 Found 81 hits of kon data for polymerid = 1043,1045,1048,1063,1064,1125,3619,3621,3974,3975,4408,49001205,49001206,4933,50000090
Found 81 hits of kon data for polymerid = 1043,1045,1048,1063,1064,1125,3619,3621,3974,3975,4408,49001205,49001206,4933,50000090 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50093799
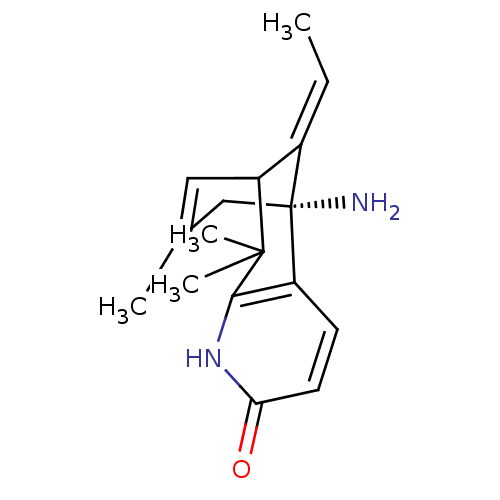
((R)-1-Amino-13-eth-(E)-ylidene-8,8,11-trimethyl-6-...)Show SMILES C\C=C1/C2C=C(C)C[C@]1(N)c1ccc(=O)[nH]c1C2(C)C |t:4,TLB:11:10:2:4.7.5,THB:15:16:2:4.7.5| Show InChI InChI=1S/C17H22N2O/c1-5-11-13-8-10(2)9-17(11,18)12-6-7-14(20)19-15(12)16(13,3)4/h5-8,13H,9,18H2,1-4H3,(H,19,20)/b11-5+/t13?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. |
Bioorg Med Chem Lett 6: 259-262 (1996)
Article DOI: 10.1016/0960-894X(96)00012-1
BindingDB Entry DOI: 10.7270/Q2R49R8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | 0.000367 | 0.0155 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant for the inhibition of fetal bovine serum acetylcholinesterase was determined.. |
Bioorg Med Chem Lett 6: 259-262 (1996)
Article DOI: 10.1016/0960-894X(96)00012-1
BindingDB Entry DOI: 10.7270/Q2R49R8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 0.0567 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic constant for inhibition of fetal bovine serum acetylcholinesterase |
Bioorg Med Chem Lett 6: 259-262 (1996)
Article DOI: 10.1016/0960-894X(96)00012-1
BindingDB Entry DOI: 10.7270/Q2R49R8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | n/a | n/a | 0.0570 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic constant for inhibition of fetal bovine serum acetylcholinesterase |
Bioorg Med Chem Lett 6: 259-262 (1996)
Article DOI: 10.1016/0960-894X(96)00012-1
BindingDB Entry DOI: 10.7270/Q2R49R8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50027340
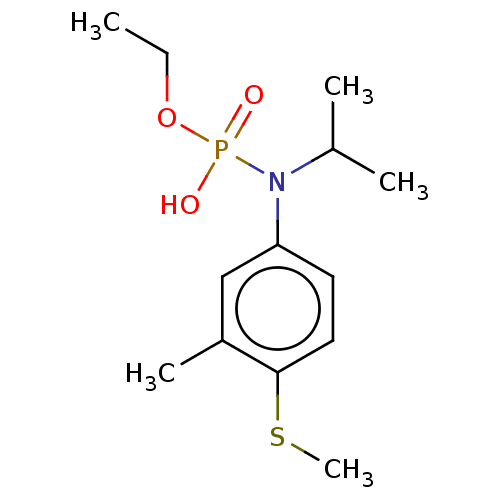
(FENAMIPHOS)Show InChI InChI=1S/C13H22NO3PS/c1-6-17-18(15,16)14(10(2)3)12-7-8-13(19-5)11(4)9-12/h7-10H,6H2,1-5H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 0.330 | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016937
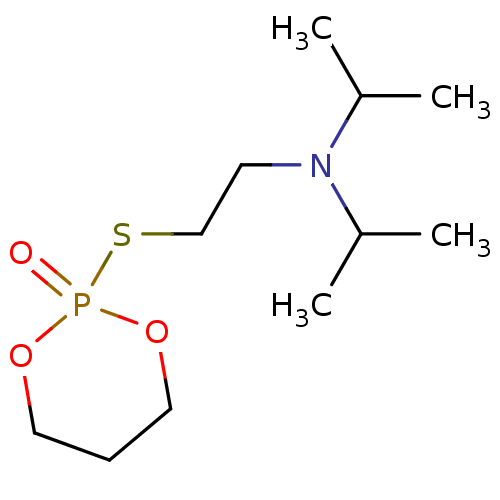
(CHEMBL3277094)Show InChI InChI=1S/C11H24NO3PS/c1-10(2)12(11(3)4)6-9-17-16(13)14-7-5-8-15-16/h10-11H,5-9H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method |
J Med Chem 19: 810-3 (1976)
BindingDB Entry DOI: 10.7270/Q2N58NX7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064505

