

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209917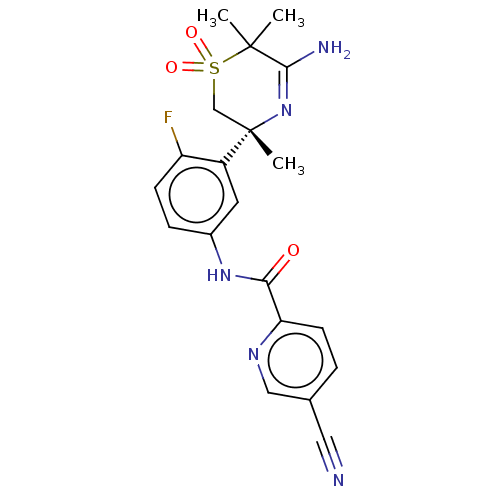 (US9273042, 6 | US9556135, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.000200 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012660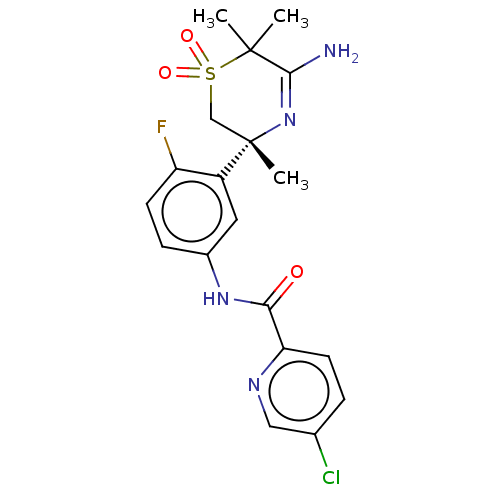 (CHEMBL3261078 | US9273042, 5 | US9556135, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.000900 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209918 (US9273042, 7 | US9556135, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.000900 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209920 (US9273042, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209919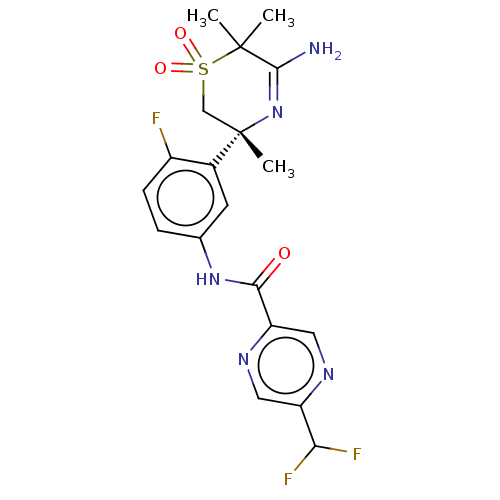 (US9273042, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00180 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209916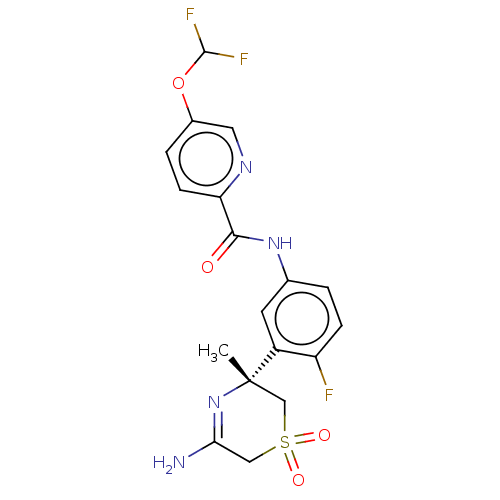 (US9273042, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00570 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM160666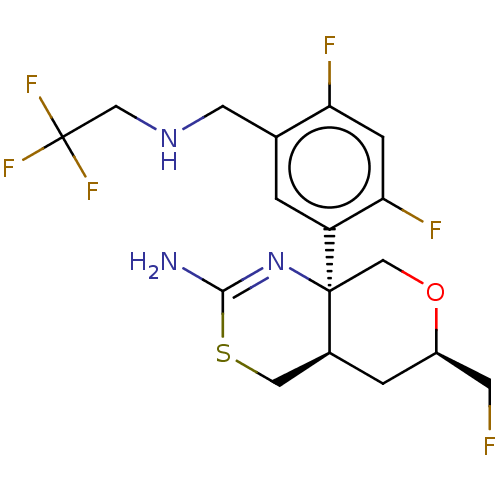 (US9045498, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209915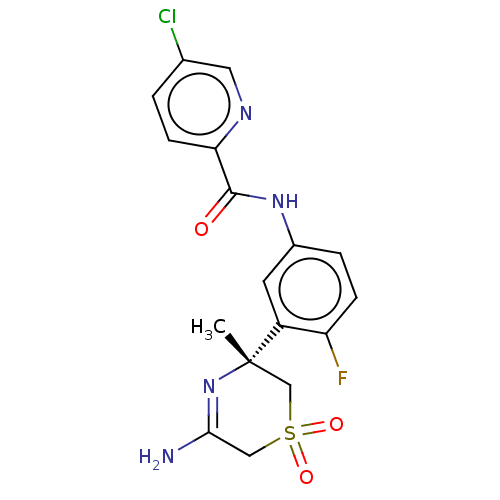 (US9273042, 3 | US9556135, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00610 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209914 (US9273042, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00820 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM209913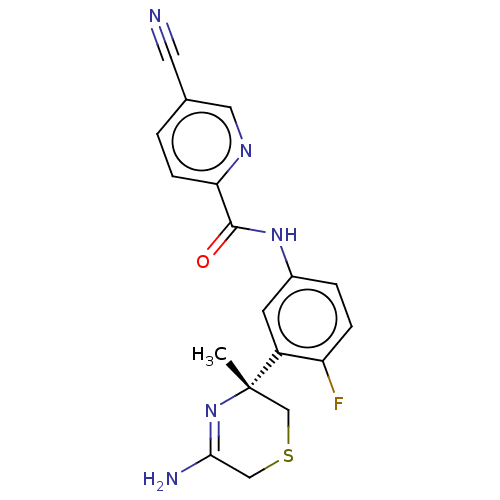 (US9273042, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10% (v... | US Patent US9273042 (2016) BindingDB Entry DOI: 10.7270/Q22Z14CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223332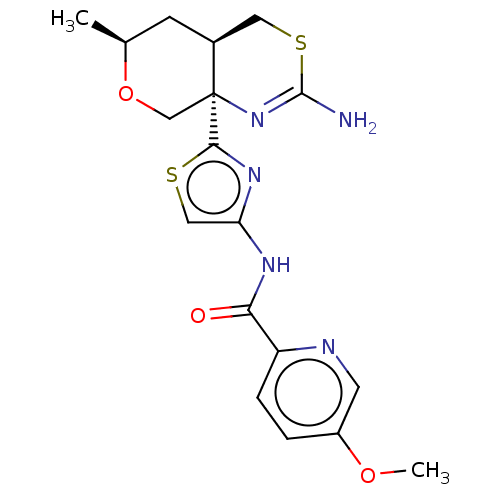 (US9315520, 19 | US9605007, Example 19 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50005021 (CHEMBL2408786 | US9650336, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as release of sAPPbeta after 17 hrs | ACS Med Chem Lett 4: 578-9 (2013) Article DOI: 10.1021/ml400177y BindingDB Entry DOI: 10.7270/Q2BR8TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452875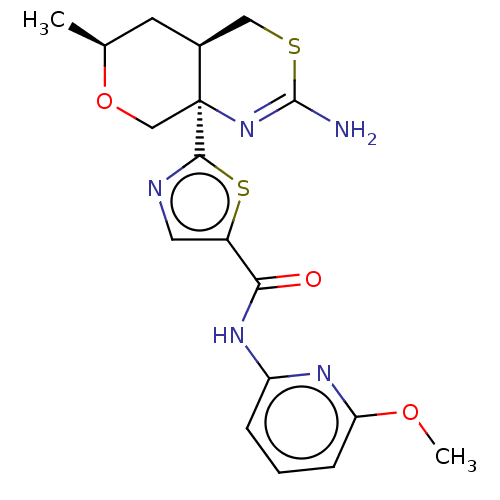 (CHEMBL4212046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452884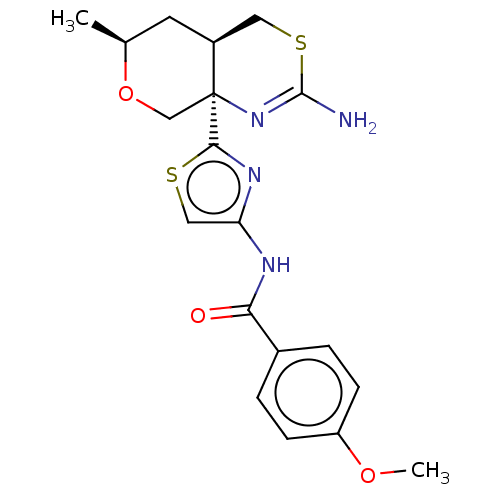 (CHEMBL4217023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510795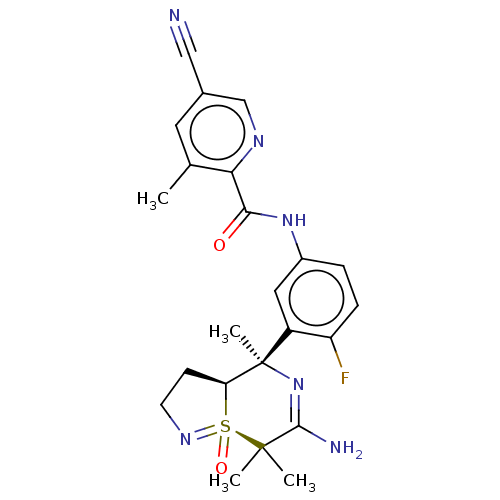 (CHEMBL4561849) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells harboring APP assessed as reduction in amyloidbeta 40 level incubated for 18 to 20 hrs by AlphaLISA Assay | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 BindingDB Entry DOI: 10.7270/Q2S185SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124179 (US8754075, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452883 (CHEMBL4203860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM589176 (US11548903, Example 109) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398266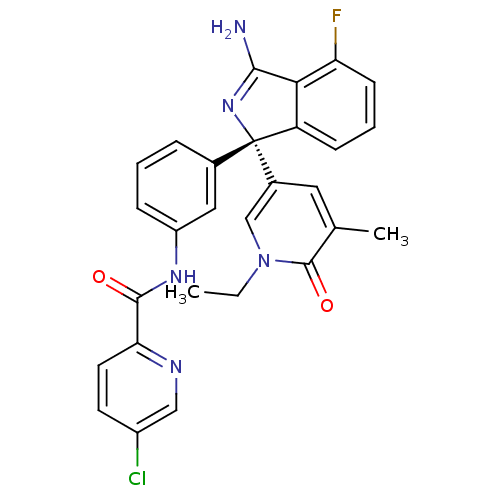 (CHEMBL2177305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassay | J Med Chem 55: 9346-61 (2012) Article DOI: 10.1021/jm3009025 BindingDB Entry DOI: 10.7270/Q2WW7JTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387062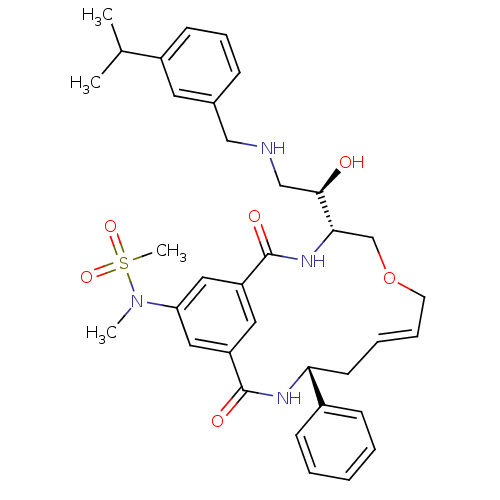 (CHEMBL2047041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Link£ping University Curated by ChEMBL | Assay Description Inhibition of BACE1 expressed in human HEK293 cells expressing Swedish mutant APP assessed as reduction on amyloid beta (1 