

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Escherichia coli) | BDBM466971 (2-fluoro-2-(((2S,5R)-3-methyl-7-oxo-2-((pyrazin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149467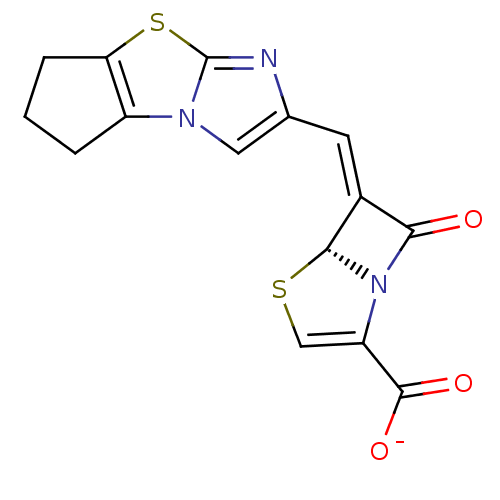 ((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149466 (CHEMBL124416 | Sodium; (R)-6-[1-(5,6-dihydro-4H-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466985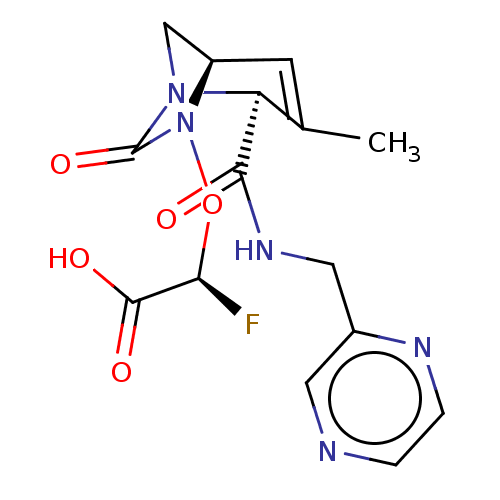 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(pyrazi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466999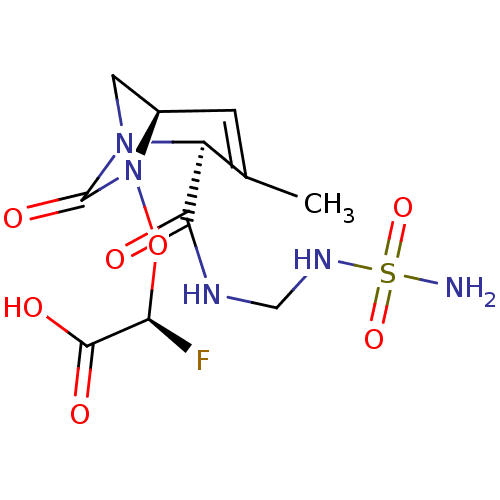 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[(sulfa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466998 ((2S)-2-fluoro-2-[[(2S,5R)-2-(acetamidomethylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466979 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(2-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466987 ((2S)-2-[[(2S,5R)-2-[(3-amino-3-oxo-propyl)carbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149468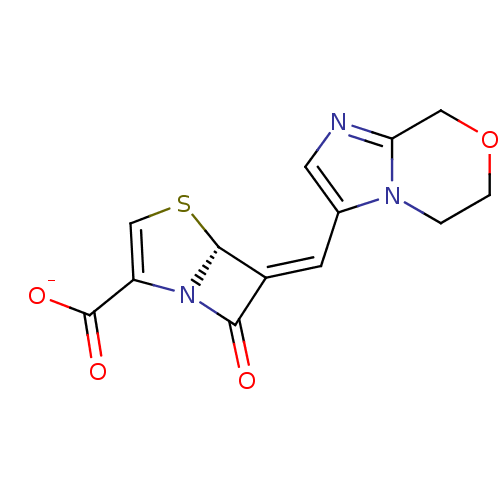 (CHEMBL263746 | Sodium; (R)-6-[1-(5,6-dihydro-8H-im...