(CHEMBL2288464)Show InChI InChI=1S/C7H10N2O3/c1-5-4-6(8-12-5)11-7(10)9(2)3/h4H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.97 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064604
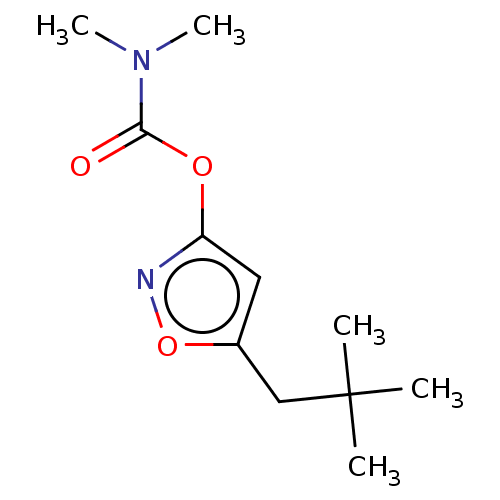
(CHEMBL3401237)Show InChI InChI=1S/C11H18N2O3/c1-11(2,3)7-8-6-9(12-16-8)15-10(14)13(4)5/h6H,7H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 7.33 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016938
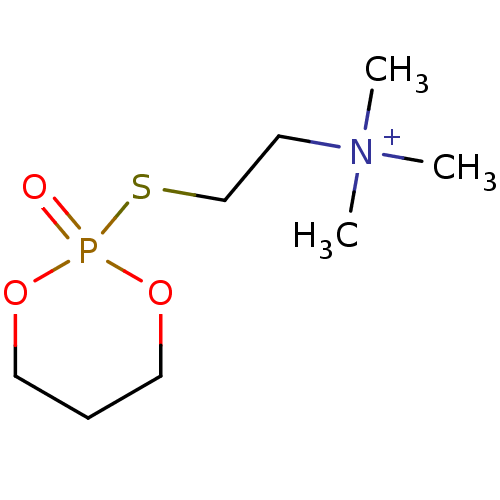
(CHEMBL3277095)Show InChI InChI=1S/C8H19NO3PS.CH4O3S/c1-9(2,3)5-8-14-13(10)11-6-4-7-12-13;1-5(2,3)4/h4-8H2,1-3H3;1H3,(H,2,3,4)/q+1;/p-1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 13.3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method |
J Med Chem 19: 810-3 (1976)
BindingDB Entry DOI: 10.7270/Q2N58NX7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016936

(CHEMBL3277093)Show SMILES CS([O-])(=O)=O.CC[N+](C)(CC)CCSP1(=O)OCCCO1 Show InChI InChI=1S/C10H23NO3PS.CH4O3S/c1-4-11(3,5-2)7-10-16-15(12)13-8-6-9-14-15;1-5(2,3)4/h4-10H2,1-3H3;1H3,(H,2,3,4)/q+1;/p-1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 18.3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method |
J Med Chem 19: 810-3 (1976)
BindingDB Entry DOI: 10.7270/Q2N58NX7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064479

(CHEMBL3401207)Show InChI InChI=1S/C7H10N2O3/c1-5-4-6(10)9(12-5)7(11)8(2)3/h4H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 20.3 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064522