to 40) production after 24... | Bioorg Med Chem 20: 4377-89 (2012) Article DOI: 10.1016/j.bmc.2012.05.039 BindingDB Entry DOI: 10.7270/Q2N29Z06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510787 (CHEMBL4564324) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells transfected with APP695 assessed as reduction in amyloidbeta (1 to 40 residues) incubated for 24 hrs by sa... | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 BindingDB Entry DOI: 10.7270/Q2S185SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149939 (US8975415, 221 | US9242973, 221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | 37 |
COMENTIS, INC. US Patent | Assay Description Test Example 3: The potency of compounds against BACE1 activity was determined in a cellular assay of Aβ production. Human SK-N-BE(2) neuroblas... | US Patent US9242973 (2016) BindingDB Entry DOI: 10.7270/Q29W0D9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393088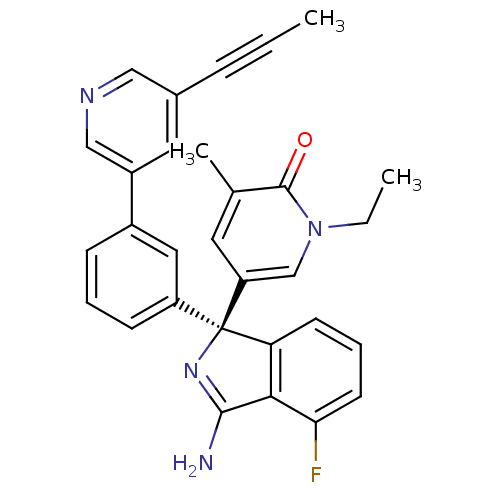 (CHEMBL2152903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassay | J Med Chem 55: 9346-61 (2012) Article DOI: 10.1021/jm3009025 BindingDB Entry DOI: 10.7270/Q2WW7JTP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM589190 (US11548903, Example 120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312923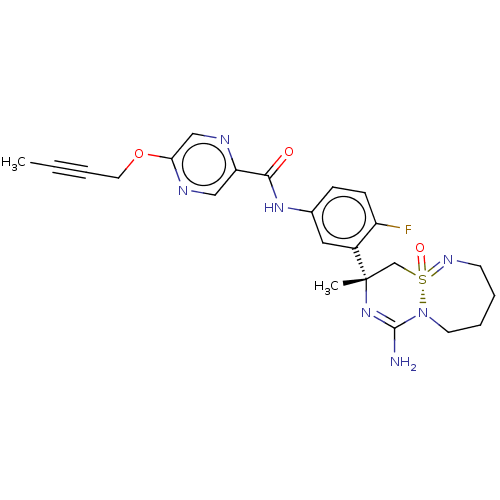 (5-But-2-ynyloxy-pyrazine-2-carboxylic acid [3-((3R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells expressing APP assessed as decrease in amyloid beta-40 level after 18 to 20 hrs | ACS Med Chem Lett 6: 1031-4 (2015) Article DOI: 10.1021/acsmedchemlett.5b00321 BindingDB Entry DOI: 10.7270/Q2BP05S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510993 (CHEMBL4437161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510977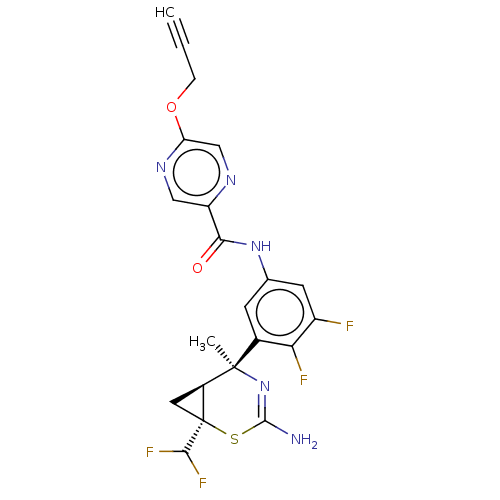 (CHEMBL4577527) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510794 (CHEMBL4588608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells harboring APP assessed as reduction in amyloidbeta 40 level incubated for 18 to 20 hrs by AlphaLISA Assay | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 BindingDB Entry DOI: 10.7270/Q2S185SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312923 (5-But-2-ynyloxy-pyrazine-2-carboxylic acid [3-((3R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in HEK293 cells harboring APP assessed as reduction in amyloidbeta 40 level measured after 18 to 20 hrs by AlphaLISA Assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00960 BindingDB Entry DOI: 10.7270/Q29W0K46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312923 (5-But-2-ynyloxy-pyrazine-2-carboxylic acid [3-((3R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The Abeta 40 AlphaLISA Assay can be used. The HEK293 APP cells were seeded in 96 well Microtiter plates in cell culture medium (Iscove's, plus 10... | US Patent US9605006 (2017) BindingDB Entry DOI: 10.7270/Q2PG1TS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012629 (CHEMBL3261045) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of Fc domain of IgG1-fused human BACE (1 to 460) expressed in HEK293 cells preincubated for 10 mins followed by substrate addition measure... | Bioorg Med Chem Lett 24: 2033-45 (2014) Article DOI: 10.1016/j.bmcl.2014.03.025 BindingDB Entry DOI: 10.7270/Q2H41T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393088 (CHEMBL2152903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 by secreted AAPbeta release assay | ACS Med