

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118233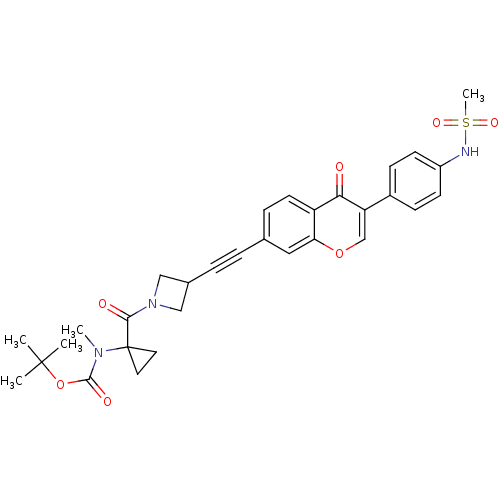 (US8673966, tert-butyl (1-(3-((3-(4-(methylsulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM50093535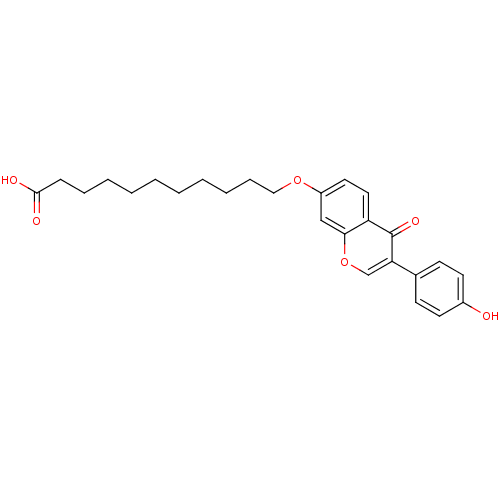 (11-(3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yloxy)u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Hamster Liver mitochondrial ALDH-2 | J Med Chem 43: 4169-79 (2000) BindingDB Entry DOI: 10.7270/Q2GX49T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118229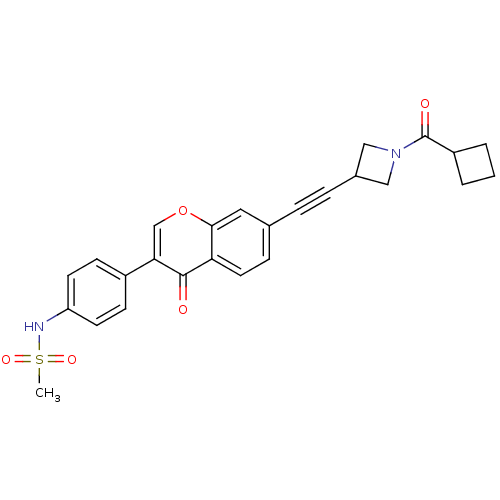 (US8673966, N-(4-(7-((1-(cyclobutanecarbonyl)azetid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM50093537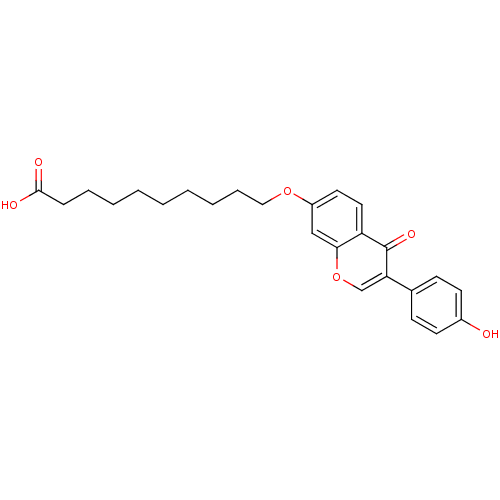 (10-(3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yloxy)d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Hamster Liver