(CHEMBL3401235)Show InChI InChI=1S/C10H16N2O3/c1-7(2)5-8-6-9(11-15-8)14-10(13)12(3)4/h6-7H,5H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 26.5 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064615
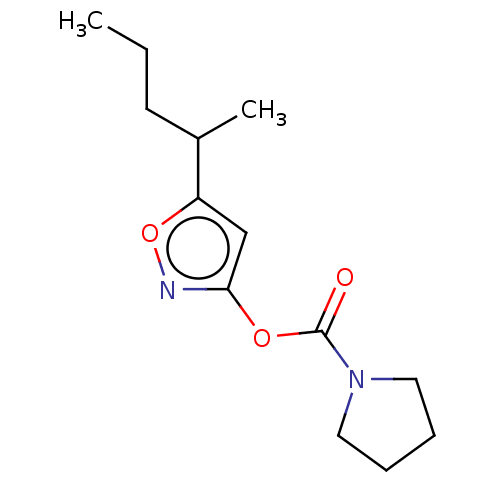
(CHEMBL3403722)Show InChI InChI=1S/C13H20N2O3/c1-3-6-10(2)11-9-12(14-18-11)17-13(16)15-7-4-5-8-15/h9-10H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 27 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064542
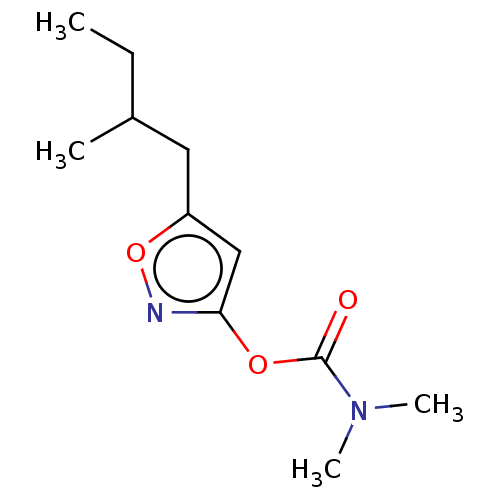
(CHEMBL3401236)Show InChI InChI=1S/C11H18N2O3/c1-5-8(2)6-9-7-10(12-16-9)15-11(14)13(3)4/h7-8H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 28.3 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064509
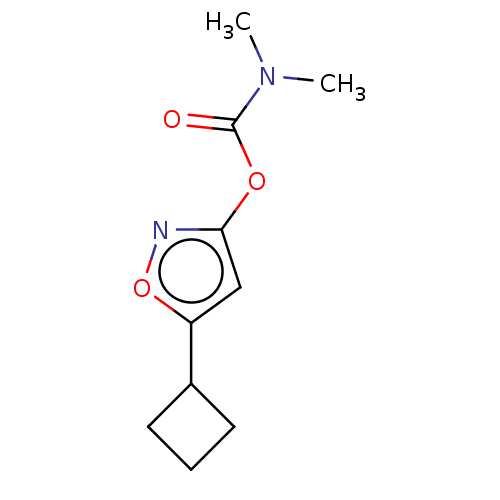
(CHEMBL3401229)Show InChI InChI=1S/C10H14N2O3/c1-12(2)10(13)14-9-6-8(15-11-9)7-4-3-5-7/h6-7H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 31 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064605
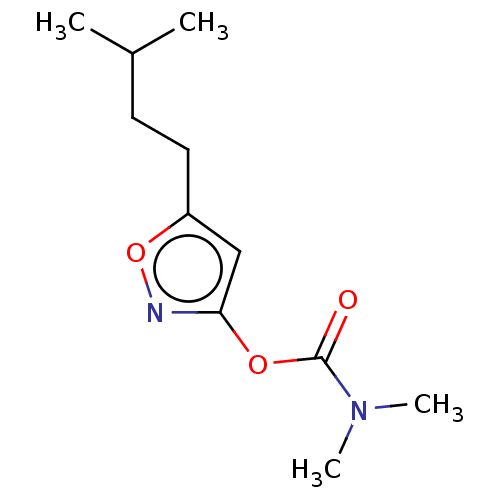
(CHEMBL3401238)Show InChI InChI=1S/C11H18N2O3/c1-8(2)5-6-9-7-10(12-16-9)15-11(14)13(3)4/h7-8H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 31.8 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50027341