Chem Lett 3: 869-870 (2012) Article DOI: 10.1021/ml300317n BindingDB Entry DOI: 10.7270/Q27S7PVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061732 (CHEMBL3394218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061780 (CHEMBL3394226) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061831 (CHEMBL3394227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM147165 (US8957083, 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc. US Patent | Assay Description The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... | US Patent US8957083 (2015) BindingDB Entry DOI: 10.7270/Q25Q4TS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012615 (CHEMBL3260839) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 1 hr prior to testing measured after 1 hr by FRET assay | Bioorg Med Chem Lett 24: 2033-45 (2014) Article DOI: 10.1016/j.bmcl.2014.03.025 BindingDB Entry DOI: 10.7270/Q2H41T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM372815 ((1R,5S,6S)-5-(5-((Z)-2-(5-(but-2-yn-1-yloxy)pyrazi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | J Med Chem 49: 6652-5 (2006) BindingDB Entry DOI: 10.7270/Q23J3G8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM589169 (US11548903, Example 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.241 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50569350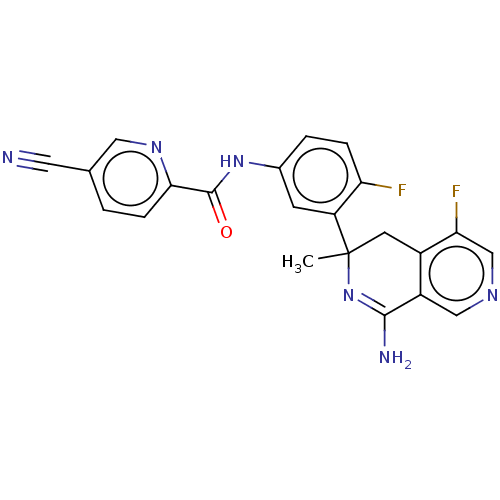 (CHEMBL4861687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in human SH-SY5Y cells expressing APP assessed as reduction in Abeta40 by HTRF assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113270 BindingDB Entry DOI: 10.7270/Q21R6V8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM400979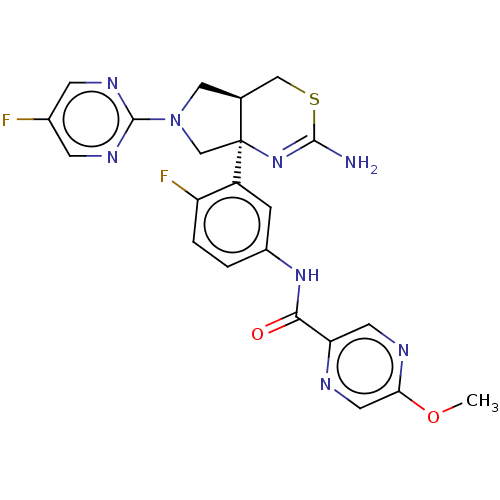 (US9999624, Compound 4) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393099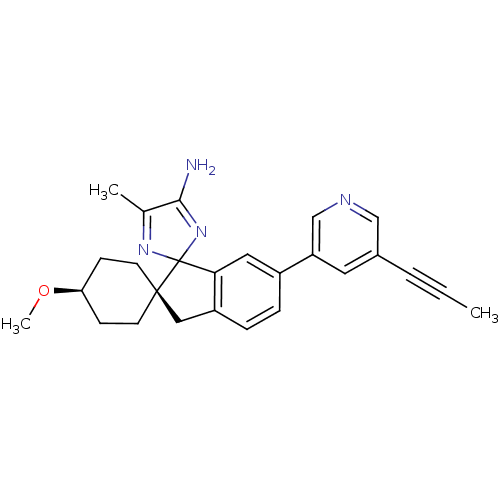 (CHEMBL2152914 | US10231967, Example 122 | US991898...