mitochondrial ALDH-2 | J Med Chem 43: 4169-79 (2000) BindingDB Entry DOI: 10.7270/Q2GX49T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118196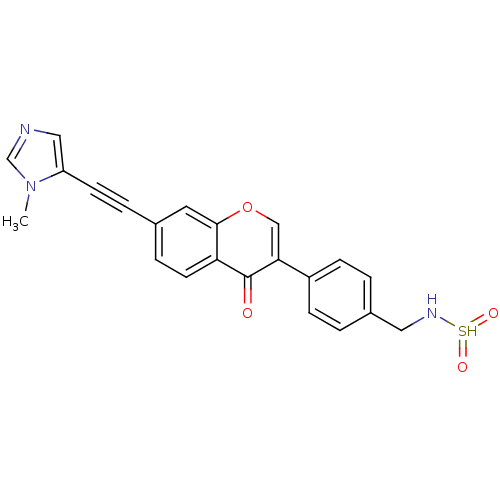 (US8673966, N-(4-(7-((1-methyl-1H-imidazol-5-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456309 (CHEMBL4207222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118209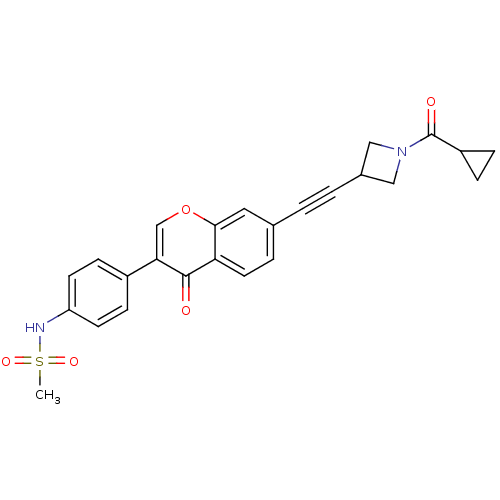 (US8673966, N-(4-(7-((1-(cyclopropanecarbonyl)azeti...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118232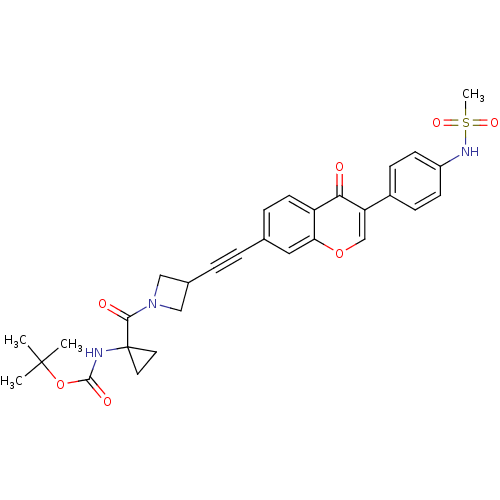 (US8673966, tert-butyl 1-(3-((3-(4- (methylsulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118228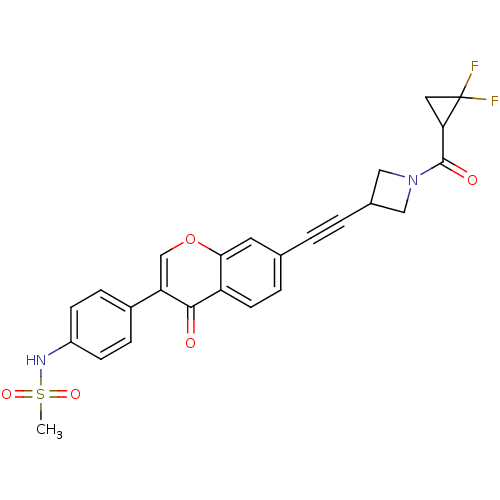 (US8673966, N-(4-(7-((1-(2,2-difluorocyclopropane- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118223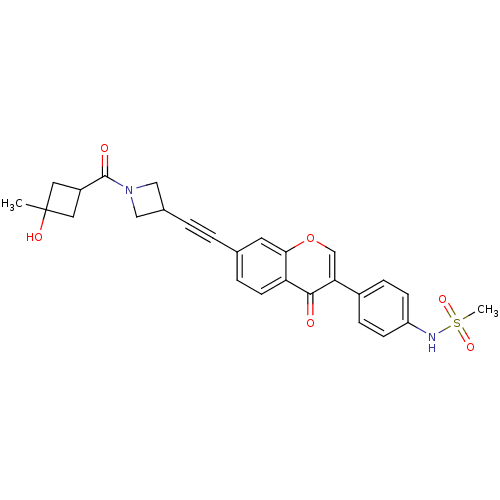 (US8673966, N-(4-(7-((1-(3-hydroxy-3-methylcyclobut...