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149469 (CHEMBL331090 | Sodium; (R)-7-oxo-6-[1-(5,6,7,8-tet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466983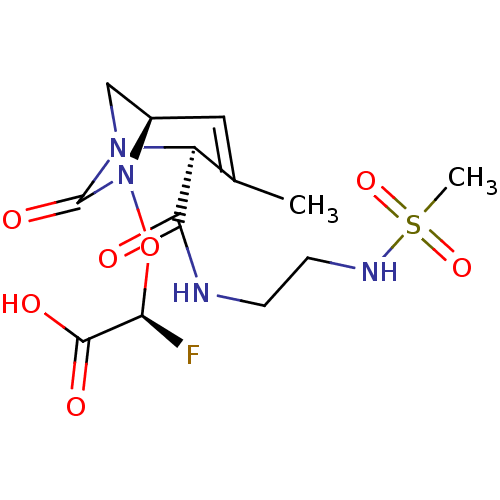 ((2S)-2-fluoro-2-[[(2S,5R)-2-[2-(methanesulfonamido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114518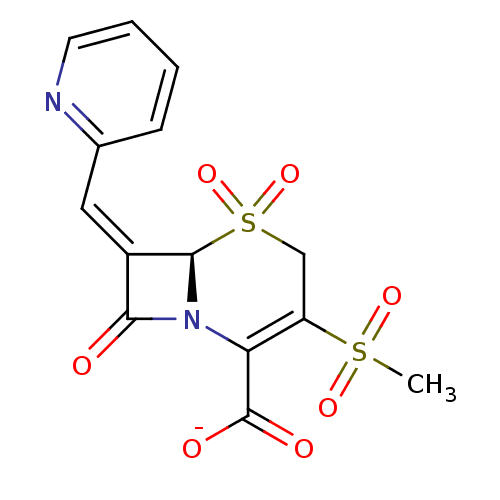 (CHEMBL297805 | Sodium; (R)-3-methanesulfonyl-5,5,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114514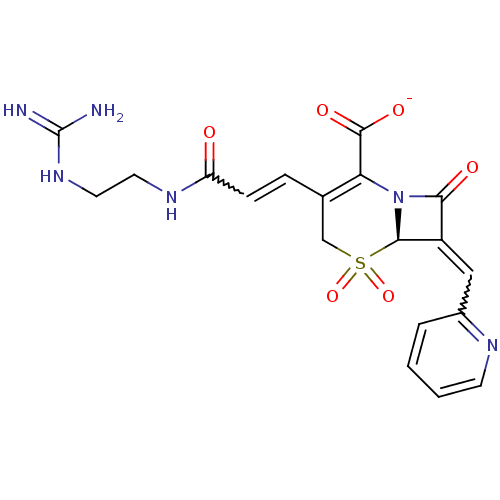 (CHEMBL44932 | Sodium; (R)-3-[(E)-2-(2-guanidino-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466970 (2-(((2S,5R)-2-(2-acetylhydrazinecarbonyl)-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466984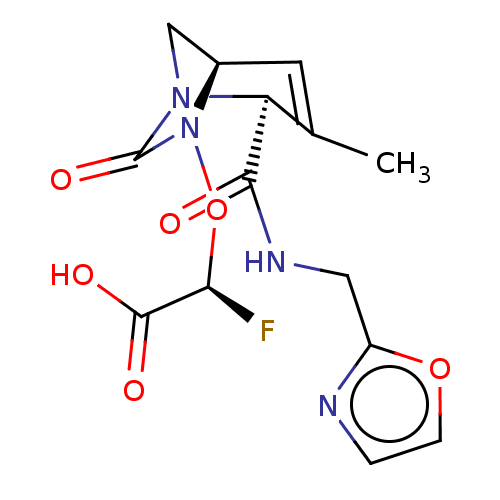 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-2-(oxazol-2-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114516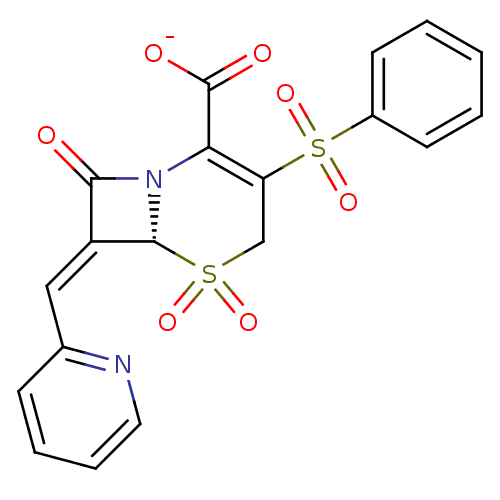 (CHEMBL295322 | Sodium; (R)-3-benzenesulfonyl-5,5,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466989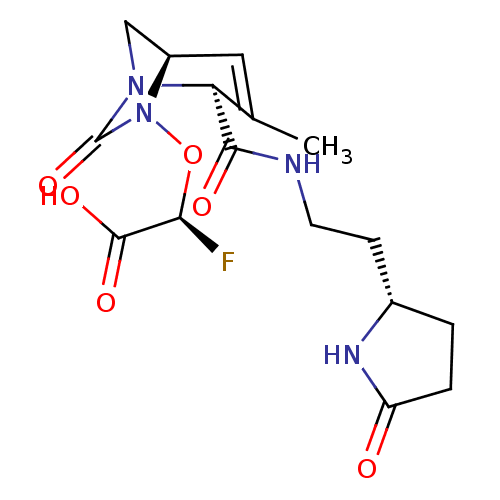 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[2-(5-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466965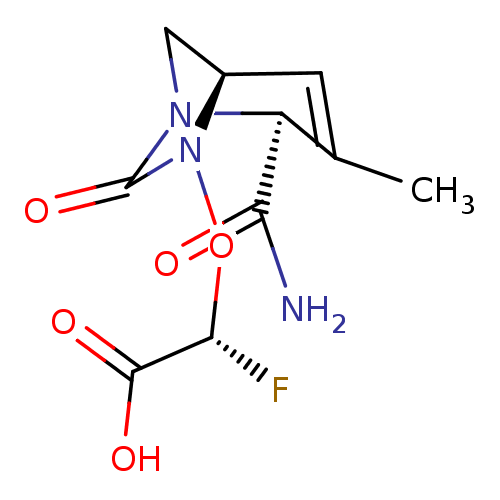 ((2R)-{[(2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114521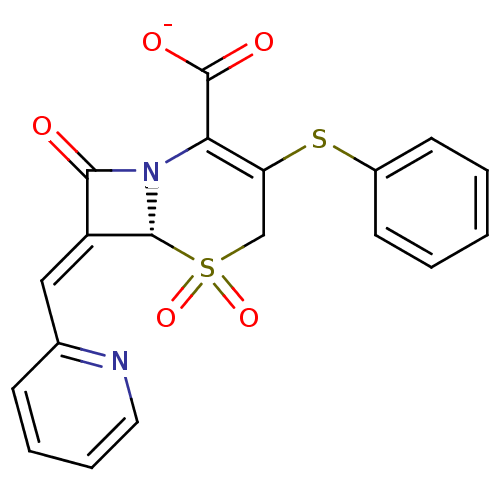 (CHEMBL42528 | Sodium; (R)-5,5,8-trioxo-3-phenylsul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466967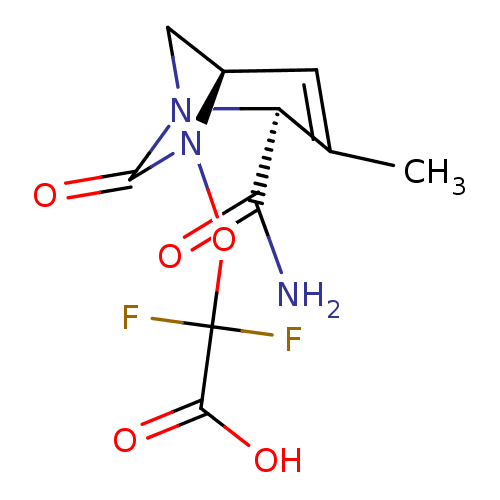 (US10800778, Example 8 | {[(2S,5R)-2-carbamoyl-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466996 ((2S)-2-fluoro-2-[[(2S,5R)-2-(cyanomethylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466991 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[2-(sul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466997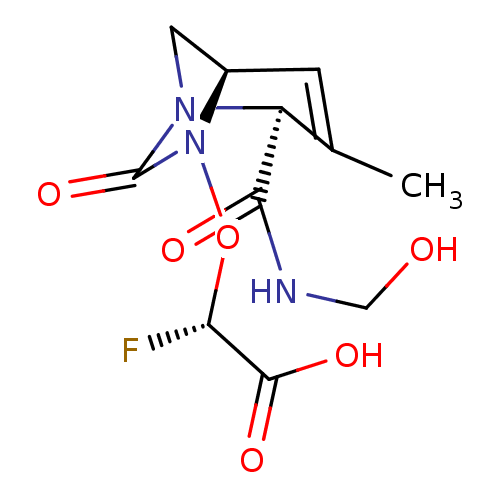 ((2S)-2-fluoro-2-[[(2S,5R)-2-(hydroxymethylcarbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466966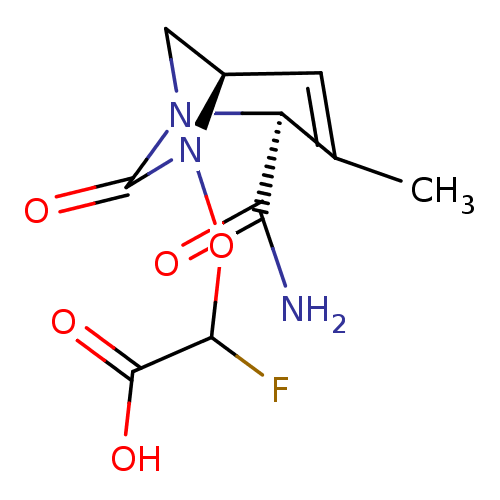 (US10800778, Example 5 | {[(2S,5R)-2-carbamoyl-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466974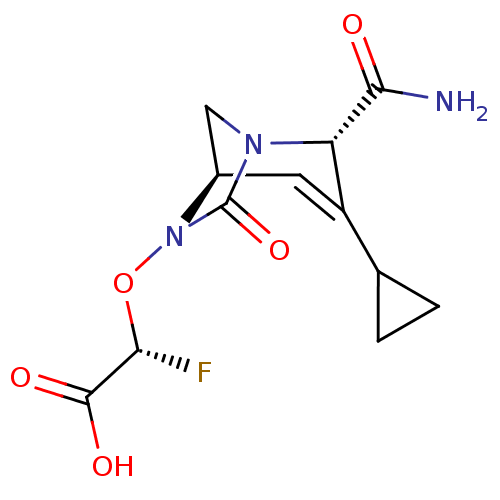 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466990 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(3-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067053 (CHEMBL127782 | Sodium; (1S,5R)-2-benzyloxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067066 (CHEMBL340707 | Sodium; (1S,4R,5S)-2-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM467000 (2-(((2S,5R)-2-carbamoyl-4-methyl-7-oxo-1,6-diazabi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466981 ((2S)-2-(((2S,5R)-2-((((S)-4,4-difluoropyrrolidin-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114515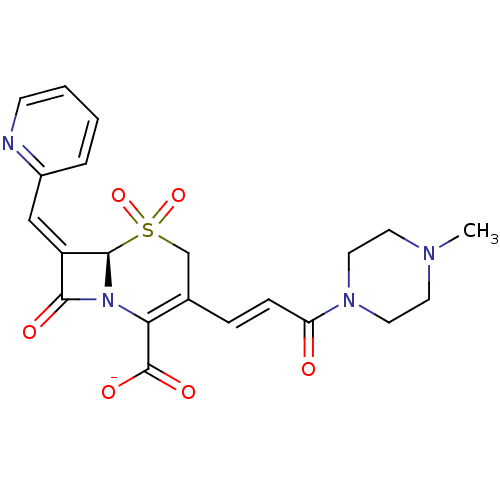 (CHEMBL416447 | Sodium; (R)-3-[(E)-3-(4-methyl-pipe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466988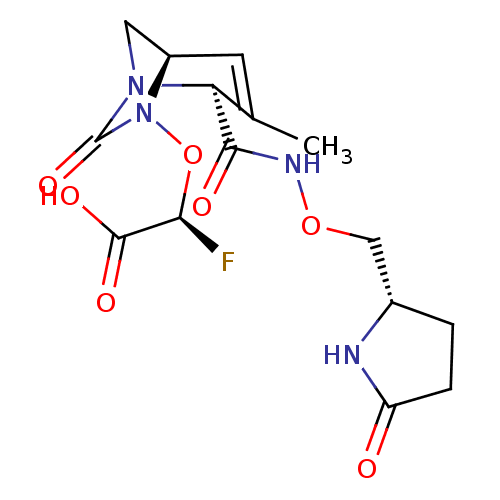 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[(5-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466982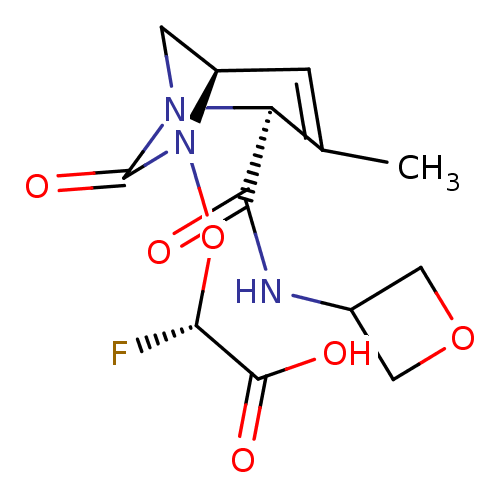 