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE by secreted APPbeta release assay | ACS Med Chem Lett 3: 875-876 (2012) Article DOI: 10.1021/ml300324q BindingDB Entry DOI: 10.7270/Q2VM4DC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM589185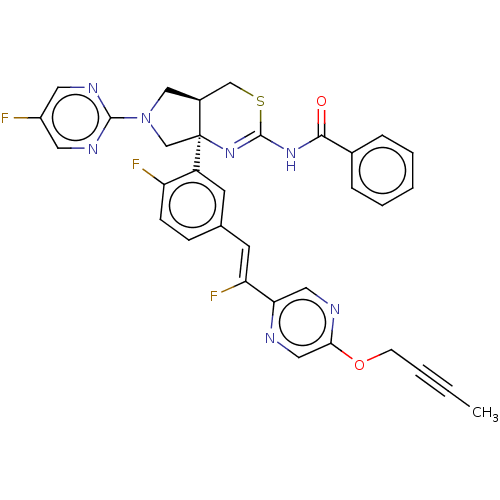 (US11548903, Example 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.287 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510987 (CHEMBL4472708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012614 (CHEMBL3260838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as inhibition of amyloid beta (1 to 40) production after 48 hrs by HTRF immunoassay | Bioorg Med Chem Lett 24: 2033-45 (2014) Article DOI: 10.1016/j.bmcl.2014.03.025 BindingDB Entry DOI: 10.7270/Q2H41T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510987 (CHEMBL4472708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE in HEK293 cells harboring APP Swedish mutant assessed as reduction in amyloid beta 40 level measured after overnight incubation by... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50032737 (CHEMBL3354718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) by fluorescence assay | Bioorg Med Chem Lett 25: 767-74 (2015) Article DOI: 10.1016/j.bmcl.2014.12.092 BindingDB Entry DOI: 10.7270/Q21G0NXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061730 (CHEMBL3394216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061734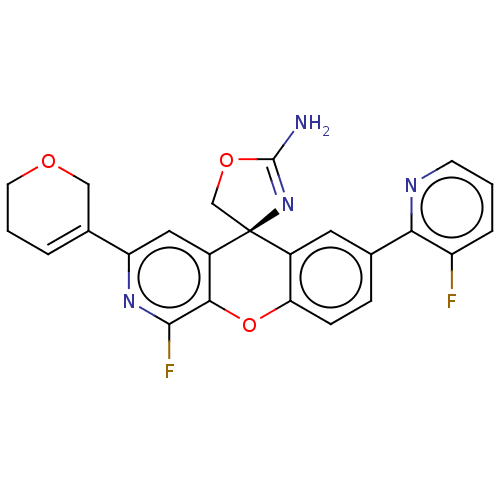 (CHEMBL3394220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 15152 total ) | Next | Last >> |