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456224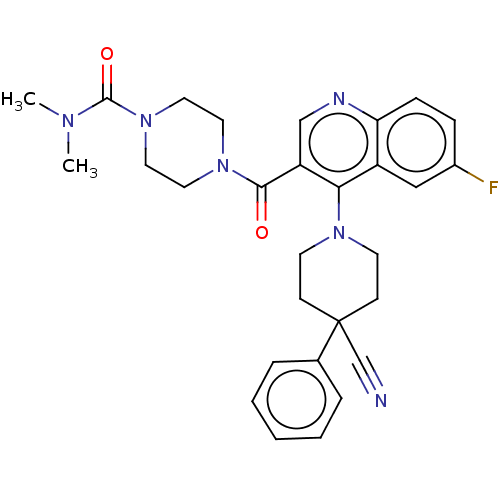 (CHEMBL4207514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118230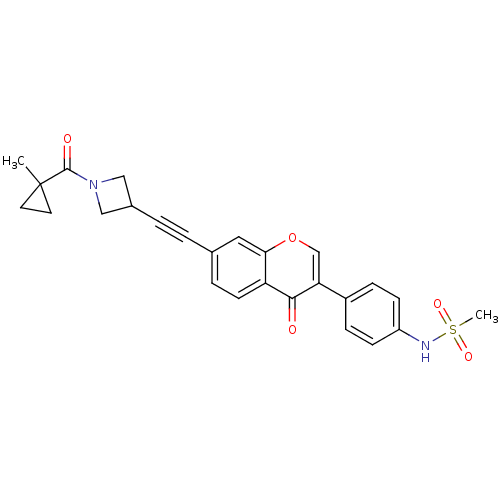 (US8673966, N-(4-(7-((1-(1-methylcyclopropanecarbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456249 (CHEMBL4212671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456228 (CHEMBL4215704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456233 (CHEMBL4211904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118231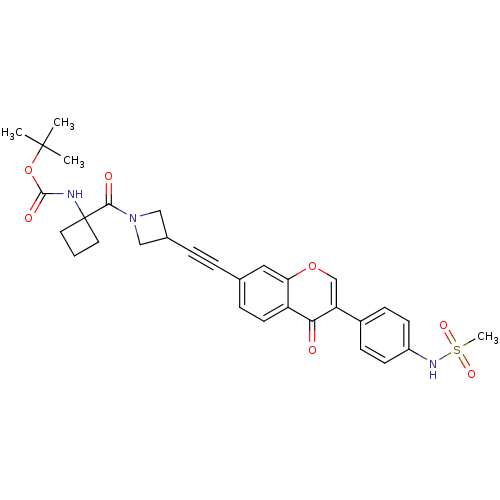 (US8673966, tert-butyl 1-(3-((3-(4-(methylsulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118220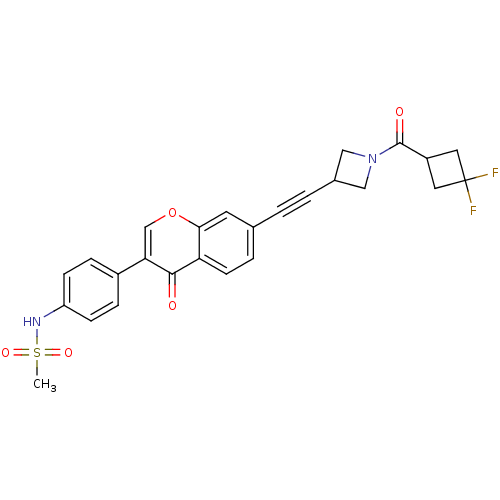 (US8673966, N-(4-(7-((1-(3,3-difluorocyclobutanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118225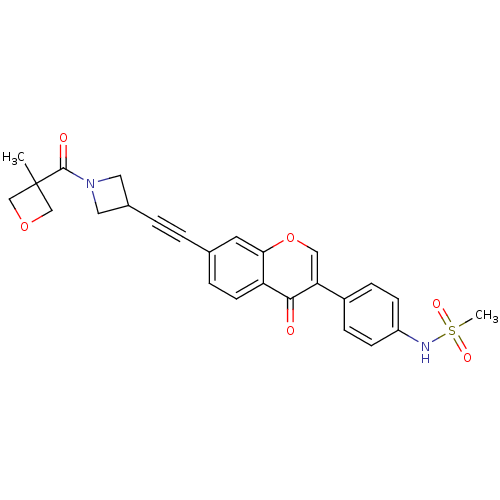 (US8673966, N-(4-(7-((1-(3-methyloxetane-3- carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118211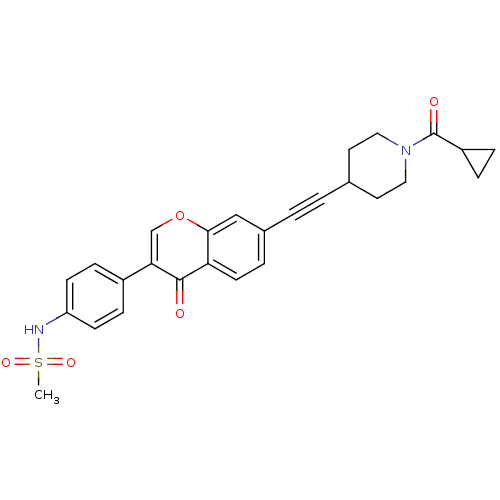 (US8673966, N-(4-(7-((1-(cyclopropanecarbonyl)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456223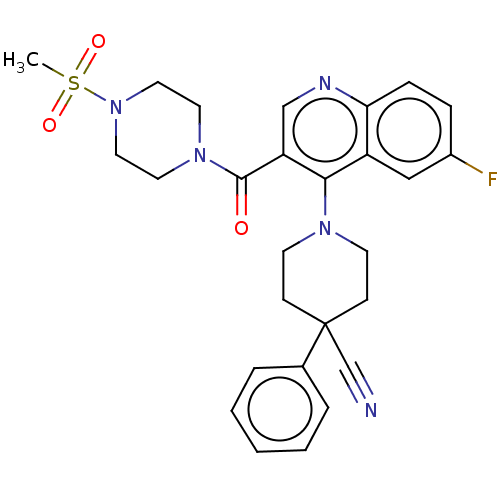 (CHEMBL4206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 30 uM after... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456222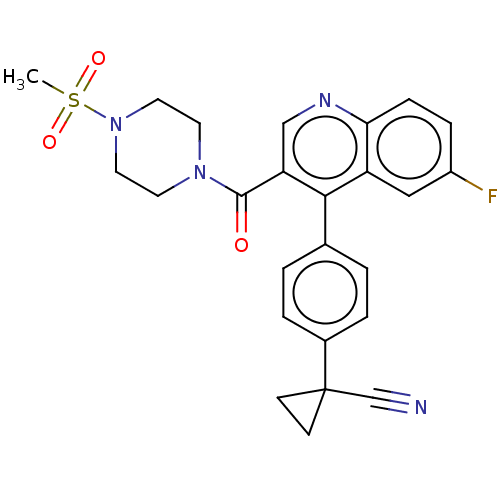 (CHEMBL4206272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456223 (CHEMBL4206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456254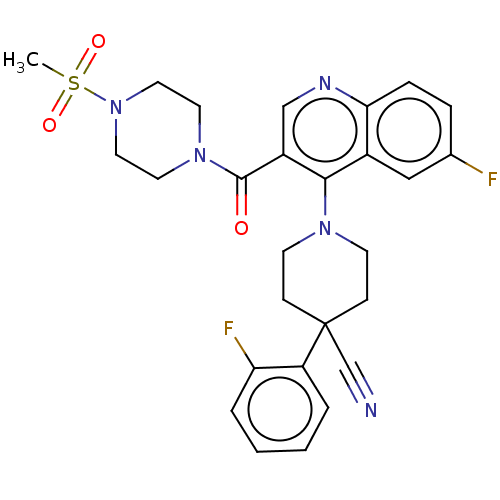 (CHEMBL4202680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118213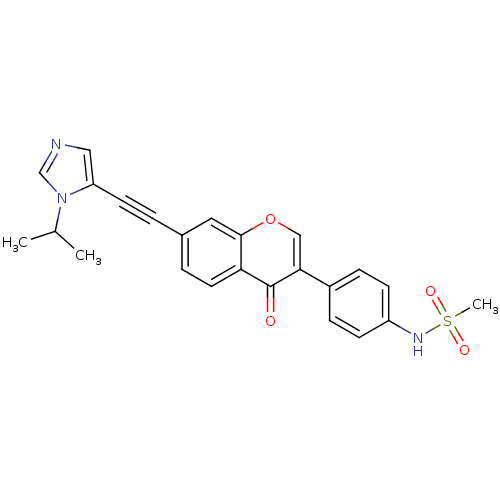 (US8673966, N-(4-(7-((1-isopropyl-1H-imidazol-5- yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456235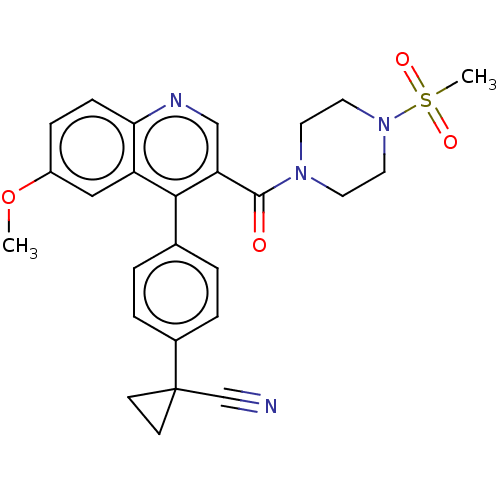 (CHEMBL4209722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456232 (CHEMBL4207617) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456216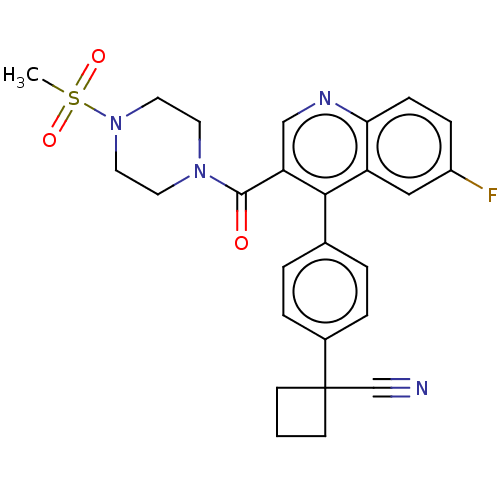 (CHEMBL4206606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456247 (CHEMBL4213848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118219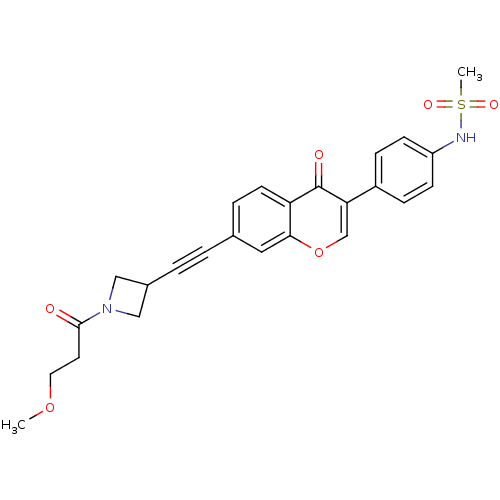 (US8673966, N-(4-(7-((1-(3-methoxypropanoyl)azetidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456267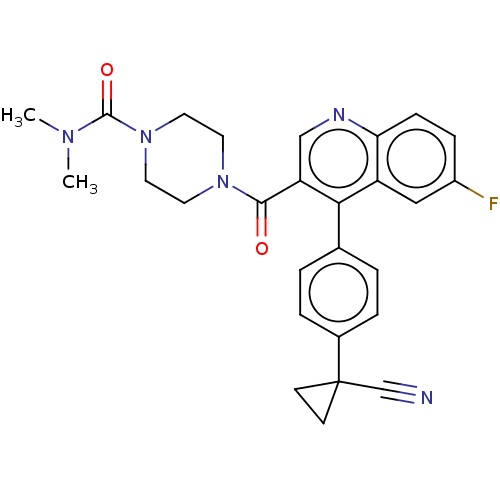 (CHEMBL4218688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456265 (CHEMBL4212891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456312 (CHEMBL4217115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118207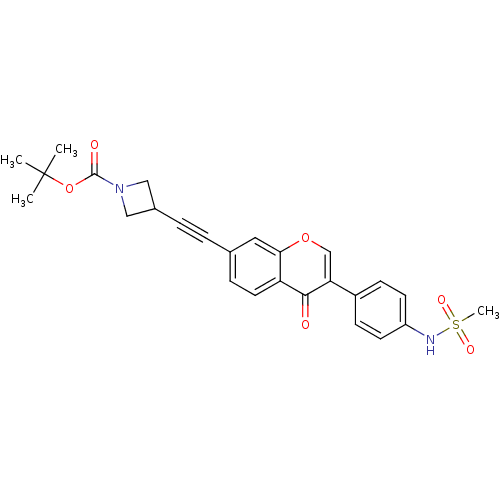 (US8673966, tert-butyl 3-((3-(4-(methylsulfonamido)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456217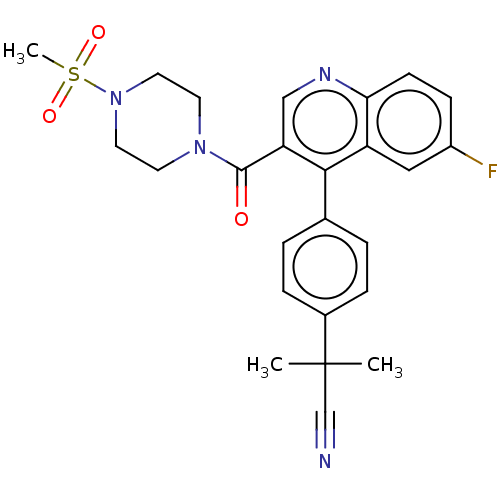 (CHEMBL4214724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456244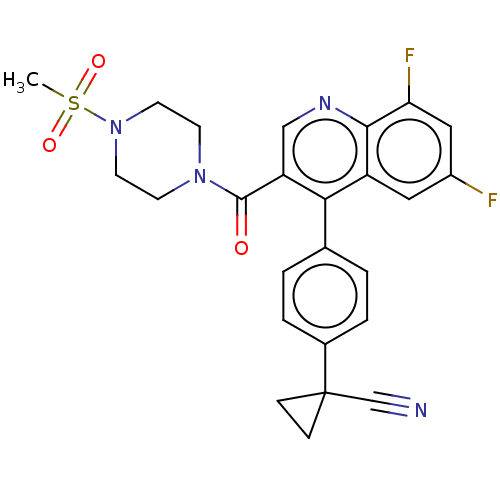 (CHEMBL4213604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM50093531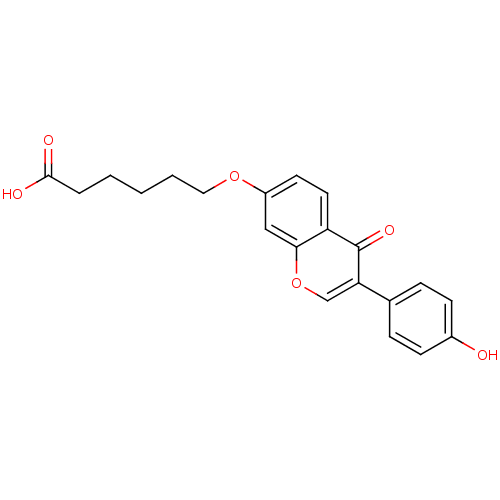 (6-[3-(4-Hydroxy-phenyl)-4-oxo-4H-chromen-7-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Hamster Liver mitochondrial ALDH-2 | J Med Chem 43: 4169-79 (2000) BindingDB Entry DOI: 10.7270/Q2GX49T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118208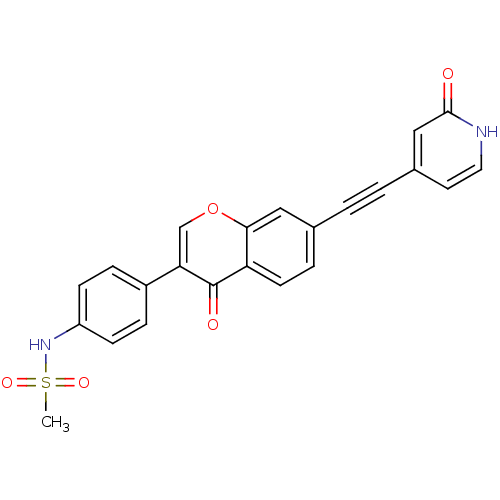 (US8673966, N-(4-(4-oxo-7-((2-oxo-1,2-dihydropyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM118216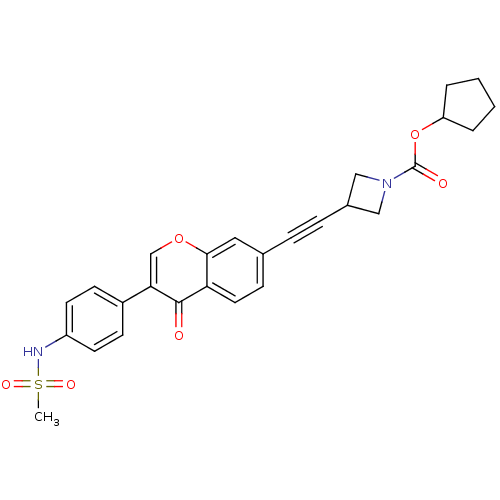 (US8673966, cyclopentyl 3-((3-(4- (methylsulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US8673966 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM50093534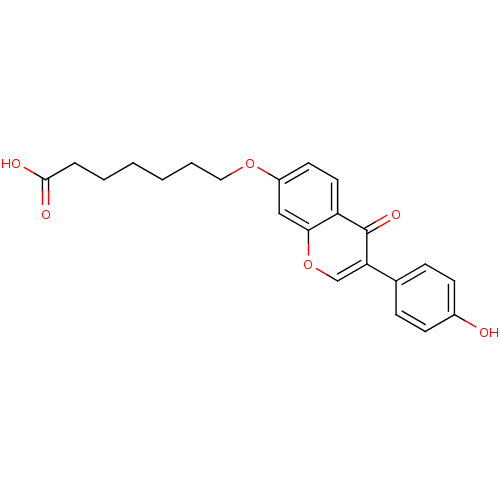 (7-[3-(4-Hydroxy-phenyl)-4-oxo-4H-chromen-7-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Hamster Liver mitochondrial ALDH-2 | J Med Chem 43: 4169-79 (2000) BindingDB Entry DOI: 10.7270/Q2GX49T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456223 (CHEMBL4206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 20 uM after... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456328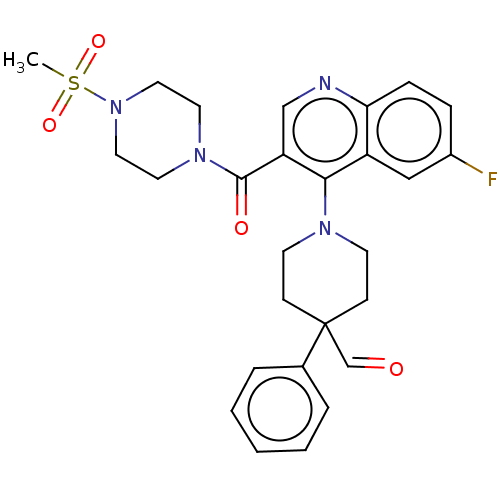 (CHEMBL4210671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456261 (CHEMBL4207953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM22794 (1-benzyl-2,3-dihydro-1H-indole-2,3-dione | Isatin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456256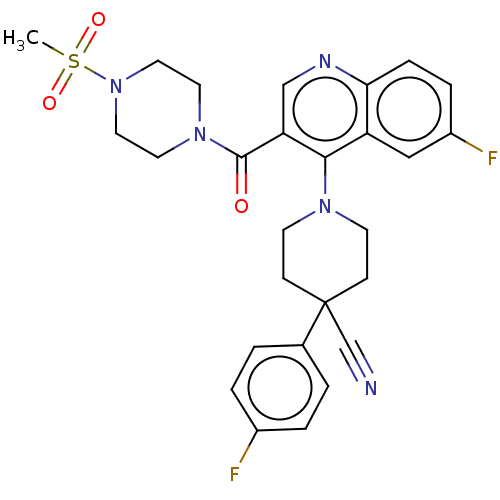 (CHEMBL4205368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456250 (CHEMBL4215957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456258 (CHEMBL4205805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456259 (CHEMBL4216229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456326 (CHEMBL4205051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456252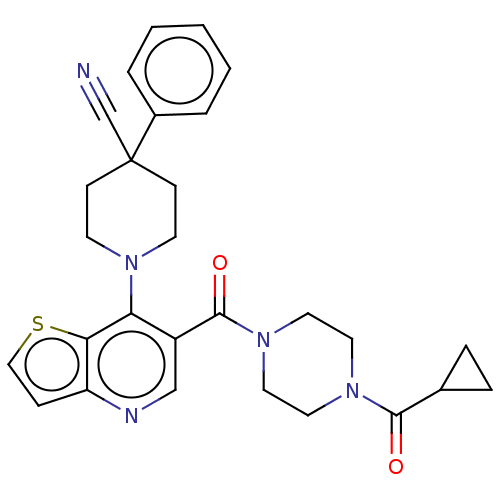 (CHEMBL4210811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase 1A1 (Homo sapiens (Human)) | BDBM50456251 (CHEMBL4213304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... | J Med Chem 61: 4883-4903 (2018) Article DOI: 10.1021/acs.jmedchem.8b00270 BindingDB Entry DOI: 10.7270/Q2SB48BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1235 total ) | Next | Last >> |