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-2-(oxetan-3-ylc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076671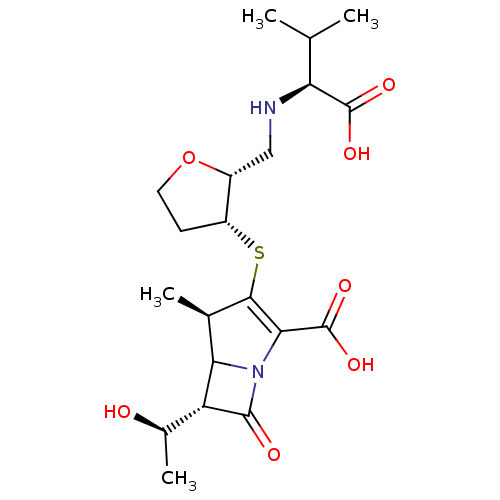 ((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114524 (CHEMBL47198 | Sodium; (R)-3-[(E)-2-(2-hydroxy-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466964 ((2S)-{[(2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149467 ((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class A (Imi-1) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114513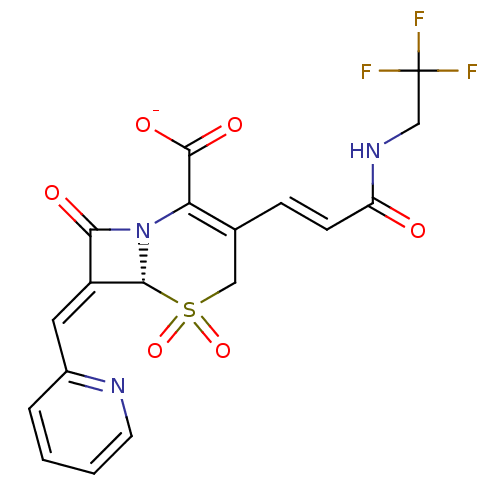 (CHEMBL44813 | Sodium; (R)-5,5,8-trioxo-7-[1-pyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM178203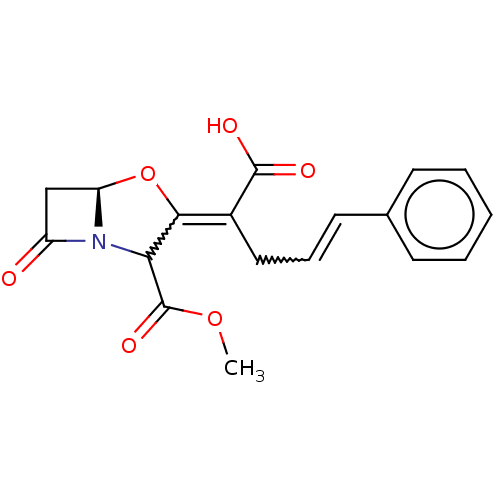 (US9120808, Example 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 88.4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
NABRIVA THERAPEUTICS AG US Patent | Assay Description The inhibitory concentrations (IC50, [uM]) of the beta -lactamase inhibitors against purified TEM-1, SHV-1 and AmpC beta -lactamases are assessed by ... | US