

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395732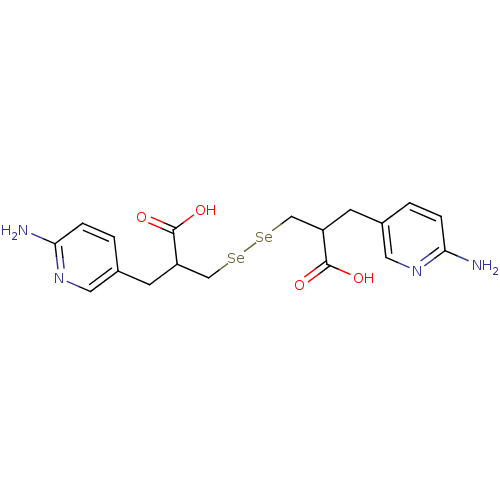 (CHEMBL2164462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395730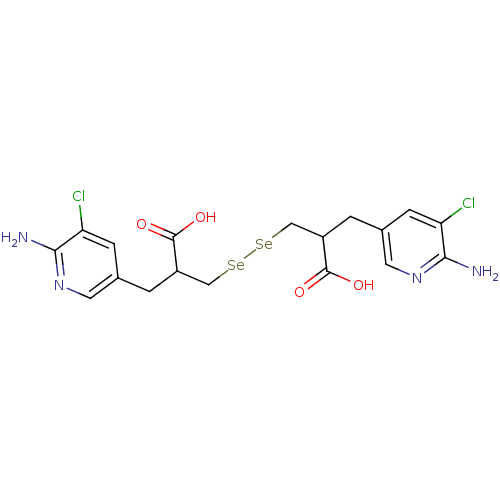 (CHEMBL2164450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50395732 (CHEMBL2164462) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM537094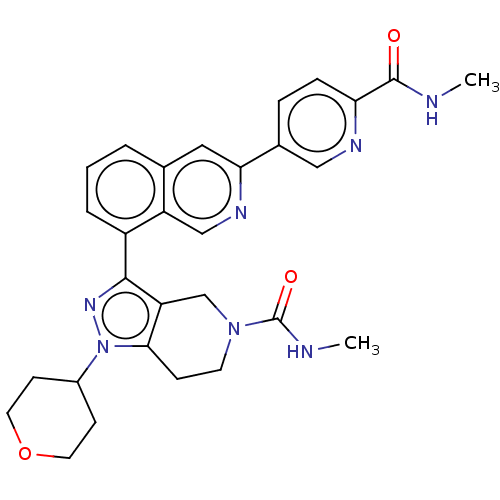 (US11247989, Example 92) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50453957 (CHEMBL4217213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged CBP (unknown origin) by TR-FRET assay | Bioorg Med Chem Lett 28: 15-23 (2018) Article DOI: 10.1016/j.bmcl.2017.11.025 BindingDB Entry DOI: 10.7270/Q2ZG6VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM537100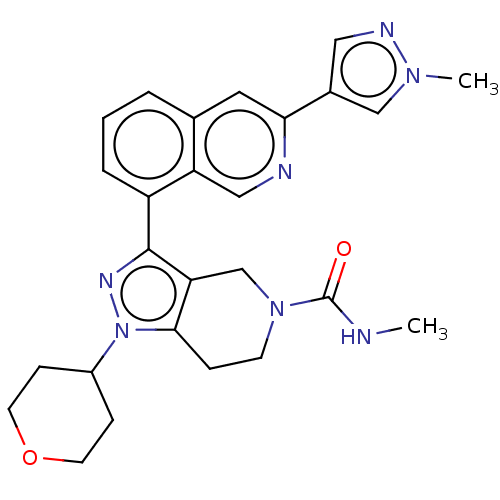 (US11247989, Example 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269847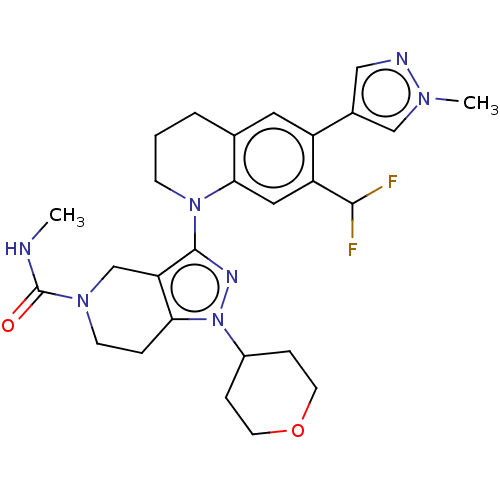 (CHEMBL4097025) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536872 (1-(1-acetylpiperidin-4-yl)-N-methyl-3-(3-(2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50453949 (CHEMBL4208820 | US11247989, Example 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.997 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511322 (3-Cyclopropyl-N-methyl-1-(3-(2-methylthiazol-5-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511316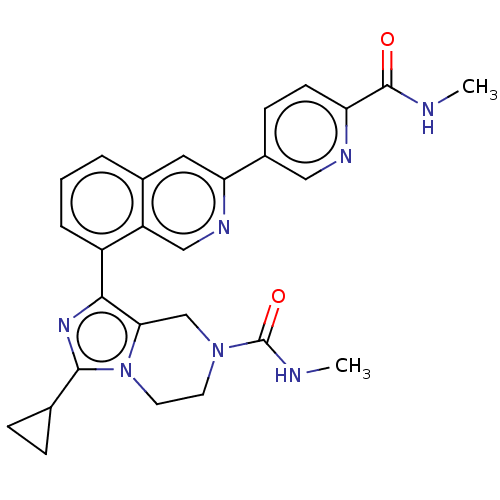 (3-Cyclopropyl-N-methyl-1-(3-(6-(methylcarbamoyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575776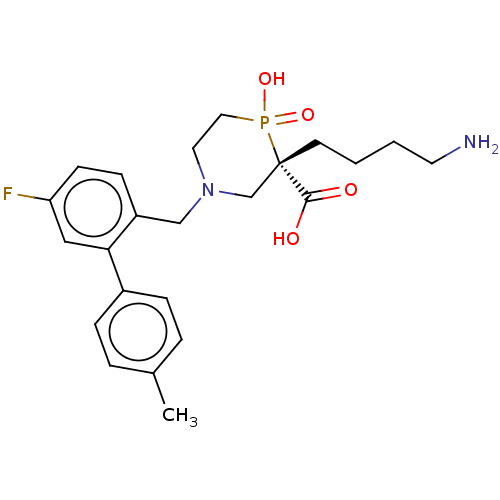 (CHEMBL4868605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50089691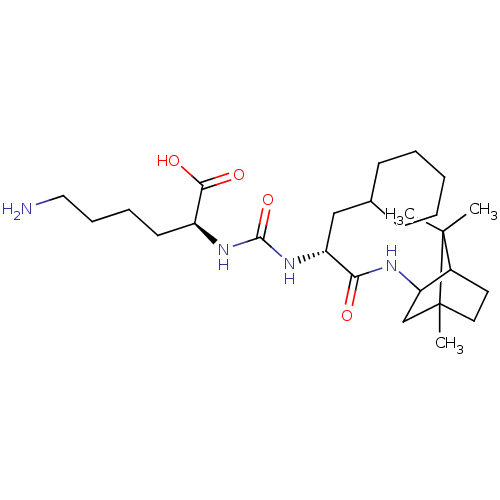 (CHEMBL3577425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of CBP (unknown origin) | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50144342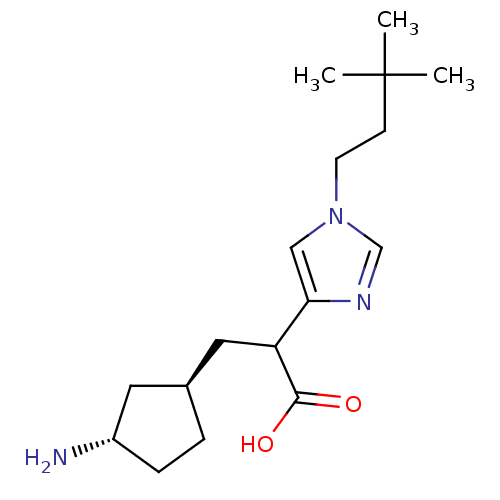 (3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carboxypeptidase B (CPB) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50144342 (3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50144336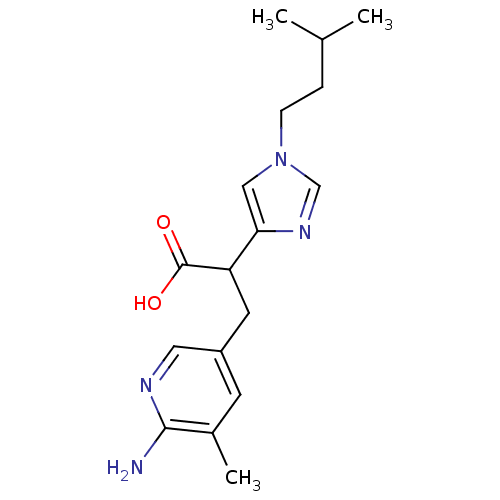 (3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(3-methyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carboxypeptidase B (CPB) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM537101 (US11247989, Example 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50144333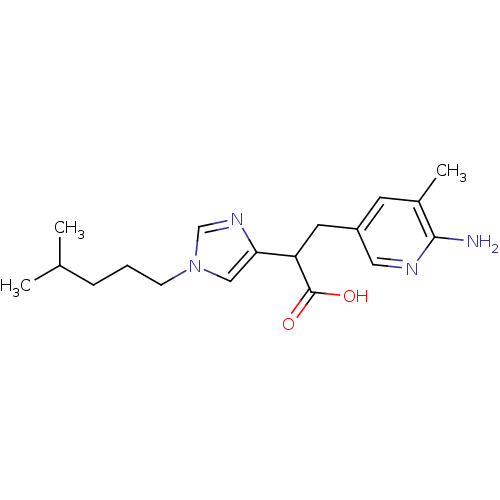 (3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(4-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carboxypeptidase B (CPB) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM340748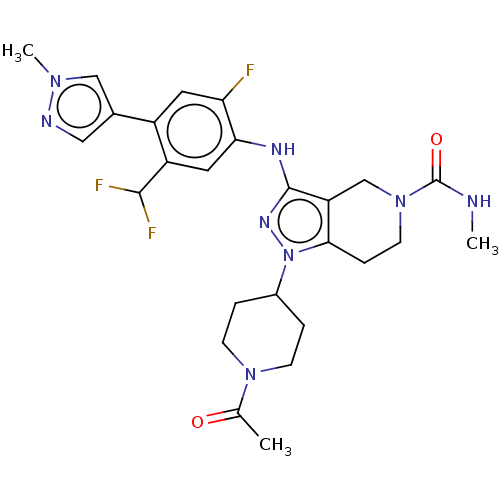 (US9763922, Example 274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GENENTECH, INC.; CONSTELLATION PHARMACEUTICALS, INC. US Patent | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | US Patent US9763922 (2017) BindingDB Entry DOI: 10.7270/Q23N25G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM340734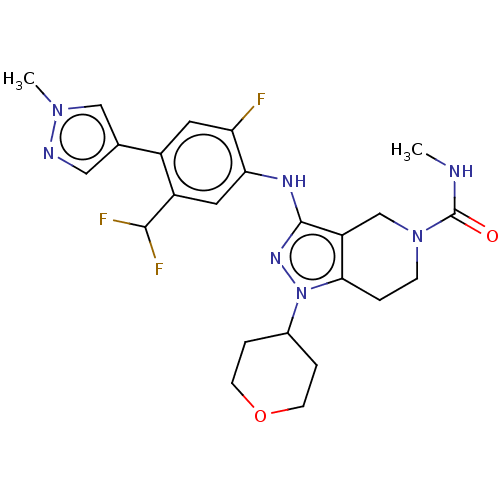 (3-[5-(difluoromethyl)-2-fluoro-4-(1-methylpyrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GENENTECH, INC.; CONSTELLATION PHARMACEUTICALS, INC. US Patent | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | US Patent US9763922 (2017) BindingDB Entry DOI: 10.7270/Q23N25G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089758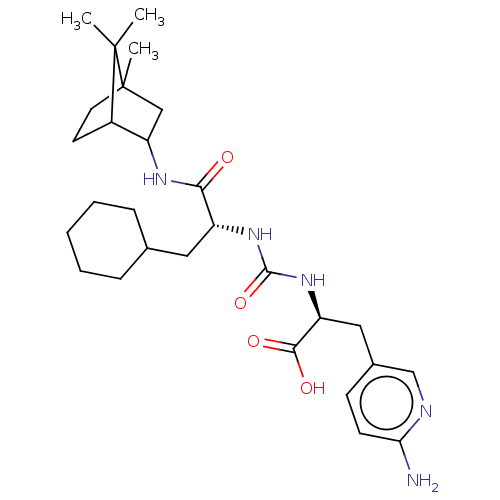 (CHEMBL3577442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269857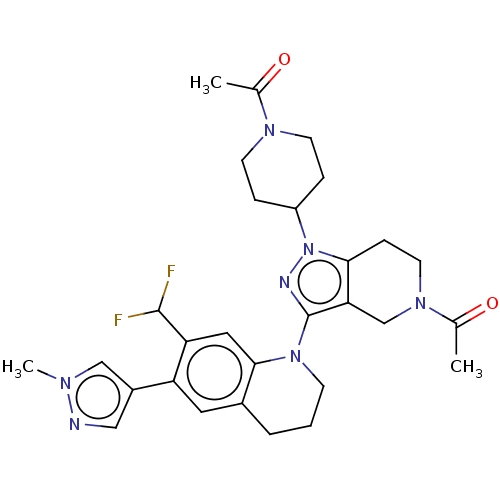 (CHEMBL4088793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269848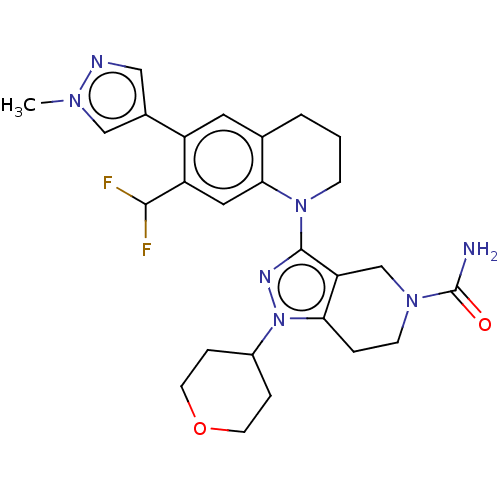 (CHEMBL4069831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511394 (3-(4,4- difluorocyclohexyl)-1-(7- fluoro-3-(2-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511390 (1-(7-fluoro-3-(2- methylthiazol-5- yl)isoquinolin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511377 (3-(4,4- difluorocyclohexyl)-N- methyl-1-(2-(1-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511356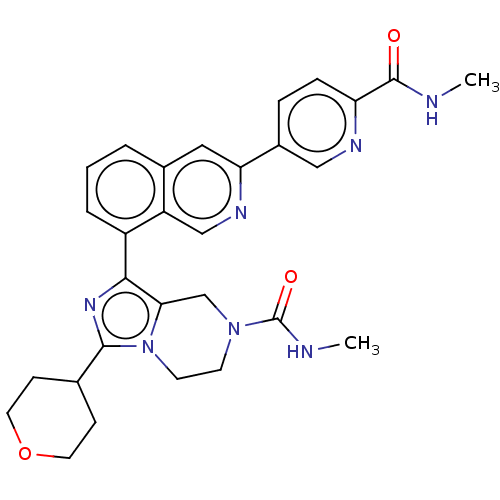 (N-methyl-1-(3-(6- (methylcarbamoyl)pyridin- 3-yl)i...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536819 (3-(6-(difluoromethyl)-7-(1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM537087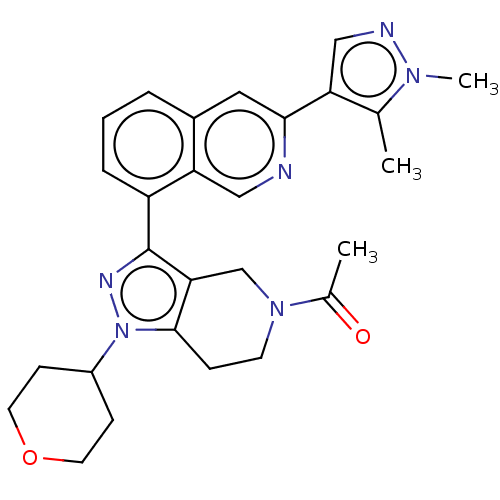 (US11247989, Example 90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269849 (CHEMBL4076748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50395737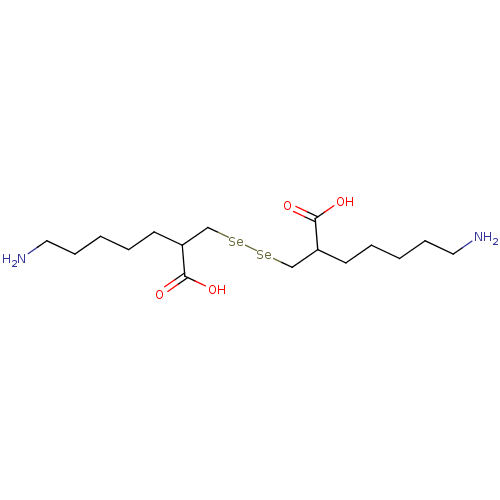 (CHEMBL2164457) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50395735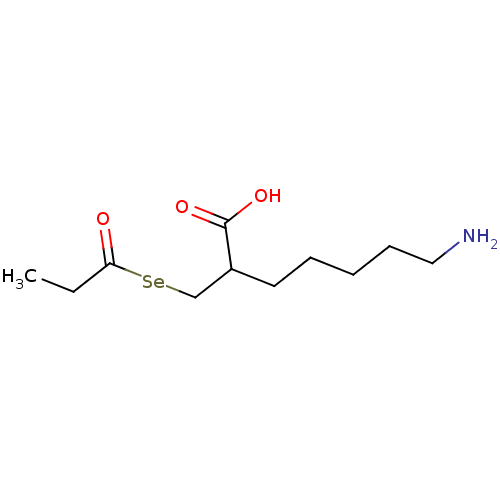 (CHEMBL2164459) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395735 (CHEMBL2164459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536735 (US11247989, Example 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536741 (US11247989, Example 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50453950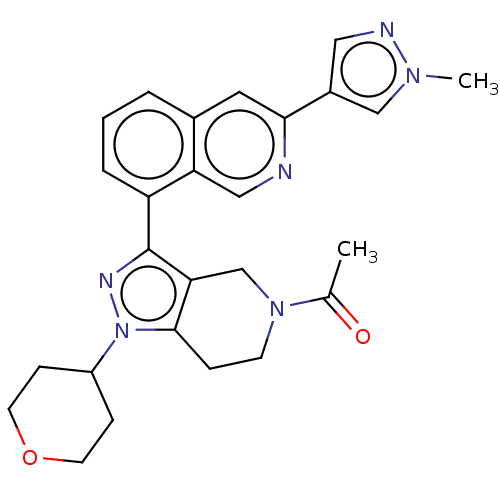 (CHEMBL4203082 | US11247989, Example 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 1.