

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM6190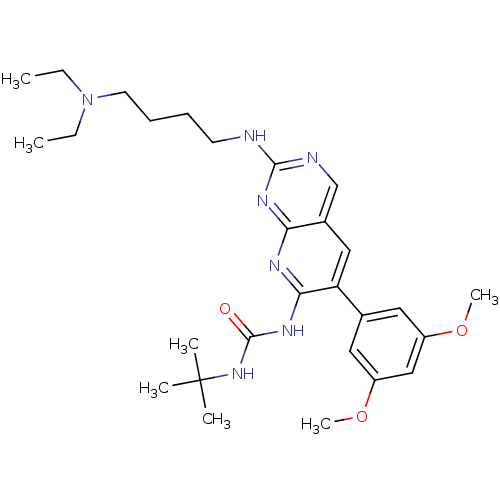 (3-tert-butyl-1-(2-{[4-(diethylamino)butyl]amino}-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of FGFR3 by cellular assay | Proc Natl Acad Sci USA 104: 19936-41 (2007) Article DOI: 10.1073/pnas.0707498104 BindingDB Entry DOI: 10.7270/Q24X58QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM482158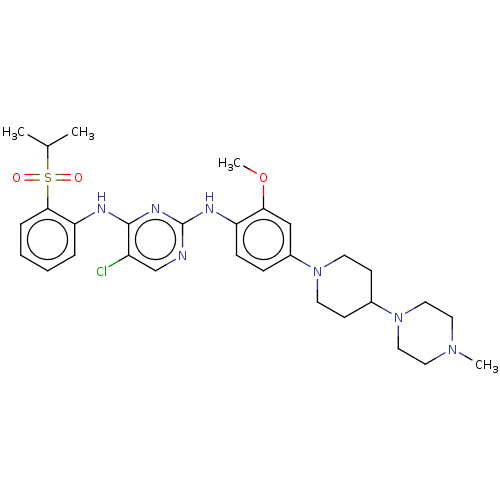 (BDBM50242742 | TAE684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of FGFR3 | Proc Natl Acad Sci USA 104: 19936-41 (2007) Article DOI: 10.1073/pnas.0707498104 BindingDB Entry DOI: 10.7270/Q24X58QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR3 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445224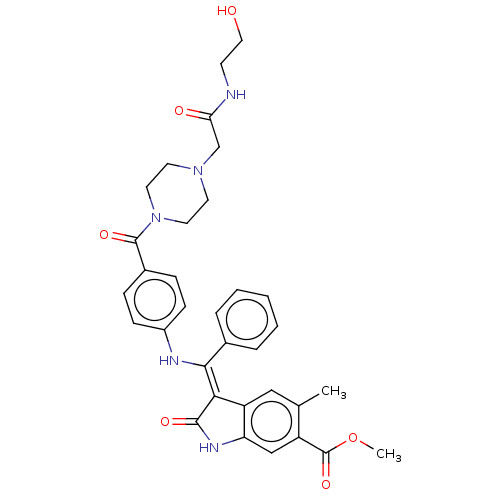 (US10669235, Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM531233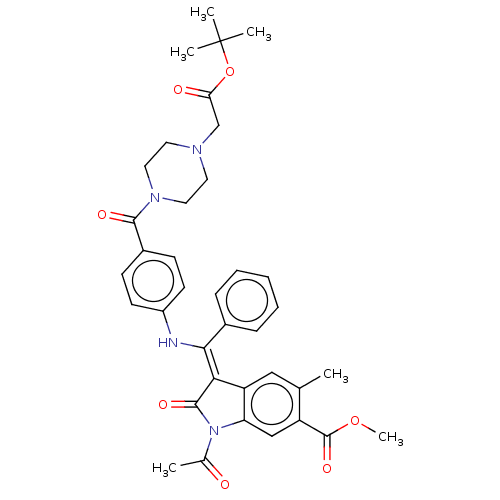 ((Z)-Methyl 3-(((4-(4-(2-((2-hydroxyethyl)amino)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445226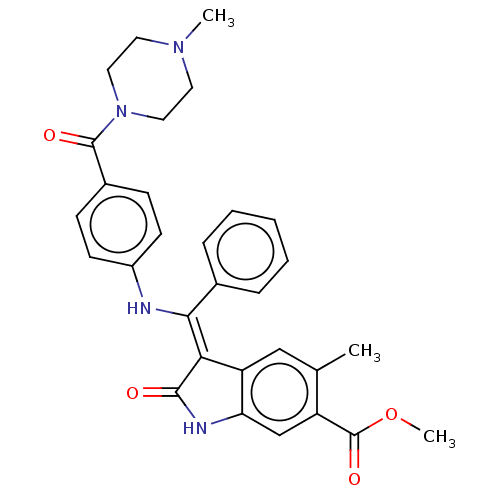 (US10669235, Example 17 | US11208381, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445226 (US10669235, Example 17 | US11208381, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445223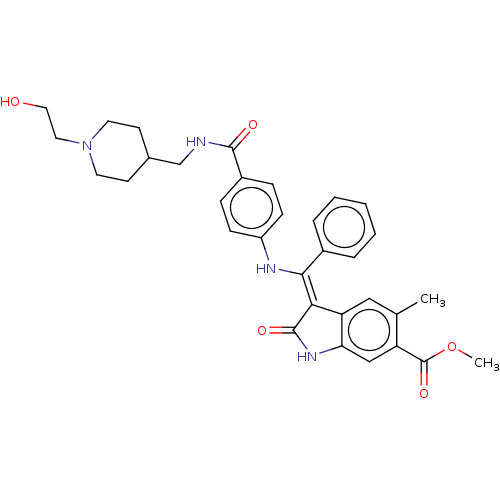 (US10669235, Example 14 | US10669235, Example 50 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445223 (US10669235, Example 14 | US10669235, Example 50 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445223 (US10669235, Example 14 | US10669235, Example 50 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445223 (US10669235, Example 14 | US10669235, Example 50 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445209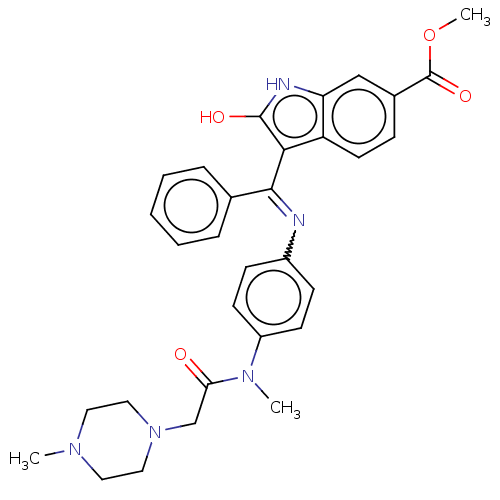 (US10669235, Nintedanib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507116 (US11046672, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445241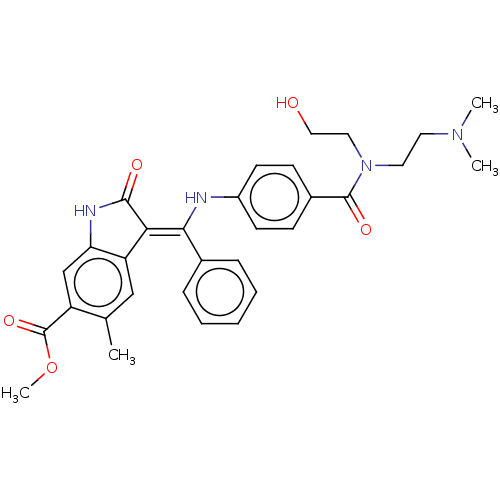 (US10669235, Example 32 | US11208381, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445241 (US10669235, Example 32 | US11208381, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507113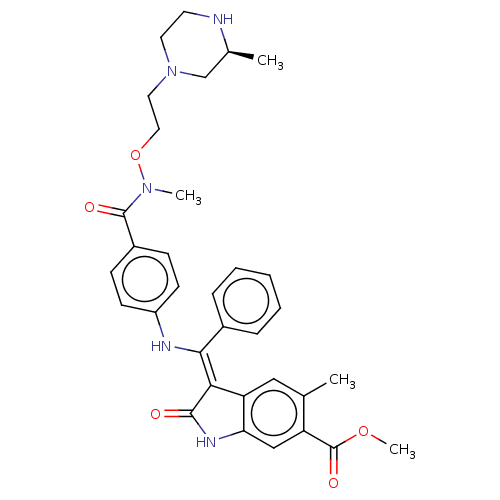 (US11046672, Example 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507056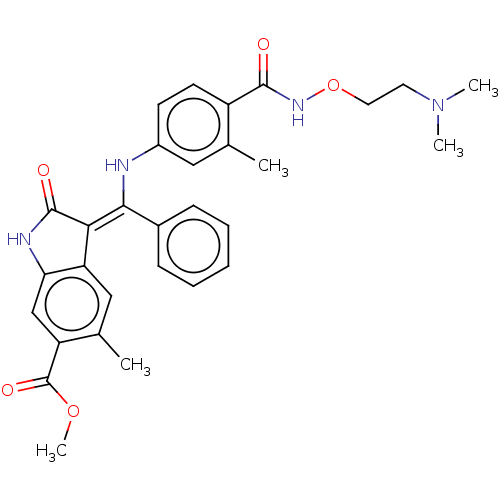 ((Z)-Methyl 3-(((4-((2-(dimethylamino)ethoxy)carbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445247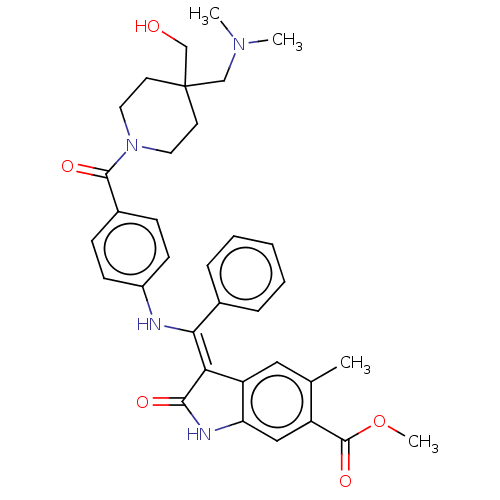 (US10669235, Example 38 | US11208381, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445247 (US10669235, Example 38 | US11208381, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507090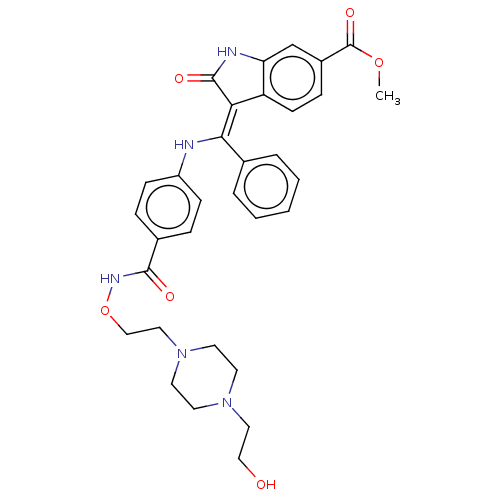 (US11046672, Example 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445222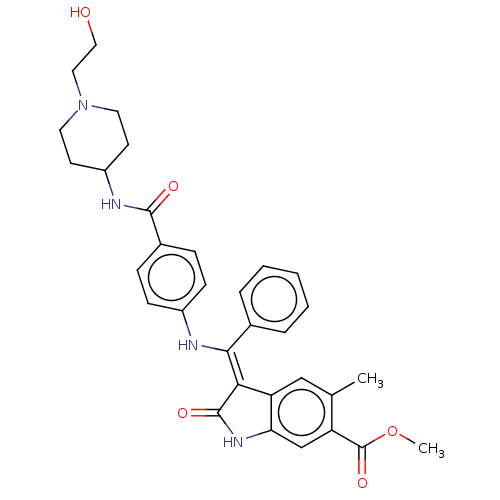 (US10669235, Example 13 | US10669235, Example 49 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445222 (US10669235, Example 13 | US10669235, Example 49 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445222 (US10669235, Example 13 | US10669235, Example 49 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445222 (US10669235, Example 13 | US10669235, Example 49 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445215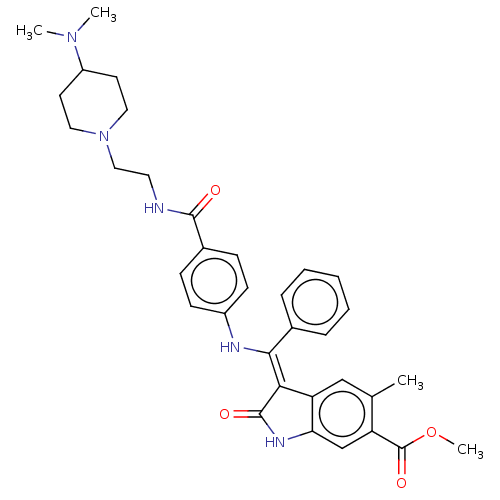 (US10669235, Example 6 | US11208381, Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445215 (US10669235, Example 6 | US11208381, Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445242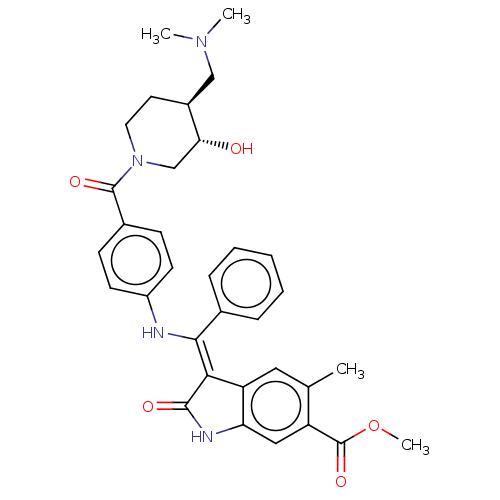 (US10669235, Example 33 | US11208381, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445242 (US10669235, Example 33 | US11208381, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507069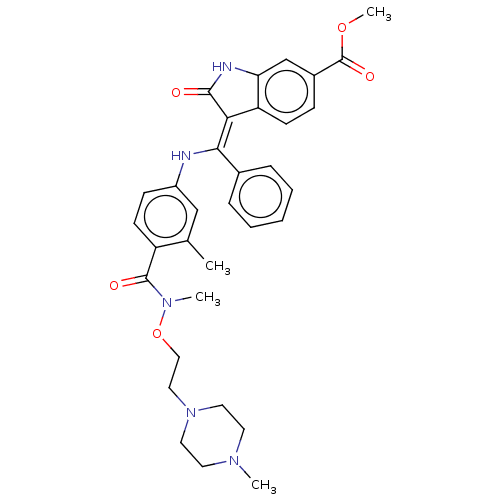 (US11046672, Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507089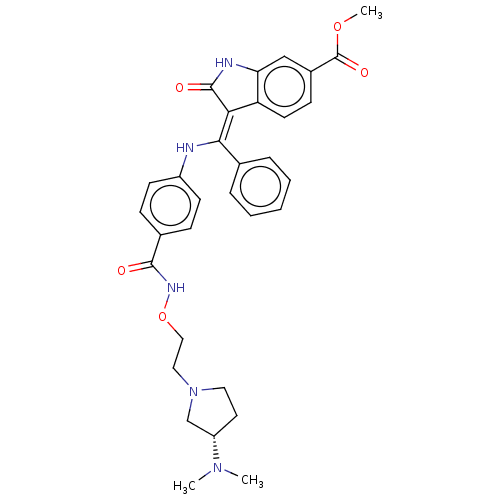 (US11046672, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445255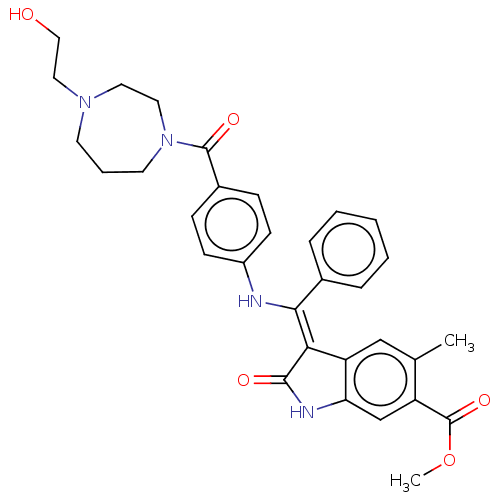 (US10669235, Example 46 | US11208381, Example 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445255 (US10669235, Example 46 | US11208381, Example 