Patent US9120808 (2015) BindingDB Entry DOI: 10.7270/Q2Q81BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466975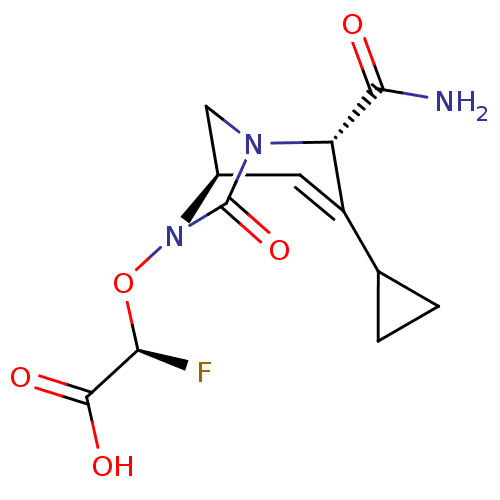 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50140673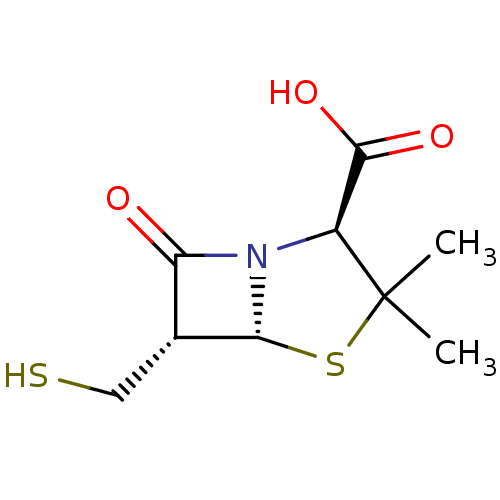 ((2S,5R,6S)-6-Mercaptomethyl-3,3-dimethyl-7-oxo-4-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Beta-Lactamase inhibitory activity against representativeclass C (P99) serine enzyme | Bioorg Med Chem Lett 14: 1299-304 (2004) Article DOI: 10.1016/j.bmcl.2003.12.037 BindingDB Entry DOI: 10.7270/Q22Z14ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114506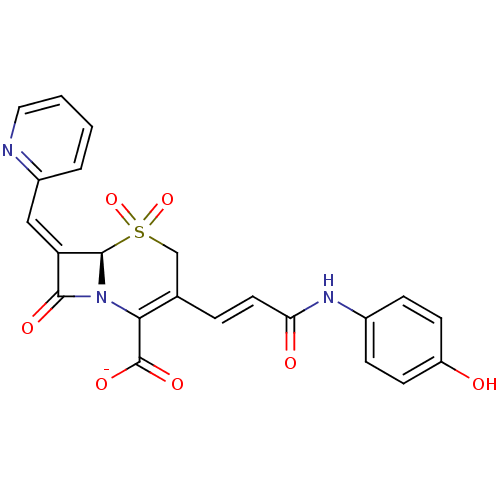 (CHEMBL44278 | Sodium; (R)-3-[(E)-2-(4-hydroxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076673 (CHEMBL175189 | Sodium; (2S,5R,6R)-6-((S)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM178165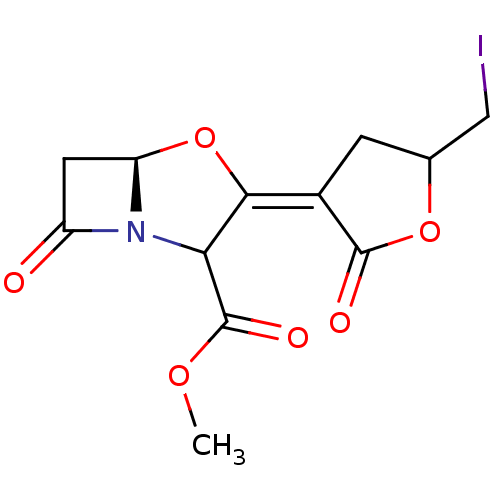 (US9120808, Mixture of Example 43 and Example 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | 7.0 | 37 |