(CHEBI:38721 | METHAMIDOPHOS)Show InChI InChI=1S/C2H8NO2PS/c1-5-6(3,4)7-2/h1-2H3,(H2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 32 | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064507
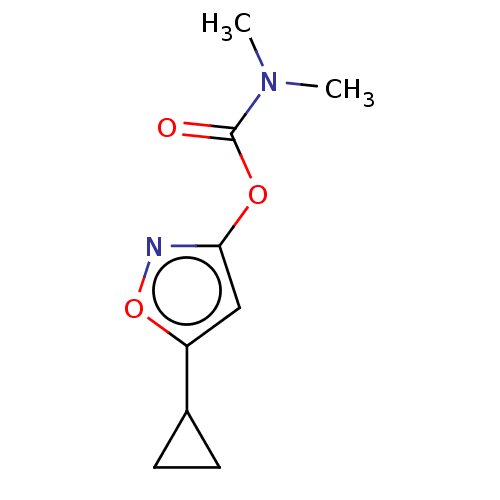
(CHEMBL3401227)Show InChI InChI=1S/C9H12N2O3/c1-11(2)9(12)13-8-5-7(14-10-8)6-3-4-6/h5-6H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 33.7 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064514
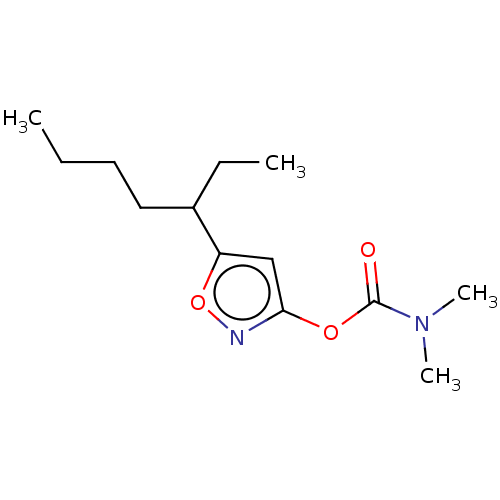
(CHEMBL3401234)Show InChI InChI=1S/C13H22N2O3/c1-5-7-8-10(6-2)11-9-12(14-18-11)17-13(16)15(3)4/h9-10H,5-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 35.5 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064616
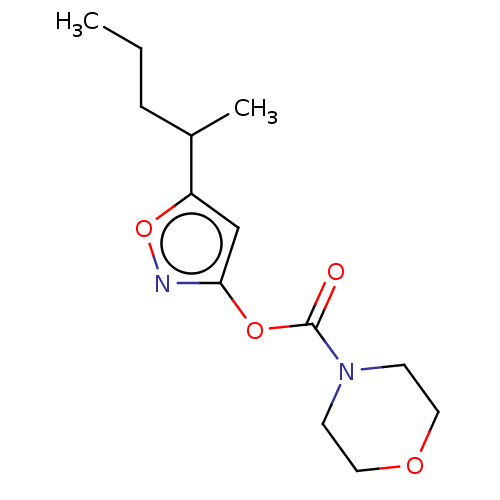
(CHEMBL3403723)Show InChI InChI=1S/C13H20N2O4/c1-3-4-10(2)11-9-12(14-19-11)18-13(16)15-5-7-17-8-6-15/h9-10H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 44.7 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 55 | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as carbamylation |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016935
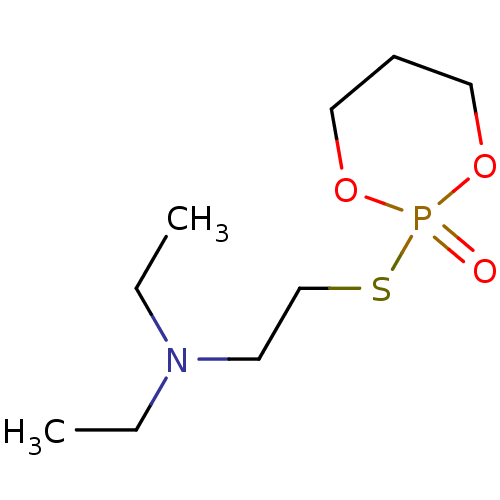
(CHEMBL3277092)Show InChI InChI=1S/C9H20NO3PS/c1-3-10(4-2)6-9-15-14(11)12-7-5-8-13-14/h3-9H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 68.3 | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method |
J Med Chem 19: 810-3 (1976)
BindingDB Entry DOI: 10.7270/Q2N58NX7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064508
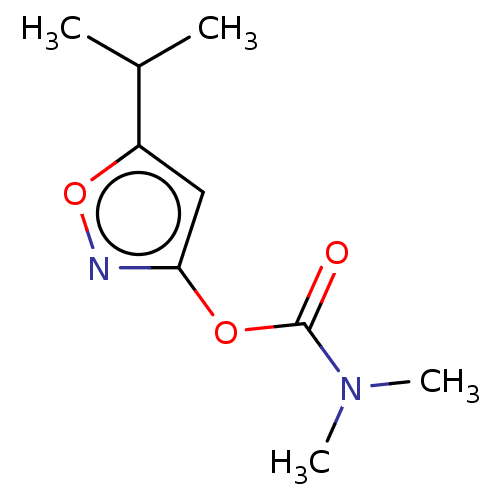
(CHEMBL3401228)Show InChI InChI=1S/C9H14N2O3/c1-6(2)7-5-8(10-14-7)13-9(12)11(3)4/h5-6H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 74.3 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064511
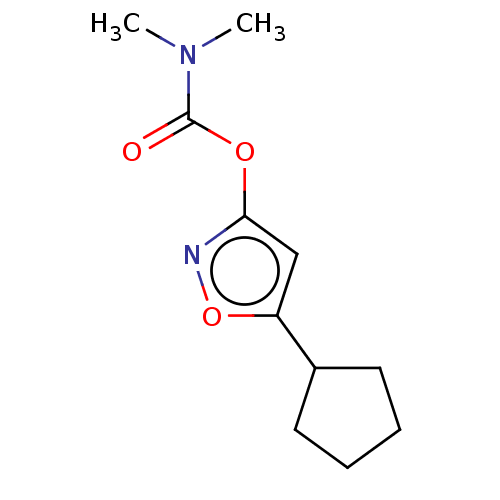
(CHEMBL3401231)Show InChI InChI=1S/C11H16N2O3/c1-13(2)11(14)15-10-7-9(16-12-10)8-5-3-4-6-8/h7-8H,3-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 77 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064478
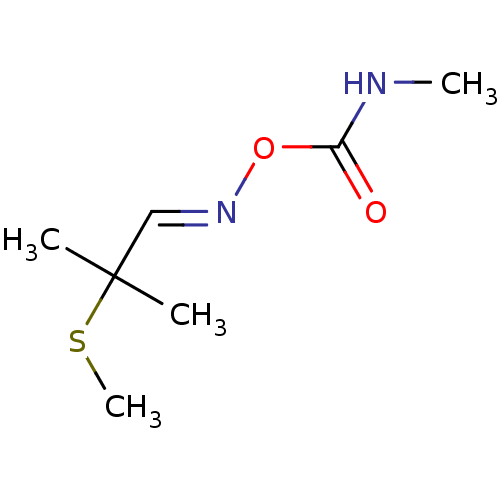
(ALDICARB)Show InChI InChI=1S/C7H14N2O2S/c1-7(2,12-4)5-9-11-6(10)8-3/h5H,1-4H3,(H,8,10)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 108 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064607