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507122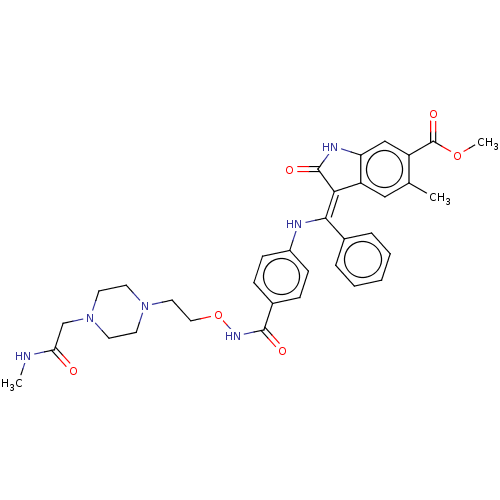 (US11046672, Example 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507115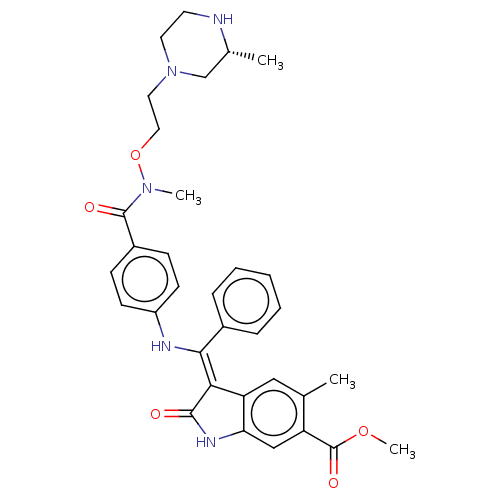 (US11046672, Example 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445213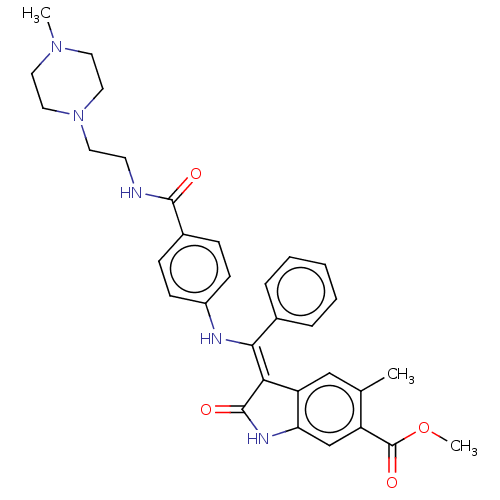 (US10669235, Example 4 | US11208381, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445213 (US10669235, Example 4 | US11208381, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507073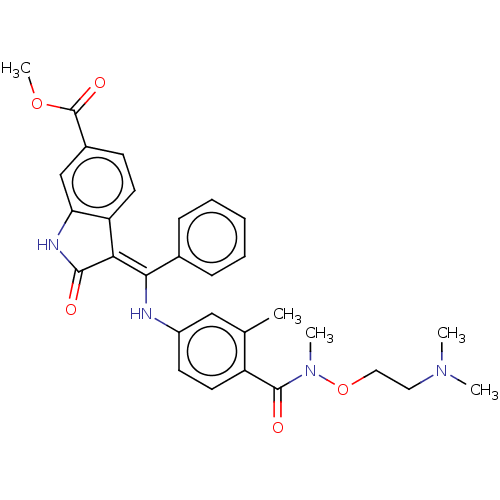 (US11046672, Example 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507054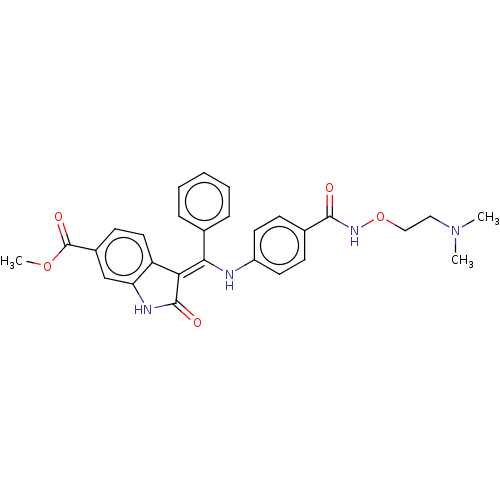 ((Z)-Methyl 3-(((4-((2-(dimethylamino)ethoxy)carbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445257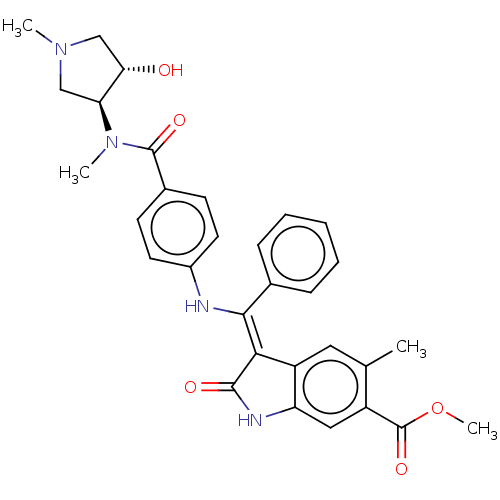 (US10669235, Example 48 | US11208381, Example 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445257 (US10669235, Example 48 | US11208381, Example 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507054 ((Z)-Methyl 