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM537088 (US11247989, Example 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269855 (CHEMBL4061161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269856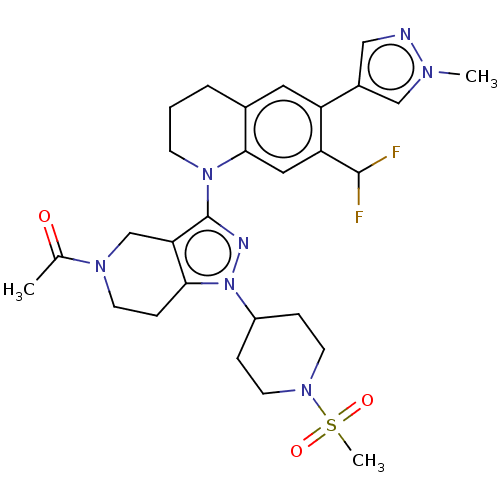 (CHEMBL4060566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50453949 (CHEMBL4208820 | US11247989, Example 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged CBP (unknown origin) by TR-FRET assay | Bioorg Med Chem Lett 28: 15-23 (2018) Article DOI: 10.1016/j.bmcl.2017.11.025 BindingDB Entry DOI: 10.7270/Q2ZG6VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50453950 (CHEMBL4203082 | US11247989, Example 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged CBP (unknown origin) by TR-FRET assay | Bioorg Med Chem Lett 28: 15-23 (2018) Article DOI: 10.1016/j.bmcl.2017.11.025 BindingDB Entry DOI: 10.7270/Q2ZG6VVZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536862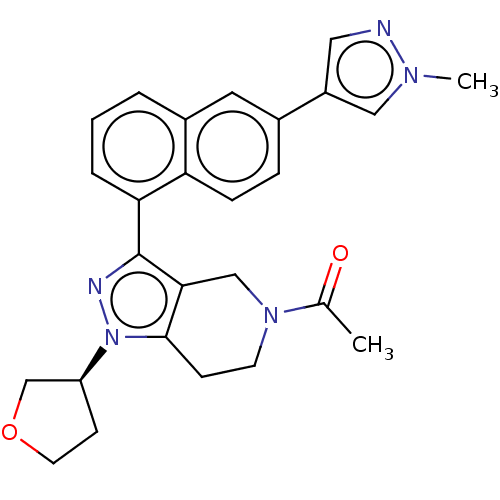 ((S)-1-(3-(6-(1-methyl-1H-pyrazol-4-yl)naphthalen-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269850 (CHEMBL4061600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536875 (2-cyano-N-methyl-3-(3-(1-methyl-1H-pyrazol-4-yl)is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM536860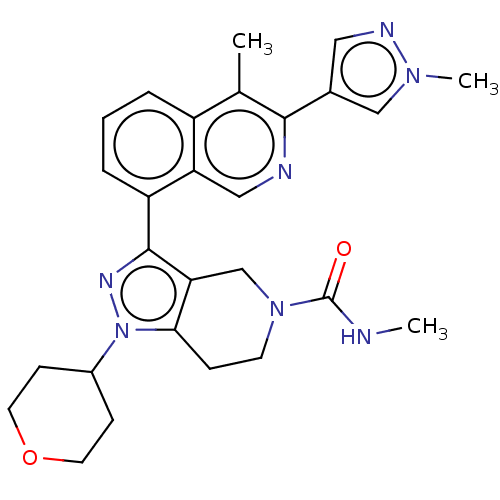 (N-methyl-3-(4-methyl-3-(1-methyl-1H-pyrazol-4-yl)i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description