(CHEMBL3401240)Show InChI InChI=1S/C12H18N2O3/c1-14(2)12(15)16-11-8-10(17-13-11)7-6-9-4-3-5-9/h8-9H,3-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 121 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064606

(CHEMBL3401239)Show InChI InChI=1S/C11H16N2O3/c1-13(2)11(14)15-10-7-9(16-12-10)6-5-8-3-4-8/h7-8H,3-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 138 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064513

(CHEMBL3401233)Show InChI InChI=1S/C11H18N2O3/c1-5-8(6-2)9-7-10(12-16-9)15-11(14)13(3)4/h7-8H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 151 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064611
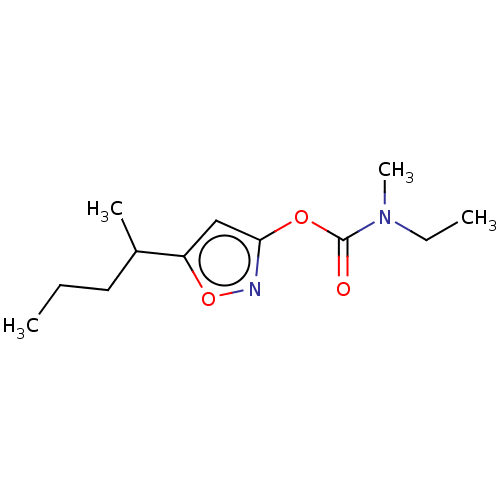
(CHEMBL3403721)Show InChI InChI=1S/C12H20N2O3/c1-5-7-9(3)10-8-11(13-17-10)16-12(15)14(4)6-2/h8-9H,5-7H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 152 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50490821
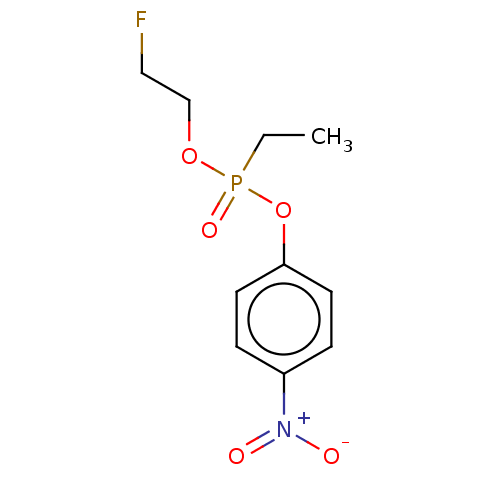
(2-Fluoroethyl 4-Nitrophenyl Ethylphosphonate | CHE...)Show InChI InChI=1S/C10H13FNO5P/c1-2-18(15,16-8-7-11)17-10-5-3-9(4-6-10)12(13)14/h3-6H,2,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 167 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50490825
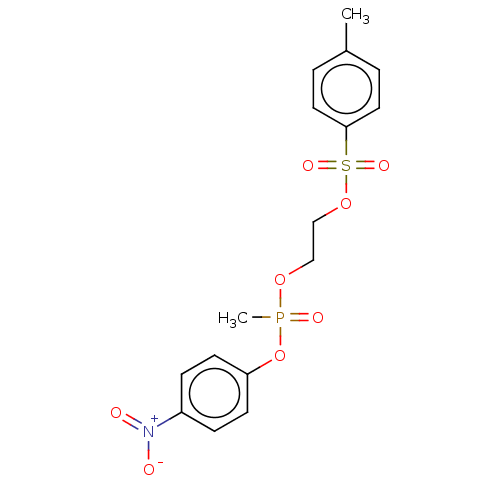
(CHEMBL2348368)Show SMILES Cc1ccc(cc1)S(=O)(=O)OCCOP(C)(=O)Oc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C16H18NO8PS/c1-13-3-9-16(10-4-13)27(21,22)24-12-11-23-26(2,20)25-15-7-5-14(6-8-15)17(18)19/h3-10H,11-12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 183 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50490821