3-(((4-((2-(dimethylamino)ethoxy)carbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507058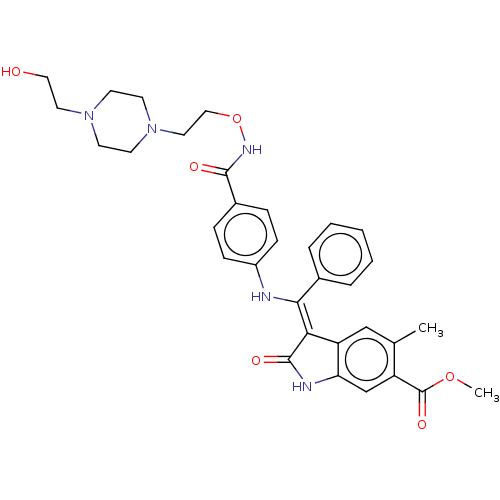 ((Z)-Methyl 3-(((4-((2-(4-(2-hydroxyethyl)piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507109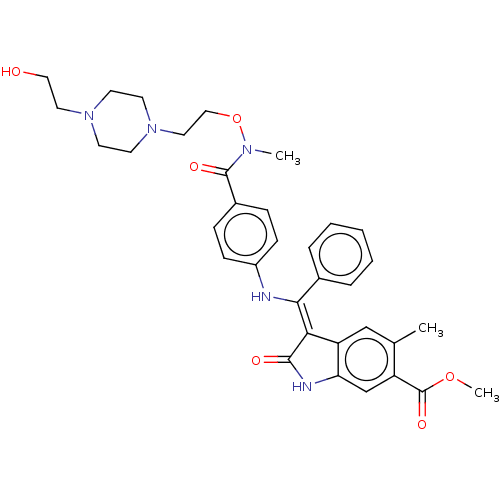 (US11046672, Example 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445228 (US10669235, Example 19 | US11208381, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445228 (US10669235, Example 19 | US11208381, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445250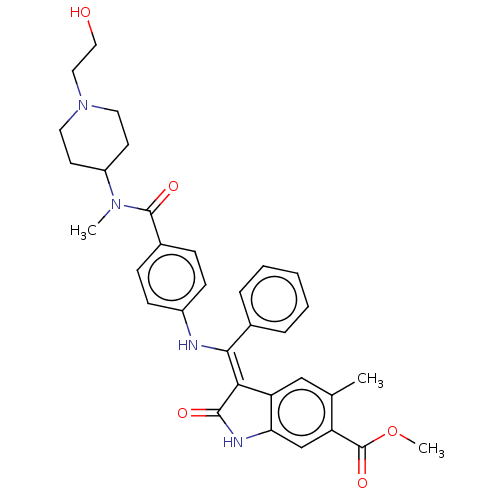 (US10669235, Example 41 | US11208381, Example 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM445250 (US10669235, Example 41 | US11208381, Example 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | US Patent US10669235 (2020) BindingDB Entry DOI: 10.7270/Q2XW4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM507102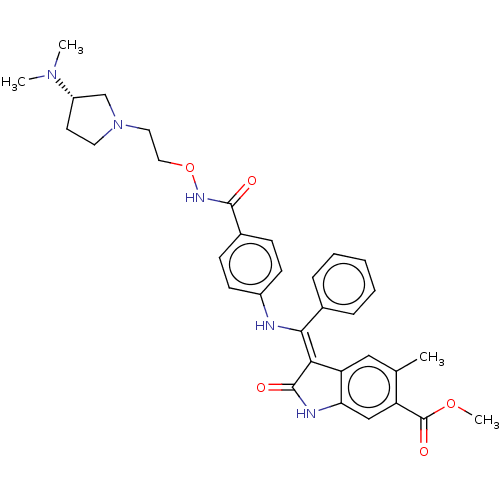 (US11046672, Example 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5096 total ) | Next | Last >> |