16?,18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 3.80E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Evaluated for kinetic dissociation constant (kon) for the inhibition of caspase-1. |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50422307

(CHEMBL273689)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C28H31Cl2N3O9/c1-15(2)24(33-28(40)42-13-17-8-5-4-6-9-17)26(38)31-16(3)25(37)32-20(12-22(35)36)21(34)14-41-27(39)23-18(29)10-7-11-19(23)30/h4-11,15-16,20,24H,12-14H2,1-3H3,(H,31,38)(H,32,37)(H,33,40)(H,35,36)/t16-,20-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 4.07E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against caspase-1 |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091580

(3-[2-(2-Benzyloxycarbonylamino-3-methyl-butyrylami...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COc1cc2ccccc2cc1C(N)=O Show InChI InChI=1S/C32H36N4O9/c1-18(2)28(36-32(43)45-16-20-9-5-4-6-10-20)31(42)34-19(3)30(41)35-24(15-27(38)39)25(37)17-44-26-14-22-12-8-7-11-21(22)13-23(26)29(33)40/h4-14,18-19,24,28H,15-17H2,1-3H3,(H2,33,40)(H,34,42)(H,35,41)(H,36,43)(H,38,39)/t19-,24?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 4.30E+5 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Evaluated for dissociation constant for the inhibition of caspase-1 |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091572
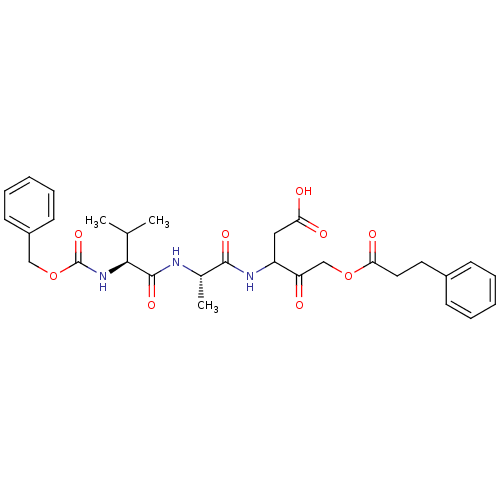
(3-[2-(2-Benzyloxycarbonylamino-3-methyl-butyrylami...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COC(=O)CCc1ccccc1 Show InChI InChI=1S/C30H37N3O9/c1-19(2)27(33-30(40)42-17-22-12-8-5-9-13-22)29(39)31-20(3)28(38)32-23(16-25(35)36)24(34)18-41-26(37)15-14-21-10-6-4-7-11-21/h4-13,19-20,23,27H,14-18H2,1-3H3,(H,31,39)(H,32,38)(H,33,40)(H,35,36)/t20-,23?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.60E+6 | n/a | n/a |
BASF Bioresearch Corporation
Curated by ChEMBL
| Assay Description
Evaluated for dissociation constant for the inhibition of caspase. |
J Med Chem 43: 3351-71 (2000)
BindingDB Entry DOI: 10.7270/Q2416W98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data