His/Flag epitope tagged CBP was cloned, expressed, and purified to homogeneity. CBP binding and inhibition was assessed by monitoring the engagement ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X63R43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50275212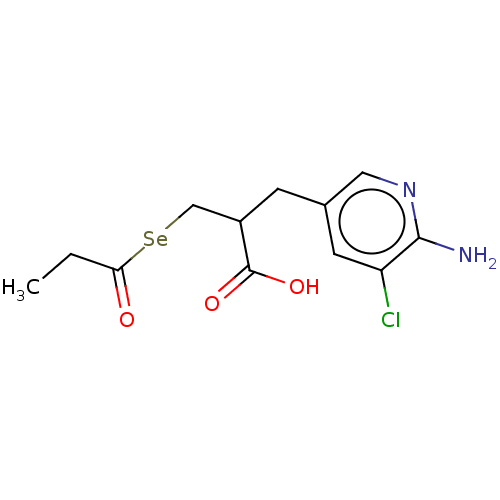 (CHEMBL4127473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human plasma activated thrombin-activatable fibrinolysis inhibitor after 10 mins in presence of DTT | Bioorg Med Chem Lett 28: 2256-2260 (2018) Article DOI: 10.1016/j.bmcl.2018.05.042 BindingDB Entry DOI: 10.7270/Q25M686N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269907 (CHEMBL4080905) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50269914 (CHEMBL4083416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay | J Med Chem 60: 9162-9183 (2017) Article DOI: 10.1021/acs.jmedchem.7b00796 BindingDB Entry DOI: 10.7270/Q23F4S4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50602881 (CHEMBL5204688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01864 BindingDB Entry DOI: 10.7270/Q2VT1X5F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089688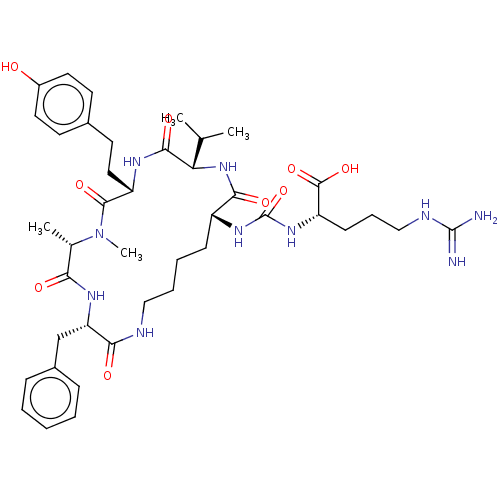 (ANABAENOPEPTIN B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5610 total ) | Next | Last >> |