(2-Fluoroethyl 4-Nitrophenyl Ethylphosphonate | CHE...)Show InChI InChI=1S/C10H13FNO5P/c1-2-18(15,16-8-7-11)17-10-5-3-9(4-6-10)12(13)14/h3-6H,2,7-8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 200 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by concentration-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50490824
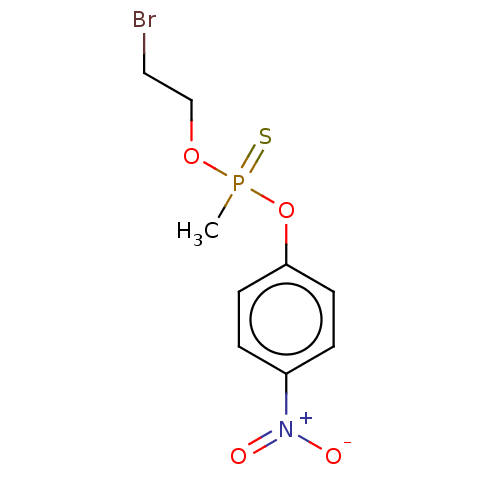
(CHEMBL2348369)Show InChI InChI=1S/C9H11BrNO4PS/c1-16(17,14-7-6-10)15-9-4-2-8(3-5-9)11(12)13/h2-5H,6-7H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 200 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064608
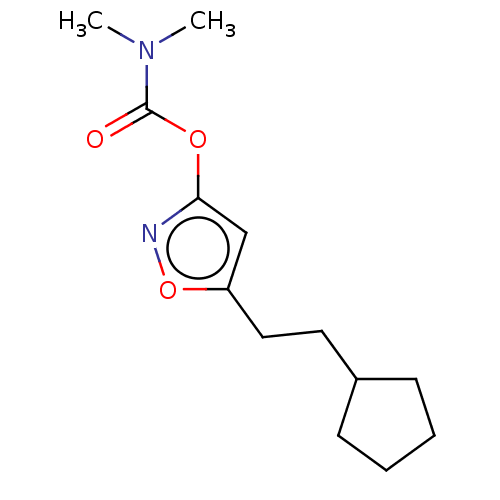
(CHEMBL3403720)Show InChI InChI=1S/C13H20N2O3/c1-15(2)13(16)17-12-9-11(18-14-12)8-7-10-5-3-4-6-10/h9-10H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 218 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50490822
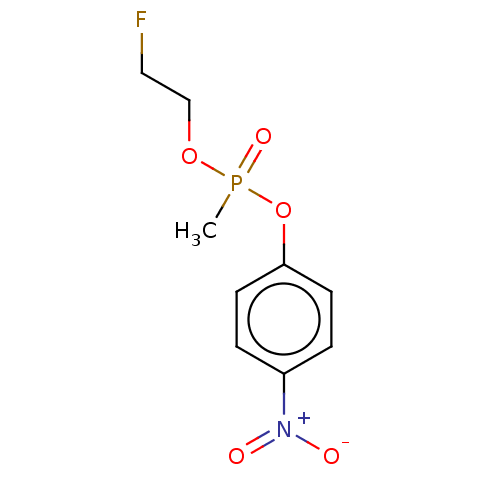
(2-Fluoroethyl 4-Nitrophenyl Methylphosphonate | CH...)Show InChI InChI=1S/C9H11FNO5P/c1-17(14,15-7-6-10)16-9-4-2-8(3-5-9)11(12)13/h2-5H,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 233 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50240416
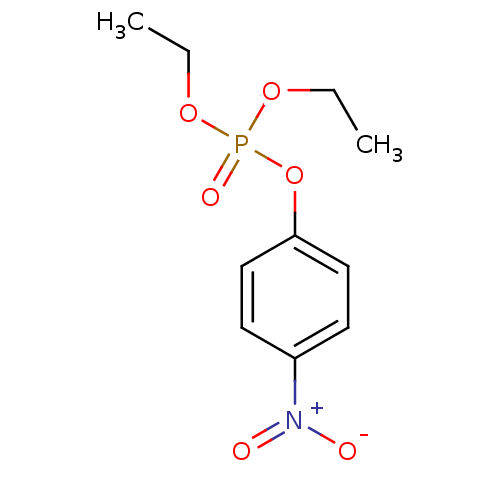
(CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...)Show InChI InChI=1S/C10H14NO6P/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13/h5-8H,3-4H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 267 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by concentration-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064617