NABRIVA THERAPEUTICS AG US Patent | Assay Description The inhibitory concentrations (IC50, [uM]) of the beta -lactamase inhibitors against purified TEM-1, SHV-1 and AmpC beta -lactamases are assessed by ... | US Patent US9120808 (2015) BindingDB Entry DOI: 10.7270/Q2Q81BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466986 ((2S)-2-[[(2S,5R)-2-(cyclopropylmethoxycarbamoyl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466978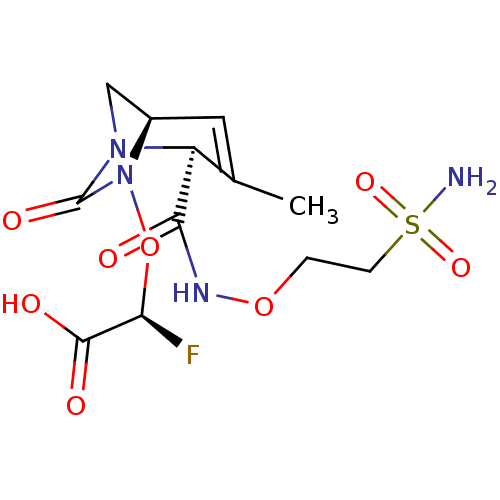 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(2-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM26139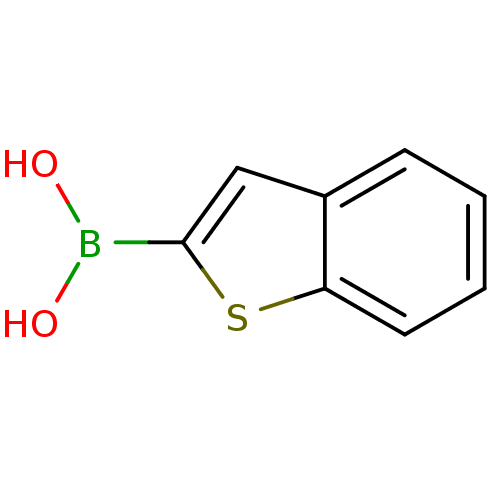 (1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Compound was tested for its specificity against AmpC beta-lactamase | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466972 ((2R)-2-(((2S,5R)-2-carbamoyl-4-methyl-7-oxo-1,6-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466977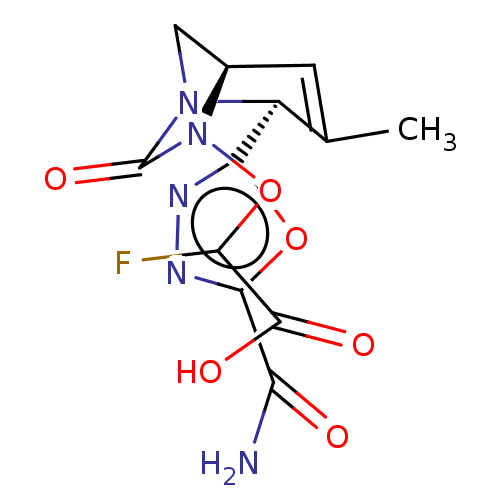 (2-[[(2S,5R)-2-(5-carbamoyl-1,3,4-oxadiazol-2-yl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 504 total ) | Next | Last >> |