(CHEBI:34938 | Propoxur)Show InChI InChI=1S/C11H15NO3/c1-8(2)14-9-6-4-5-7-10(9)15-11(13)12-3/h4-8H,1-3H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 283 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50240416

(CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...)Show InChI InChI=1S/C10H14NO6P/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13/h5-8H,3-4H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 333 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by concentration-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50490822

(2-Fluoroethyl 4-Nitrophenyl Methylphosphonate | CH...)Show InChI InChI=1S/C9H11FNO5P/c1-17(14,15-7-6-10)16-9-4-2-8(3-5-9)11(12)13/h2-5H,6-7H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 417 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064504
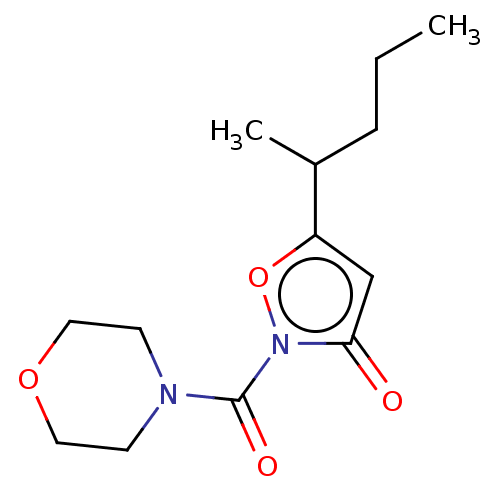
(CHEMBL3401226)Show InChI InChI=1S/C13H20N2O4/c1-3-4-10(2)11-9-12(16)15(19-11)13(17)14-5-7-18-8-6-14/h9-10H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 433 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064490
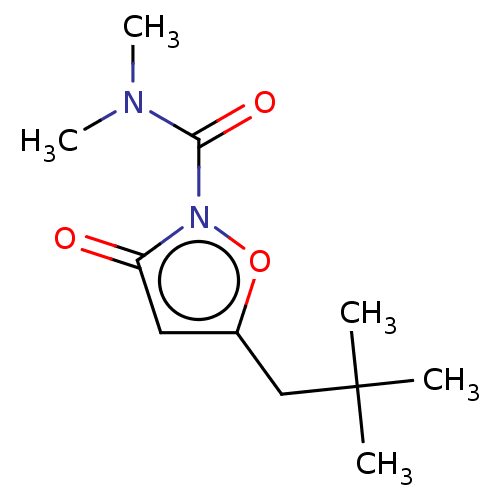
(CHEMBL3401217)Show InChI InChI=1S/C11H18N2O3/c1-11(2,3)7-8-6-9(14)13(16-8)10(15)12(4)5/h6H,7H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 437 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064488
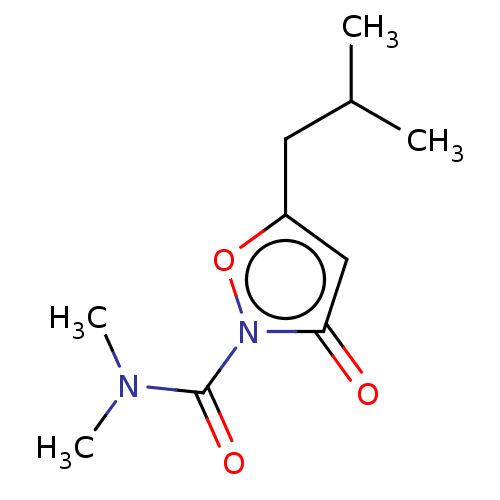
(CHEMBL3401215)Show InChI InChI=1S/C10H16N2O3/c1-7(2)5-8-6-9(13)12(15-8)10(14)11(3)4/h6-7H,5H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 515 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50240416

(CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...)Show InChI InChI=1S/C10H14NO6P/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13/h5-8H,3-4H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 567 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50490821

(2-Fluoroethyl 4-Nitrophenyl Ethylphosphonate | CHE...)Show InChI InChI=1S/C10H13FNO5P/c1-2-18(15,16-8-7-11)17-10-5-3-9(4-6-10)12(13)14/h3-6H,2,7-8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 633 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064512
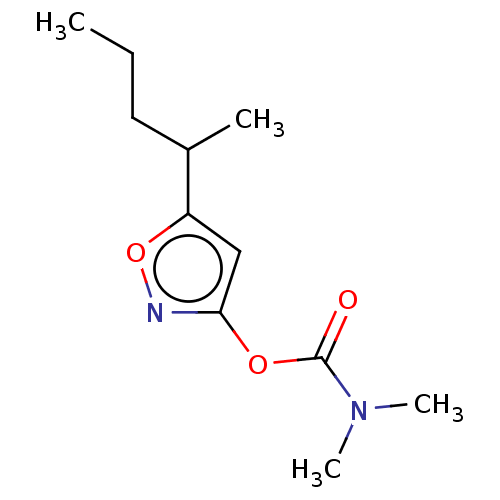
(CHEMBL3401232)Show InChI InChI=1S/C11H18N2O3/c1-5-6-8(2)9-7-10(12-16-9)15-11(14)13(3)4/h7-8H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 848 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50490821

(2-Fluoroethyl 4-Nitrophenyl Ethylphosphonate | CHE...)Show InChI InChI=1S/C10H13FNO5P/c1-2-18(15,16-8-7-11)17-10-5-3-9(4-6-10)12(13)14/h3-6H,2,7-8H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 917 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by concentration-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50490822

(2-Fluoroethyl 4-Nitrophenyl Methylphosphonate | CH...)Show InChI InChI=1S/C9H11FNO5P/c1-17(14,15-7-6-10)16-9-4-2-8(3-5-9)11(12)13/h2-5H,6-7H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 983 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by concentration-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064510
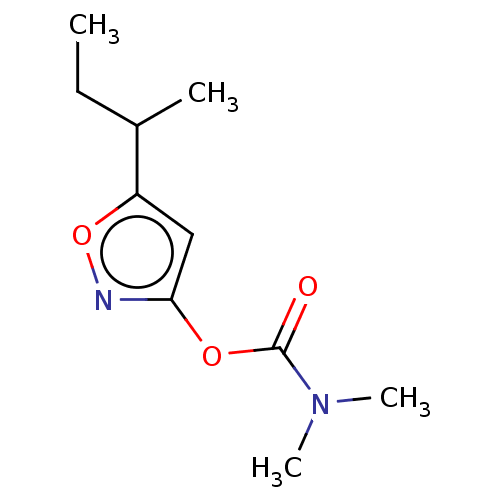
(CHEMBL3401230)Show InChI InChI=1S/C10H16N2O3/c1-5-7(2)8-6-9(11-15-8)14-10(13)12(3)4/h6-7H,5H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE |
Bioorg Med Chem 23: 1321-40 (2015)
Article DOI: 10.1016/j.bmc.2015.01.026
BindingDB Entry DOI: 10.7270/Q2VD715T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50490822

(2-Fluoroethyl 4-Nitrophenyl Methylphosphonate | CH...)Show InChI InChI=1S/C9H11FNO5P/c1-17(14,15-7-6-10)16-9-4-2-8(3-5-9)11(12)13/h2-5H,6-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.02E+3 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by concentration-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50490822

(2-Fluoroethyl 4-Nitrophenyl Methylphosphonate | CH...)Show InChI InChI=1S/C9H11FNO5P/c1-17(14,15-7-6-10)16-9-4-2-8(3-5-9)11(12)13/h2-5H,6-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by time-dependent inhibition assay |
Bioorg Med Chem Lett 23: 2048-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.010
BindingDB Entry DOI: 10.7270/Q2VH5RSV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data